Preliminary study of a traditional Chinese medicine formula in systemic lupus erythematosus patients to taper steroid dose and prevent disease flare-up
Abstract
Systemic lupus erythematosus (SLE) is a chronic systemic autoimmune disease. Prolonged complete remission is rare. Most patients with SLE need long-term treatment with glucocorticoid and immunomodulators. However, side effects because of the above medications are common. We evaluated the effect of adding-on Dan-Chi-Liu-Wei combination (DCLWC) on SLE patients with conventional therapy in tapering steroid and preventing disease flare-up. This was a double-blind and randomized controlled trial. Sixty-six SLE patients were recruited into this study and 53 patients who fulfilled the 1997 revised criteria for the classification of SLE with an SLE disease activity index (SLEDAI) score of 2–12 and a steroid (measured with prednisolone) daily dose of less than 20 mg/d were enrolled. The patients were randomized into either an experimental or control group. We checked the urine analysis, hemogram, liver function, renal function, C3, C4, erythrocyte sedimentation rate, and anti-dsDNA, evaluated the SLEDAI score, and recorded the steroid dose at 0 months, 3 months, and 6 months, respectively. After 6 months of study, the C4 and blood urea nitrogen level revealed a statistically significant difference in either group. There was a tendency toward a decreased SLEDAI score in the experimental group (p = 0.083) but not in the control group (p = 0.867). The steroid dose was not statistically significant in either group. Renal function and liver function revealed no statistically significant statistics changes in either group. Adding-on DCLWC to conventional therapy for the treatment of SLE was safe and might have a borderline effect in decreasing disease activity, but it was not possible to taper the dosage of steroid after 6 months of clinical trial. Therefore, a long-term follow-up and a large-scale study are necessary to confirm the effect of DCLWC.
Introduction
Systemic lupus erythematosus (SLE) is a chronic systemic autoimmune disease predominantly involving childbearing women. Although the 10-year survival rate for SLE patients has improved up to 80%–90%, complete cure is rare. Most patients with SLE need long-term treatment with glucocorticoids and immunomodulators to control disease activity. However, prolonged complete remission in lupus is rare [1]. Long-term steroid use can lead to a variety of side effects, including central obesity, moon face, buffalo hump, wasting of the extremities, osteoporosis, avascular necrosis of bone, and infection. Therefore, the best way to taper the steroid dose while concomitantly preventing disease flare-up is an important issue to date.
At present, antimalarial drugs and immunosuppressive agents, such as azathioprine or cyclophosphamide, have been used to resolve the above-mentioned problems. However, side effects because of these medications are common. Antimalarial drugs can cause macular damage [2] and myopathy [3]. Azathioprine can cause myelosuppression [4], hepatotoxicity [5], and lymphoproliferative disorders [6]. Cyclophosphamide can cause immunosuppression [7], infertility [8], and bladder cancer [9]. Because of their concern about these side effects, most SLE patients look desire to an alternative therapy [10], [11].
Traditional Chinese medicine (TCM) is one of the most well-organized complementary and alternative medicine systems, with more than 2,000 years of clinical experience. Therefore, we wanted to find an alternative treatment using TCM to help SLE patients achieve long-term health, with a lower steroid dose and less disease flare-up. Our research team included immunologists, TCM experts, and a pharmacy specialist who designed the Dan-Chi-Liu-Wei combination (DCLWC), which is composed of two classical formulas: Liu-Wei-Di-Huang Wan (LWDHW) and Dan-Chi San (also known as Fufang Danshen tablets). LWDHW, one of the most famous TCM formulas, was originated by Qian-Yi, a TCM physician in the Northern Sung Dynasty (960–1127 A.C.). Recent studies have revealed that LWDHW has immunosuppressive effects [12].
Dan-Chi San, also known as Fufang Danshen tablets, originated at the Shanghai Secondary Pharmaceutical Factory of Traditional Chinese Medicine. It was developed in 1975 and has been produced since 1977. It is a common TCM and has been used for the treatment of coronary artery disease in China for more than 30 years [13], and it has been documented in the Pharmacopoeia of the People's Republic of China from the third edition (1977) [14]. There are other preparations, such as Compound Danshen Dripping Pill [15] and a compound injection of Danshen. It has been reported that Dan-Chi San has biological activities that improve microcirculation, dilate coronary artery, and improve myocardial ischemia. In clinical practice, it has been used clinically for coronary artery disease, cardiac angina, diabetes microvascular complications, and atherosclerosis [16]-[18]. Women with SLE have strikingly higher rates of premature cardiovascular disease, with up to a 50-fold increase in the incidence of cardiovascular complications over age- and sex-matched control subjects [19].
