Therapy-related acute lymphoblastic leukemia with t(4;11)(q21;q23) masqueraded as marrow lymphocytosis in a patient with breast cancer
Abstract
Therapy-related acute leukemia develops in patients after chemotherapy and/or radiotherapy for a prior cancer, and most cases are acute myeloid leukemia with a much lower frequency of acute lymphoblastic leukemia (ALL). One unique feature of these therapy-related ALL (t-ALL) is an increased incidence of chromosome band 11q23 aberrations as compared with de novo ALL. In adult female patients, breast cancer is the most common primary cancer. Herein, we report the case of a 49-year-old Taiwanese lady who developed t-ALL with t(4;11)(q21;q23) 16 months after cyclophosphamide, epirubicin, and 5-fluorouracil chemotherapy for her breast cancer. The unusual feature is that the t-ALL was heralded 4 months ago by marrow lymphocytosis comprising atypical small lymphocytes with condensed chromatin mimicking a B-cell chronic lymphoproliferative disorder. Retrospective studies using additional antibodies for immunophenotyping and PCR-based clonality study for immunoglobulin gene rearrangement showed that these atypical small lymphocytes shared similar features with the leukemic blasts at the frank leukemic stage. Our results suggest that these atypical small lymphocytes are lymphoblasts in disguise and that the clinicopathological correlations with ancillary pathological studies are important to reach a definitive diagnosis of such an unusual case.
Introduction
Therapy-related acute leukemia (t-AL) develops in patients after chemotherapy and/or radiotherapy for a prior cancer, and most cases are therapy-related acute myeloid leukemia (t-AML) with a much lower frequency of therapy-related acute lymphoblastic leukemia (t-ALL) [1]. The 2008 World Health Organization classification of hematopoietic and lymphoid neoplasms defined therapy-related myeloid neoplasms as a unique clinical syndrome and included t-AML, myelodysplastic syndrome, and myelodysplastic/myeloproliferative neoplasms [2]. On the other hand, t-ALL was not defined in the World Health Organization classification, probably because it is very rare and the clinicopathological features are poorly defined. One unique feature of these t-ALL is an increased incidence of chromosome band 11q23 aberrations as compared with de novo ALL [3], [4]. Rearrangements of the MLL gene located at chromosome band 11q23 is a hallmark of t-AL and myelodysplasia that result from treatment with topoisomerase II inhibitors, which includes anthracyclines [5]-[7]. Breast cancer survivors exhibit a long-term increased risk of t-AL, most commonly t-AML and less frequently t-ALL [8], [9]. In adult females with secondary ALL, breast cancer is the most common primary cancer, irrespectively of with or without prior anti-cancer treatment [3]. Herein, we report the case of a lady who developed frank t-ALL with t(4;11)(q21;q23) 16 months after cyclophosphamide, epirubicin, and 5-fluorouracil (CEF) chemotherapy for her breast cancer. The unusual feature is that the t-ALL was heralded 4 months ago by marrow lymphocytosis comprising atypical small lymphocytes with condensed chromatin mimicking a B-cell chronic lymphoproliferative disorder (B-LPD). Retrospective studies using immunophenotyping and clonality study for immunoglobulin gene rearrangement showed that these atypical small lymphocytes shared similar features with the leukemic blasts at the overt leukemic stage and suggested that the earliest t-ALL was present 4 months before the full-blown disease.
Case presentation
A 49-year-old Taiwanese woman was admitted in February 2009 with the chief complaints of general weakness, poor appetite, and dizziness. Tracing back her history, she was diagnosed with invasive ductal carcinoma of the left breast and underwent modified radial mastectomy and axillary lymph node dissection 11 months ago in March 2008. Staging results revealed a Stage IIA disease (T2N0M0) and she received six cycles of CEF chemotherapy without radiation. At admission, there was no fever, chillness, or ecchymosis. Physical examination revealed no lymphadenopathy, skin rash, or petechia. Chest X-ray was negative for any mass lesion or mediastinal lymphadenopathy. Hemogram showed pancytopenia with hemoglobin level at 4.2 g/dL, a white blood cell (WBC) count at 1,600/μL (band 0%, segment 14%, lymphocyte 84%, atypical lymphocyte 2%, monocyte 0%, eosinophil 0%, basophil 0%), and a platelet count at 10,000/μL. First bone marrow (BM) aspiration and biopsy in March 2009 revealed marrow lymphocytosis.
