Frequent neurally mediated reflex syncope in a young patient with dextrocardia: Efficacy of catheter ablation of the superior vena cava–aorta ganglionated plexus
Abstract
Neurally mediated reflex syncope is the most common cause of syncope in young individuals without cardiac or neurological pathology. We report a case of successful catheter ablation in a 17-year-old male with neurally mediated syncope (NMS) of the cardioinhibitory type. The patient had dextrocardia situs inversus totalis with a mirror-image reversal of the thoracic and abdominal organs. Because he experienced multiple syncope episodes despite pharmacological intervention, we performed endocardial ablation of the superior vena cava–aorta ganglionated plexus. Shortly afterwards, his heart rate increased from 40 to 76 beats per minutes. He has not experienced syncope during the 1-year follow-up.
1 Introduction
Neurally mediated syncope (NMS) is the most common cause of transient loss of consciousness, and is a clinical condition that reduces the quality of life [1]. Cardioinhibition with asystole or a transitory atrioventricular block induced by a massive vagal reflex is commonly observed in severe cases [2], [3]. No proven effective pharmacological therapy exists for the prevention of NMS [4]. Dual-chamber permanent pacing is effective in reducing the occurrence of syncope, but involves permanent device placement [5]. Recently, Pachon et al. reported that endocardial catheter ablation showed excellent long-term results in well-selected NMS patients [6]. In the present case, we report the outcome of a patient with severe NMS associated with significant cardioinhibition who was treated by endocardial ablation with an approach through the superior vena cava (SVC).
2 Case Report
A 17-year-old male was referred to the emergency department of our hospital for multiple episodes of syncope that were refractory to pharmaceutical therapy. He had experienced some syncopal episodes in childhood. Since 2011, he had been experiencing syncope accompanied by convulsions several times a week.
His physical examination and basic laboratory data were normal. No organic heart disease other than dextrocardia was detected on electrocardiography, chest radiography, and echocardiography (Fig. 1).
Twelve-lead electrocardiography and chest radiography.
The patient's 1231-metaiodobenzylguanidine cardiac uptake was slightly decreased (heart-to-mediastinum count ratio=1.81).
A head-up tilt test (HUT) was performed according to the ESC/ACC guidelines [7], [8]. Three minutes of tilting induced sinus arrest with a 4-s duration. We therefore diagnosed the patient with the cardioinhibitory type of NMS. Treatment with oral disopyramide (200 mg) and metligine (4 mg) was initiated. One month later, a repeat HUT demonstrated only severe hypotension (from 124/52 mmHg to 80/46 mmHg) with dizziness occurring 3 min after the start of isoproterenol infusion (0.01 μg/kg/min) and an increase in heart rate from 92 to 106 beats per minute. The patient was educated about his medications, tilt-training, and a lifestyle geared towards preventing syncope. However, similar episodes of syncope continued to occur and another HUT confirmed the occurrence of an 8.5-s sinus arrest associated with syncope 13 min after tilting (Fig. 6). In accordance with current guidelines, we recommended pacemaker implantation [9], which was refused by the patient and his family. However, they did agree to endocardial ablation and written consent was obtained.
The ablation procedure was performed under mild conscious sedation. Skin adhesives of the CARTO 3 3D mapping system (Biosense Webster, Diamond Bar, CA, USA) were applied (Fig. 2A). A single circular mapping catheter (Lasso, Biosense Webster) was placed in the SVC. The ablations were anatomically guided using an irrigated catheter with a temperature-controlled system (NaviStar ThermoCool, Biosense Webster). Endocardial ablation was also performed through the SVC in the area between the aorta and the SVC. Temperature-controlled radiofrequency energy limited to 25 W/50 °C was applied to a total of 10 points while anatomical locations along with local potentials representing the fibrillar myocardium were identified using contact bipolar recording. The heart rate quickly increased at 4 out of the 10 ablated points (Fig. 2B and C). After ablation, the basic cycle length (BCL) increased from 1475 ms to 791 ms and the heart rate fluctuation stabilized (Fig. 3). There were no procedure-related complications.
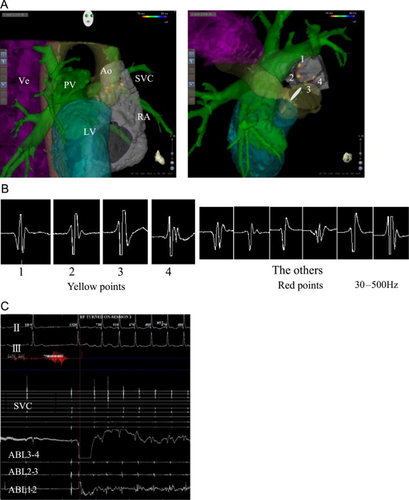
Catheter ablation using a three-dimensional system. (A) The left panel shows the right lateral view and the right panel shows the cranial view. The three-dimensional mapping system shows the superior vena cava (SVC), right atrium (RA), aorta (Ao), pulmonary vein (PV), vertebrae (Ve), and left ventricle (LV). The yellow and red points indicate the sites of ablation. (B) and (C) Heart rates are increased at the yellow points (B1–4), at which potentials are recorded by the contact bipolar catheter. Heart rates are not increased at the red points.

Pre- and post-ablation 12-lead electrocardiograms are shown. The basic cycle length increased from 1475ms to 791ms and the heart rate fluctuation stabilized.