Taken together, the DCLWC is accepted to be beneficial in tapering the steroid dose and decreasing the frequency of disease flare-up in SLE patients. Therefore, we evaluated the effect of DCLWC on SLE patients, using a double-blind and randomized controlled trial.
Material and methods
Recruitment of patients
This trial was performed at Chang Gung Memorial Hospital-Kaohsiung Medical Center and Chung-Ho Memorial Hospital, Kaohsiung Medical University from February 2007 to December 2007 (Clinical trial registration number: ISRCTN73842582). The study protocol was evaluated and approved by the Institutional Review Boards of Chang Gung Memorial Hospital-Kaohsiung Medical Center and Chung-Ho Memorial Hospital. We recruited SLE patients from the outpatient clinic. All participants gave their written informed consent, which was approved by the Institutional Review Board. Inclusion criteria were all of the following: patients meeting the 1997 revised criteria for the classification of SLE [20], SLE disease activity index (SLEDAI) score [21] of 2–12, and a steroid (measured with prednisolone) daily dose less than 20 mg/d, the minimum dose being 2.5 mg/d.
Exclusion criteria were as follows: pregnancy, age under 18 or more than 60 years, and renal function impairment (serum creatinine level higher than 1.4 mg/dL).
Study design
This was a double-blind, randomized controlled trial. SLE patients with mild-to-moderate disease activity (SLEDAI 2–12) were recruited and randomly assigned to either a control or an experimental group. Simple randomization was achieved using a sequence of random numbers from a computer-generated sequence. “T” was allocated to “experiment” and “C” to “control”. In the “T” group, patients were treated with conventional medicines and 100% TCM. In the “C” group, patients were treated with conventional medicines and 10% TCM. All TCM packages, including both experimental and control groups, were marked with sequence number only. Neither patients nor doctors or research assistants knew the content of TCM packages. The sequence of random numbers was sealed in a box with signature by the principle investigator until the study was completed. All SLE patients were allowed to continue their original therapy using Western medicine, including glucocorticoid, antimalaria drugs, and/or other immunomodulators.
DCLWC is composed of two well-known formulas: LWDHW (Table 1) and Dan-Chi San (Table 1). The study medication was administered as pills within a package to both experimental and control groups. The experimental group received a package of TCM each time via oral intake in total three times per day as an add-on therapy. Each package contained 2.7 g of LWDHW and 125 mg of Dan-Chi San (100%) together. The dosage was given according to the manufacturer's instructions. In the control group, 10% of LWDHW with 90% starch and 10% Dan-Chi San with 90% starch were used.
| Liu-Wei-Di-Huang Wan | Dan-Chi San | ||
|---|---|---|---|
| Scientific name of Chinese herbs | Amount of every 25 g extractum (g) | Scientific name of Chinese herbs | Amount per pill (25 mg each pill) (mg) |
| Radix Rehmanniae Preparata∗ | 8 | Radix Salviae Miltiorrhizae | 17.5 |
| Rhizoma Dioscoreae∗ | 4 | Radix Notoginseng | 3.4 |
| Poriae∗ | 3 | Borneolum Syntheticum | 0.2 |
| Cortex Moutan Radicis∗ | 3 | Polyethylene glycolsa | 3.9 |
| Rhizoma Alismatis∗ | 3 | ||
| Fructus Corni∗ | 4 | ||
| Extractum† |  |
||
| Starch | |||
- Every 8 g of extract fine granules contained 4 g starch and 4 g extractum from Chinese herbs. The ratio of the six Chinese herbs (marked “∗”) and the extractum (marked “†”) is 25:4 = 6.25:1. Starch was used as an excipient in Liu-Wei-Di-Huang Wan combination.
- a Polyethylene glycols were used as an excipient in Dan-Chi San.
The primary outcome was the change of steroid dosage after 6 months of combined therapy (DCLWC and regular medications). The secondary outcome was the frequency of disease flare-up and the change in the immunologic index (C3, C4, anti-dsDNA) after 6 months of combined therapy.
Evaluation of adverse effects
Adverse effects, including abdominal pain, dyspepsia, constipation, diarrhea, edema, headache, abnormal renal function test, abnormal liver function test, and abnormal hematologic test were recorded. All patients were quested systemically at the 3rd and 6th months of this study, respectively.
