Demonstration of a hidden interaction between the aortomitral continuity and the conduction system in a case of idiopathic left ventricular outflow tract ventricular tachycardia
Abstract
We describe a case of idiopathic left ventricular outflow ventricular tachycardia. Monomorphic ventricular arrhythmia (VA) with a right bundle branch block morphology and an inferior axis was induced through exercise or isoproterenol infusion. During VA bigeminy, a spiked presystolic potential (PP) preceding the VA with a qrS pattern in the unipolar electrogram was recorded at the aortomitral continuity (AMC). Radiofrequency catheter ablation eliminated the VA despite the persistence of sinus-PP bigeminy. Furthermore, these PP rates were dependent on the preceding sinus rhythm rates. The present case suggests a hidden interaction between the AMC and the conduction system.
1 Introduction
Idiopathic ventricular arrhythmias (VAs) have become curable as improved knowledge and ablative techniques have been established. However, VA originating from the left ventricular outflow tract (LVOT) remains a challenge because of the complexity of the involved anatomic structures and the unique electrophysiological properties of the region. The unique feature is not fully understood, although some reports suggest a possible link between the aortomitral continuity (AMC) and the conduction system [1]. We present a case of LVOT-VT that demonstrated the missing link between the AMC and the conduction system.
2 Case Report
A 60-year-old man was referred for examination and treatment of a documented VT during exercise (Fig. 1a). The VT showed a right bundle branch block with an inferior axis pattern, but without a precordial transition. In addition, lead I showed an rS pattern, but an apparent S-wave was not observed in V6, suggesting that the focus of the VT is located on the left coronary cusp, not beneath the aortic valve [2]. An electrophysiological study was performed, in which quadripolar electrode catheters were attached in the high right atrium and right ventricular apex by using a decapolar electrode for recording the His bundle electrogram and a 3.5-mm Navistar ThermoCool Catheter (Biosense-Webster, Diamond Bar, CA, USA) for LVOT recording. The clinical VA was inducible by isoproterenol infusion (0.02 μg/[kg min]). A pacemap, obtained at the bottom of left coronary cusp (LCC), showed a near-perfect match (11/12; Fig. 1a). During the VA, a spiked presystolic potential (PP) preceded the onset of the QRS complex (Vo) by −16 ms, and a QS pattern was demonstrated in the unipolar recordings. Several radiofrequency catheter ablation (RFCA) attempts in this region (up to 25 W) yielded only partial success. Subsequently, we examined the endocardial side of the AMC and found a highly preceding PP relative to the onset of the QRS complex (PP-Vo=68 ms) of the VA (Figs. 1b and 2a). The unipolar recordings showed a qrS activation pattern that corresponded to the PP and VAs. A near perfect pacemap was obtained at this earliest activation (Fig. 1a). Soon after the RF application, non-sustained VT occurred and turned into the bigeminy of a single ventricular premature complex (VPC). The PP-Vo interval was gradually prolonged during RFCA, followed by the disappearance of the VA, with a PP-VPC block (Fig. 2a). However, the sinus-PP bigeminy remained even after the disappearance of the VA. In addition, a late potential (LP), recorded immediately after the ventricular potential of the sinus beats, disappeared simultaneously with the elimination of the VPC (Fig. 2a). After five more applications, the VA was totally eliminated, whereas the sinus-PP, bigeminal rhythm remained. It is interesting that the PP rates were dependent on the rates of the preceding sinus rhythm and the coupling intervals of the sinus-PP bigeminy were constant (Fig. 2b).
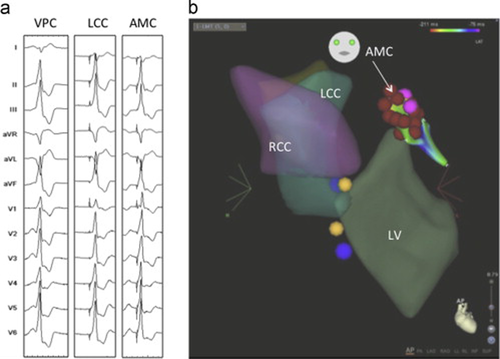
(a) Twelve-lead electrocardiographic morphology of the clinical ventricular premature complex (VPC) is demonstrated in the left column. The left coronary cusp (LCC) and aortomitral continuity (AMC) describe pacemaps at the left coronary cusp and aortomitral continuity. (b) Carto imaging of the left ventricular (LV) outflow tract is demonstrated. The red dots at the AMC denote the successful ablation site. RCC, right coronary cusp.