Subsequent hemogram on April 20, 2009 showed persistent anemia (hemoglobin level at 11.9 g/dL) and leukocytosis with numerous blasts (WBC count at 47,100/μL with 87% blasts). She received daily oral cyclophosphamide and prednisolone (100 mg and 15 mg, respectively) with a temporary control of leukocytosis but pancytopenia developed later. She was readmitted in the middle of July 2009 because of fever and pancytopenia with increased blasts (WBC count at 1,200/μL with 38% blasts). Second BM aspiration and biopsy revealed a pro B-cell ALL. The patient received hyperfractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone, cytarabine, and methotrexate chemotherapy in late July. Unfortunately, her general condition deteriorated with pneumonia, septic shock, and multiple organ failure, and she passed away on August 13, 2009, 5 months after the initial presentation of atypical marrow lymphocytosis. An autopsy was not performed.
The first BM biopsy was hypercellular with 70% cellularity and an increased number of small lymphocytes accounting for 70% of marrow cells (Fig. 1A–C). The proliferation fraction was low at less than 10% by MIB1 (Ki-67) immunohistochemistry. Flow cytometric immunophenotyping using a B-LPD panel showed that these small lymphocytes expressed CD19, CD38, CD43, and cytoplasmic CD79a (cCD79a) but not CD3, CD5, CD20, cCD22, or CD23. Cytogenetic study was not performed. The second BM biopsy revealed 100% cellularity and the marrow was near totally replaced by blasts expressing CD43, cCD79a, IgM, and terminal deoxynucleotidyl transferase (TdT) with focal and weak expression of CD7 by immunohistochemistry (Fig. 1D–F). The proliferation index was 70%. The blasts did not express CD3, CD10, CD15, CD20, CD34, IgD, or cyclin D1. Flow cytometry of the marrow aspirate showed a phenotype of CD10− CD15+ CD19+ CD20− cCD22− CD43+ cCD79a+ TdT+ and immunoglobulin light chain (surface and cytoplasmic)−. These findings indicated B-lineage ALL at pro-B cell stage. The karyotype was 47,XX,t(4;11)(q21;q23)[cp7]/46,XX[cp13] (Fig. 2).
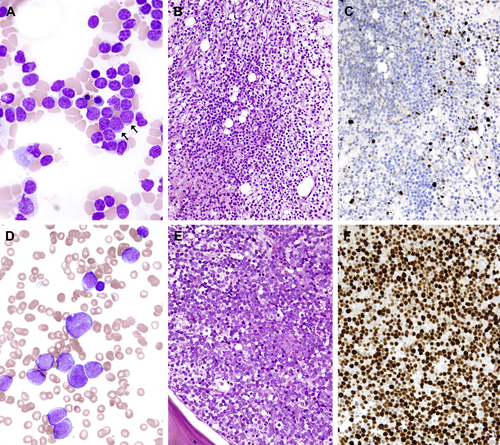
Photomicrographs of the (A–C) first and (D–F) second marrow aspiration and biopsy in March and July 2009, respectively. (A) In the first marrow aspirate, there are many small lymphocytes with occasional immature cells indicated by arrows (Liu stain; ×1,000). (B) The marrow biopsy shows diffuse infiltration by small lymphocytes [hematoxylin and eosin (HE) stain, ×400] and (C) scattered TdT-positive cells are highlighted (immunohistochemical stain, ×400). (D) In contrast, in the second aspirate, the marrow is filled with numerous blasts (Liu stain; ×1,000). (E and F) Biopsy shows sheets of blasts (HE stain, ×400) expressing TdT (immunohistochemical stain, ×400).
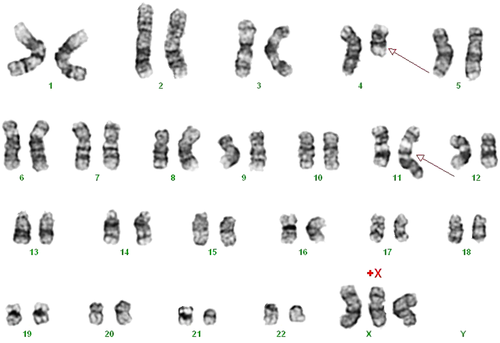
Cytogenetic study of the bone marrow with G-banding shows reciprocal translocation involving chromosomes 4q21 and 11q23, t(4;11)(q21;q23).
Retrospective review of the first BM aspirate showed a small amount of immature cells (around 10%) in a background of small lymphocytes, and additional immunohistochemical stain with anti-TdT revealed scattered positive cells (Fig. 1C). Additional antibodies were added for flow cytometric study using the thawed specimen from the first BM aspiration. The gated cells in both the first and second marrow aspirates shared the phenotype of CD3− CD5− CD10− CD19+ CD20− cCD22− CD23− CD33+ CD38+ cCD79a+ and HLA-DR+. The first specimen differed from the second by the absence of CD34 and TdT expression, and furthermore, only one-fourth of the gated cells in the first sample expressed CD15, whereas the expression in the second sample was more extensive (Fig. 3). Clonality study using BIOMED-2 DH-JH primers showed two distinct clonal bands of the same size in both specimens (Fig. 4). Although morphologically the tumor cells in the first specimen was small with condensed chromatin in contrast to the medium-sized blasts in the second specimen, the immunophenotype and clonality study suggested that the tumor cells in both specimens were probably of the same clonal origin.