Laboratory test and evaluation of disease activity
Complete blood routine, erythrocyte sedimentation rate, urine analysis, blood urea nitrogen (BUN), creatinine, alanine transaminase, aspartate transaminase (AST), C3, C4, anti-dsDNA were measured at baseline, 3 months, and 6 months, respectively. The disease activity of SLE was evaluated at baseline, 3 months, and 6 months, using the SLEDAI developed by Bombardier et al. [21]. If SLEDAI score increased more than 3 compared with previous SLEDAI score, it was regarded as disease flare-up [22].
Statistics
Statistical analyses were performed with Statistics Package for Social Science software 11.5 for windows (SPSS Inc., Chicago, IL, USA). Descriptive data were presented as mean ± standard deviation as required according to the normal distribution of the parameters. Figures were pictured with repeated measurements. Statistical comparison between DCLWC experimental or control group was performed using the analysis of variance, whereas the paired t test was used for comparison between pre- and posttreatment. Statistically significant probability was expressed as p value less than 0.05.
Results
Patient enrollment and assignment
We opened this trial, including inclusion criteria by poster, to 502 patients with SLE; followed up at outpatient department of Chang Gung Memorial Hospital-Kaohsiung Medical Center and Chung-Ho Memorial Hospital, Kaohsiung Medical University. Sixty-six volunteers volunteered for the trial. After screening, 53 patients who fulfilled the enrollment criteria were randomly assigned to either the control or the experimental group (Fig. 1). Forty-eight patients completed 3 months of the trial, and 46 completed 6 months. The baseline characteristics of both groups that completed 6 months study showed no significant differences (Table 2). No patients changed any disease-modifying antirheumatic drugs, such as hydroxychloroquine and azathioprine; only steroid dose was adjusted according to disease activity during the study period.
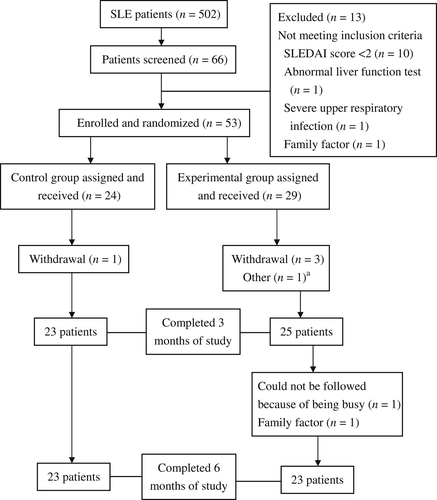
Flow chart of the patients who participated in the clinical trial. a One patient was lost to follow-up at the 3rd month but came back again at the 6th month. SLE = systemic lupus erythematosus; SLEDAI = SLE disease activity index.
| Characteristics | Control (n = 23) (mean ± SD) | Experimental (n = 23) (mean ± SD) | p |
|---|---|---|---|
| Age (yr) | 36.26 ± 9.70 | 36.21 ± 8.90 | 0.987 |
| Systolic blood pressure (mmHg) | 108.52 ± 11.52 | 111.21 ± 14.16 | 0.483 |
| Diastolic blood pressure (mmHg) | 68.60 ± 9.25 | 72.08 ± 9.65 | 0.219 |
| Heart rate (bpm/min) | 77.56 ± 7.93 | 79.39 ± 9.21 | 0.475 |
| Body height (cm) | 158.52 ± 5.08 | 160.43 ± 5.79 | 0.241 |
| Body weight (kg) | 51.78 ± 9.22 | 56.39 ± 7.86 | 0.075 |
| BMI (kg/m2) | 20.55 ± 3.16 | 21.96 ± 3.28 | 0.146 |
- Basic data of the experimental and control group patients who completed the 6-month trial. BMI = body mass index; bpm = beats per minute; SD = standard deviation.
Steroid dosage change
There was no significant change of steroid dose after 6 months in the experimental (p = 0.715) (Table 3) or control group compared with baseline (p = 0.947) (Table 3).