(a) An intracardiac tracing during successful ablation at the aortomitral continuity (AMC) is shown. The presystolic potential (PP) preceded the onset of the QRS complex (Vo) by 68ms during a ventricular premature complex (VPC). The PP-Vo interval gradually increased during the radiofrequency catheter ablation (RFCA; 68–132ms). Consequently, the VPC disappeared with the conduction block between these deflections (PP-VPC block; white reversed arrow). Notably, this finding was accompanied with the disappearance of the late potential (LP; white reversed triangle). Uni, unipolar recordings; SR, sinus rhythm beat. (b) A tracing of sinus rhythm after successful RFCA is demonstrated. Despite the elimination of the VPCs, the bigeminy of local depolarization (PP) still remained during sinus rhythm. In particular, each of the PP rates was dependent on the preceding sinus rhythm rate (A–A).
3 Discussion
Compared to the right ventricular outflow tract, the LVOT has a more complicated anatomic structure, comprising the mitral annulus, aortic cusps, and LV basal myocardium. The AMC bridges the aortic and mitral valves with fibrous tissue, called the left fibrous trigone [3], and the extent of the fibrous tissue varies. A remnant of the atrioventricular conduction system (the “dead-end pathway”) was reported to be closely located, or even reaching, the AMC in some autopsied neonates or infants [4].
Particularly in the present case, the PP persists after the disappearance of the VA. A Purkinje fiber network may be present in the AMC that forms a dead-end pathway. The AMC could serve as an independent arrhythmogenic substrate like focal Purkinje VT or verapamil-sensitive VT. Because we did not show response of the PP and VPC to the drug challenge test with adenosine, we could not tell what the PP really was. However, to our knowledge, no clear pathological or anatomical evidence demonstrates that the Purkinje fiber network forms a dead-end pathway in the AMC. Kurosawa et al. [3] described the direct continuation of the conduction axis itself, not referring the Purkinje fibers. In the present report, we demonstrated that the depolarization of the AMC was exceptionally directed by the upper conduction system based on the finding after successful RFCA. We speculated that the PP is a depolarization of the “dead-end” of the conduction axis continued from the AV node. The unique feature of the qrS pattern of the unipolar recordings would be formed by the depolarization of the focus deep inside the myocardium that propagated away from the catheter tip (“q”) and simultaneously toward the tip (“r”), and the depolarization of the endocardium, showing a deep S-wave. The r-S interval suggested a preferential pathway between the VA focus and the endocardial exit.
The disappearance of the LP accompanied with the VPCs also suggests an interaction between the intramural focus and the endocardial exit. Thus, the activation input into the VT exit site should be different from that of the endocardium via the His-Purkinje system. We hypothesized that the LP was depolarized not from the endocardium via an ordinary His-Purkinje system but directly from the AV node via the preferential pathway connected to the AMC.
Furthermore, the consecutive PP–PP intervals were consistent with the intervals of the preceding consecutive sinus rhythm, suggesting that depolarization of the VA focus depended on the sinus rhythm. We speculated that the AMC might provide a “missing” link to the conduction system. With an increased sinus rate, both the His-Purkinje and His-AMC axes can be activated. Non-sustained VT or frequent VPCs might be the result of an increased input frequency into the His-AMC conduction axis, manifesting as a non-reentrant mechanism [1].
To the best of our knowledge, the present report is the first to demonstrate a possible relationship between the AMC-VT and normal conduction in a clinical setting. A hidden connection between the conduction system and the AMC region may serve as an arrhythmogenic substrate.
Conflict of interest
None to declare.