Flow cytometric immunophenotyping of the first (upper panels) and second (lower panels) marrow aspirates. The gated cells in both specimens share the same phenotype (CD10− CD13− CD19+) except that the cells in the first specimen are negative for both surface CD34 and nuclear TdT, and furthermore, only one-fourth of the gated cells in the first sample expressed CD15, whereas the expression in the second sample was more extensive. cCD = cytoplasmic CD; FITC = fluorescein isothiocyanate; PerCP = peridinin chlorophyll protein.
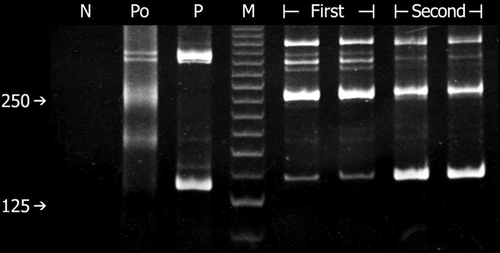
Polyacrylamide gel electrophoresis for clonality study using polymerase chain reaction method with BIODED-2 primers directed for immunoglobulin heavy chain gene rearrangement (DH-JH) shows the same clonal band patterns between the first and second bone marrow biopsies, indicating that the same tumor clone is present in both specimens. M = molecular size marker with the brightest band at 125 base pairs; N = negative control (water); P = positive control; Po = polyclonal control.
Discussion
Our case is a t-ALL at pro-B stage with t(4;11)(q21;q23) after CEF chemotherapy for breast cancer. The latency period and cytogenetic findings are typical for t-ALL after alkylating agent and anthracycline, a topoisomerase II inhibitor [3], [10]-[12]. Interestingly, the unique feature is the presence of atypical marrow lymphocytosis at 12 months with progression to overt pro-B ALL 4 months later. Marrow lymphocytosis in our patient may indicate a coexisting but previously unrecognized B-LPD, B-lymphocyte precursors (hematogones), or leukemic blasts with atypical morphology mimicking small lymphocytes.
The possibility of a B-LPD in our patient is not likely as there was no lymphocytosis in the peripheral blood and there was no monoclonal gammopathy in the serum to suggest lymphoplasmacytic lymphoma. Furthermore, the same clonal bands persisted in the second biopsy when there was no residual marrow lymphocytosis. Hematogones may mimic lymphoblasts both morphologically and immunophenotypically, and they often pose a diagnostic challenge particularly in patients with precursor B-cell ALL after chemotherapy. Reactive lymphocytosis admixed with hematogones may occur in patients with infections, lymphoma, marrow regenerative states, immune cytopenias, and acquired immunodeficiency syndrome or other unknown etiologies [13], [14]. Immunophenotypically, hematogones express CD10 and always exhibit a typical complex spectrum of antigen expression that defines the normal antigenic evolution of B-cell precursors and lack aberrant antigen expression. On the other hand, lymphoblasts show maturation arrest and exhibit incomplete maturation and immunophenotypic asynchrony and aberrancy that deviate from the normal, continuous, and complete B-lineage maturation spectrum observed for hematogones [13]. In our case, the atypical marrow lymphocytosis in the first aspirate aberrantly expressed myeloid markers (CD15 and CD33) but not CD10, excluding the possibility of hematogones.
Marrow lymphocytosis was reported in patients with precursor B-cell ALL after induction chemotherapy, and the evolution of these patients to frank leukemic relapse in a short time along with disappearance of marrow lymphocytosis indicated that these lymphocytes might be lymphoblasts in disguise [15]. In retrospect, in our patient, a minor component of immature cells suggesting blasts were present among a background of numerous small lymphocytes in the first BM aspiration/biopsy, and these immature cells probably corresponded to the scattered TdT-positive cells by immunohistochemistry. Furthermore, these small lymphocytes were phenotypically similar to the blasts in the frank leukemia by flow cytometric immunophenotyping. We concluded that these atypical marrow lymphocytes were probably lymphoblasts with atypical morphology, and that clinicopathological correlation with ancillary pathological studies are important to reach a definitive diagnosis of such an unusual case.
Acknowledgment
This work was supported by the research grant CMFHR9913 from Chi-Mei Medical Center.