| Experimental (n = 23) | Control (n = 23) | |||||||
|---|---|---|---|---|---|---|---|---|
| Parameters | 0 month (mean ± SD) | 3rd month (mean ± SD) | 6th month (mean ± SD) | p | 0 month (mean ± SD) | 3rd month (mean ± SD) | 6th month (mean ± SD) | p |
| C3 (mg/dL) | 73.86 ± 16.49 | 73.86 ± 13.61 | 74.26 ± 16.09 | 0.978 | 77.65 ± 14.90 | 72.35 ± 16.33 | 74.78 ± 16.70 | 0.123 |
| C4 (mg/dL) | 14.65 ± 5.56 | 16.08 ± 7.19 | 26.00 ± 8.64 | <0.01a | 15.65 ± 8.27 | 16.21 ± 8.05 | 24.68 ± 10.70 | <0.01a |
| BUN (mg/dL) | 16.32 ± 6.69 | 16.87±8.61 | 13.73 ± 4.42 | 0.048a | 14.41 ± 5.45 | 14.09 ± 4.48 | 11.96 ± 3.60 | 0.048a |
| Cr (mg/dL) | 0.90 ± 0.15 | 0.93 ± 0.18 | 0.92 ± 0.20 | 0.622 | 0.83 ± 0.14 | 0.83 ± 0.11 | 0.85 ± 0.12 | 0.515 |
| AST (mg/dL) | 19.21 ± 7.21 | 20.86 ± 7.58 | 23.78 ± 10.69 | 0.102 | 20.43 ± 11.63 | 17.56 ± 4.51 | 21.69 ± 7.71 | 0.162 |
| ALT (mg/dL) | 22.13 ± 17.16 | 23.86 ± 20.54 | 25.73 ± 28.47 | 0.713 | 20.39 ± 15.51 | 17.39 ± 10.30 | 18.95 ± 9.30 | 0.550 |
| ESR (mm/hr) | 51.60 ± 72.40 | 33.60 ± 38.36 | 36.30 ± 30.76 | 0.241 | 43.65 ± 40.99 | 27.82 ± 24.20 | 34.73 ± 23.07 | 0.116 |
| Anti-dsDNA (IU/mL) | 62.83 ± 106.47 | 49.40 ± 83.70 | 44.92 ± 53.93 | 0.157 | 45.97 ± 86.79 | 48.23 ± 83.40 | 44.67 ± 86.77 | 0.942 |
| SLEDAI | 4.30 ± 2.28 | 3.52 ± 0.84 | 3.47 ± 1.08 | 0.083 | 3.65 ± 2.22 | 3.52 ± 2.62 | 3.34 ± 2.97 | 0.867 |
| Prednisolone (mg/d) | 8.36 ± 3.16 | 8.26 ± 3.14 | 8.49 ± 4.15 | 0.715 | 8.26 ± 2.96 | 8.26 ± 3.72 | 8.47 ± 4.81 | 0.947 |
- ALT = alanine transaminase; AST = aspartate transaminase; BUN = blood urea nitrogen; Cr = creatinine; ESR = erythrocyte sedimentation rate; SD = standard deviation; SLEDAI = systemic lupus erythematosus disease activity index.
- a Refers to p less than 0.05 compare from 0 month to 6th month in experimental or control group.
Frequency of disease flare-up
During the 6-month period of DCLWC add-on therapy, the frequency of disease flare-up in the experimental group was 8.6% (2/23, 2 patients with 2 flare-ups). This was lower than the 13% (3/23, 3 patients with 4 flare-ups) in the control group. There was no significant difference between the two groups. However, there was one very severe disease flare-up resulting in admission in the control group, in a patient who suffered two flare-ups in the course of study, but there were no severe disease flare-ups in the experimental group.
SLEDAI score
In the experimental group, there was a tendency toward a decreased SLEDAI scores after 6 months of DCLWC add-on therapy compared with baseline (p = 0.083) (Table 3). In contrast, there was no significant change in the control group compared with the base line (p = 0.867) (Table 3).
Serologic change
Anti-dsDNA
There was no significant change of serum level of anti-dsDNA after 6 months in the experimental (p = 0.157) (Table 3) or control group compared with base line (p = 0.942) (Table 3).
C3 and C4 level
There was no significant change in the serum level of C3 after 6 months of DCLWC add-on therapy in the experimental group (p = 0.978) (Table 3) or in the control group (p = 0.123) (Table 3). There was a significant elevation of the serum level of C4 after 6 months of DCLWC add-on therapy in the experimental group (p < 0.01) (Table 3) and in the control group (p < 0.01) (Table 3).
Erythrocyte sedimentation rate
There was a no significant change in the serum level of erythrocyte sedimentation rate after 6 months of DCLWC add-on therapy in the experimental group (p = 0.241) (Table 3) or in the control group (p = 0.116) (Table 3).
Liver function test
In the experimental group, there was a mild increase of the serum level of AST after 6 months of DCLWC add-on therapy (p = 0.102) (Table 3), but the serum level of AST was still in the normal range. However, there was no significant change in the serum level of AST in the control group (p = 0.162) (Table 3). In addition, there was no significant change in the serum level of alanine transaminase in the experimental group (p = 0.713) (Table 3) or in the control group (p = 0.550) (Table 3).