We assessed changes in heart rate variability by comparing pre-ablation and post-ablation 24-h Holter electrocardiographic recordings.
High frequency (HF, 0.15–0.4 Hz) power, representing respiratory sinus arrhythmia, is shown in Fig. 4. The ablation procedure reduced HF (an example is shown in the left panel of Fig. 4), and the low frequency (LF, 0.05–0.1 Hz)/HF ratio slightly increased. After ablation, there were minor increases in the minimal and mean heart rates, but the maximal heart rate was not increased (Fig. 5). A post-ablation HUT conducted during the 12-month follow-up period was negative (Fig. 6), and the patient did not experience any episodes of NMS.
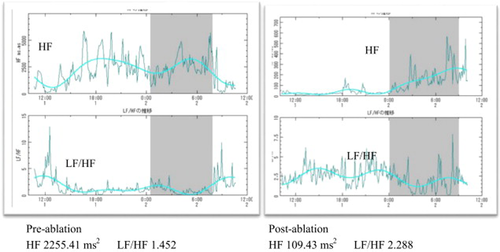
Comparison of pre- and post-ablation heart rate variability (HRV). HF, high frequency; LF, low frequency. HF range: 0.15–0.4Hz; LF range: 0.05–0.1Hz.
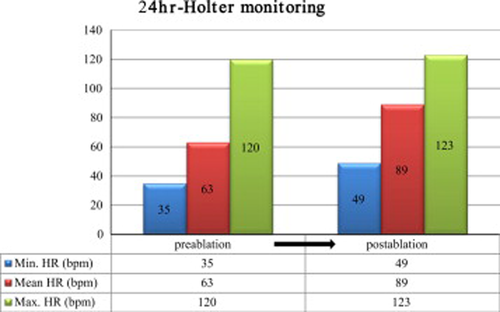
Comparison between the minimal, mean, and maximal heart rates on Holter monitoring pre- and post-ablation.
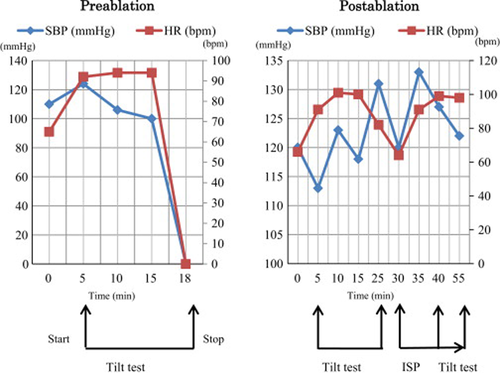
Pre- and post-ablation comparison illustrating changes in systolic blood pressure (SBP) and heart rate (HR) during the tilt procedure. Pre- and post-ablation head-up tilt testing results are shown in the left and right panels, respectively. An increased sympathetic discharge and withdrawal of vagal activity are known to be the physiological responses to the head-up tilt table test. Pre-ablation, syncope associated with asystole is observed 13min after tilting. After ablation, hypotension, bradycardia, and asystole did not occur. ISP, isoproterenol.
3 Discussion
Ordinarily, a pacemaker should have been implanted in such a case if the medical treatment option for syncope was insufficient. However, the patient and his family refused such treatment and we empirically predicted that the mean heart rate would increase after ablation of the cardiac ganglionic plexi (GP). Pachon et al. reported on the treatment of NMS with endocardial catheter ablation, which showed excellent long-term outcomes in well-selected patients. In successfully treated patients, permanent pacemaker implantation was not necessary [6]. They performed spectral mapping-guided ablation in three anatomical areas: between the aorta and SVC, between the right pulmonary veins and the right atrium, and in the inferior–posterior interatrial septum. In the present case, however, our endocardial ablation target was determined anatomically only via direct visualization of the SVC–aorta GP between the aorta and the SVC through the SVC. Afterwards, we did not ablate other areas as the patient had dextrocardia and the risks of complications such as trans-septal puncture were higher. Indeed, heart rates after ablation through the SVC obviously increased and the heart rate fluctuation stabilized, suggesting that ablation from a single approach was sufficient in this patient. We considered these responses to constitute the endpoint of this procedure.
The cardiac GP are a collection of autonomic nervous systems comprising both afferent and efferent as well as sympathetic and parasympathetic fibers [10], [11]. Radiofrequency catheter ablation eliminates the most elementary level of vagal innervation by destroying the visceral efferent limb of the parasympathetic system, thereby attenuating neural reflexes. In contrast to the parasympathetic neuron, the post-ganglionic sympathetic neuron is preserved, as its neural body is located far from the heart in the paravertebral sympathetic chain and its axon usually recovers [6].
Several studies showed that a high number of parasympathetic neurons and ganglia exist in the fibrillar myocardium areas, which are targeted by endocardial radiofrequency ablation aimed at cardiac autonomic innervation [12]. Although we could not find obvious fibrillar potentials, radiofrequency delivery caused immediate autonomic reactions with an increase in heart rate. Mapping guided by HF stimulation might have allowed a vagal response to be elicited more efficiently from the GP embedded in the epicardial fat pads.
After ablation, denervation was indicated by the significant HF reduction. Effective denervation was also supported by the disappearance of vasovagal syncope episodes at least up to 12 months after the ablation.
4 Conclusion
We presented a case of NMS in which catheter ablation at the SVC was safe and effective.
Conflict of interest
The authors have no conflict of interest to disclose.