Renal function test
After 6 months of add-on therapy, there was a significant decrease of the serum level of BUN in the experimental group (p = 0.048) (Table 3) and in the control group (p = 0.048) (Table 3). There was no significant change in the serum level of creatinine in the experimental group (p = 0.622) (Table 3) or in the control group (p = 0.515) (Table 3).
Adverse effects
During the study, very few patients experienced slightly adverse effects. These adverse effects included gingivitis, fever, skin rash, diarrhea, and so on (Table 4). There was no difference in the frequency of adverse effects between the experimental and control groups.
| Adverse effects | Control (n = 23) | Experimental (n = 23) | Total |
|---|---|---|---|
| Gingivitis, headache, and cold extremities | 1 | 0 | 1 |
| Fever | 0 | 1 | 1 |
| Skin rash | 2 | 0 | 2 |
| Constipation | 1 | 0 | 1 |
| Diarrhea and urinary tract infection | 1 | 0 | 1 |
| Upper respiratory infection | 1 | 1 | 2 |
| Dysmenorrhea | 2 | 0 | 2 |
| Gastrointestinal tract dysfunction | 0 | 2 | 2 |
| Number of adverse effects (%) | 8 (34.8) | 4 (17.4) | 12 |
Real compliance of patients
During the study, the accountability of returned drug in the experimental group was 15.02% and in the control group was 11.08%. There was no difference in the accountability of returned drug between the experimental and control groups.
Discussion
This was the first double-blind randomized controlled trial using TCM add-on therapy to taper steroid dose, while concomitantly preventing disease flare-up. Only C4 and BUN improved significantly, but these improvements were also found in the control group. This phenomenon might be because of the therapeutic effect from conventional treatment with steroid and immunomodulators. The steroid dose showed no significant decrease after 6 months of TCM add-on therapy, but the SLEDAI score showed a decreasing tendency in the experimental group after 6 months of treatment. This suggested that TCM add-on therapy might have a borderline effect to patients with SLE. However, another possibility was that the finding might be biased because of the SLEDAI score appeasing to be higher in the experimental group at the beginning of the study. Between these two possibilities, the former was favored by our team based on clinical observation. The number of the patients who completed 6 months study was too small (total 46 patients) and the duration of study was only 6 months. This might be the major reason to explain why there was no significant effect between groups.
The frequency of disease flare-up in the experimental group after 6 months of therapy was 8.6% (2/23), which was less than the 13% (3/23) in the control group. There was one very severe flare-up resulting in the admission of a patient in the control group who suffered two flare-ups during the study period, but there was none in the experimental group. Although the incidence of flare-ups did not reach statistical significance between the two groups, a longer follow-up (1 year or more) in a future study may provide a more solid answer as to know whether DCLWC can decrease the frequency of disease flare-up or not.
The safety of TCM therapy is a major concern to date. Some herbs, such as Aristolochia fangchi (known as “Guang Fang Ji” in Chinese medicine) and Aristolochia manschuriensis (known as “Guan Mutong” in Chinese medicine) have been reported to cause renal toxicity [23]; however, no herbs used in DCLWC have been reported to cause renal toxicity. After 6 months of observation, DCLWC was shown to be really safe and without renal toxicity. Although AST was slightly elevated in the experimental group, it was still within the normal range. However, a further work-up is mandatory to ensure the safety of DCLWC in relation to the liver.
One clinical trial has been conducted using TCM in SLE patients. It was reported that the Liuwei Dihuang pill can improve the therapeutic effectiveness and counteract the adverse effects of steroid and immunosuppressive agents in the treatment of SLE, and reduce the recurrence of the disease [24]. There were several drawbacks of the study: first, it lacked of double-blind design; second, there was a lack of disease activity scores before and after treatment; and third, the steroid dose was tapered by schedule, despite the disease activity of SLE.
In conclusion, add-on therapy of DCLWC to the conventional therapy for the treatment of SLE was safe and might have a borderline effect on decreasing disease activity, but it was unable to assist in tapering the dosage of steroid after 6 months of clinical trial. However, long-term follow-up and large-scale studies are necessary in the future.
Acknowledgments
This study was funded by Committee on Chinese medicine and Pharmacy, Department of Health, Executive Yuan, Taiwan (Serial number at source CCMP95-TP-025). The authors thank their research assistants Miss Ming-Chi Yang, Miss Bo-Yin Pan, and Miss Wei-Rong Chang for their help.




