Influence of coronary artery disease and coronary revascularization status on outcomes in patients with acute heart failure syndromes: A report from OPTIMIZE-HF (Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure)*
Abstract
Background:
Coronary artery disease (CAD) is frequent among patients hospitalized with acute heart failure syndromes (AHFS).
Aims:
To describe the influence of coronary revascularization status on survival in patients with AHFS.
Methods and results:
OPTIMIZE-HF enrolled 48,612 patients with AHFS from 259 U.S. hospitals. In-hospital data were obtained for all patients and post-discharge 60–90 day follow-up in a pre-specified 10% sample. CAD was associated with higher in-hospital (3.7% vs. 2.9%, OR 1.14, 95% CI 1.00–1.31) and post-discharge mortality (9.2% vs. 6.9%, HR 1.37, 95% CI 1.03–1.81) compared to no CAD. Post-discharge, patients with CAD who were not revascularized had higher mortality compared to patients without CAD (10.6% vs. 6.9%, HR 1.56, 95% CI 1.15–2.11). This association was similar in patients with left ventricular systolic dysfunction (EF <40%, adjusted HR 1.52, 95% CI 0.98–2.35) and preserved systolic function (EF ≥40%, adjusted HR1.58, 95% CI 1.05–2.39). Patients with CAD who were revascularized had similar mortality to patients without CAD (HR 1.06, 95% CI 0.62–1.80 for PSF, HR 1.13, 95% CI 0.71–1.80 for LVSD).
Conclusions:
In AHFS, patients with CAD have a higher 60–90 day post-discharge mortality compared to no-CAD patients. However, patients with CAD who are revascularized appear to have similar post-discharge mortality when compared to the no-CAD group. This suggests that revascularization status may confer a survival advantage in this high risk population.
1. Introduction
Acute heart failure syndromes (AHFS) are a leading cause of hospitalization in the United States, with more than 1 million episodes annually 1. The aetiology of AHFS is often multi-factorial, but recent analyses of registries and randomized controlled trials have demonstrated that the majority of patients carry a diagnosis of coronary artery disease (CAD) 2 3 4 5. In single centre studies of selected patients, CAD in patients with AHFS has been shown to be a strong predictor of early post-discharge mortality 6,7. Despite the high prevalence of CAD and the associated adverse outcomes, coronary angiography and revascularization are uncommonly performed in this population. Registry data suggests that only 2% of patients hospitalized with AHFS undergo a revascularization procedure during the same hospitalization 2,8,9. Although revascularization for AHFS in the setting of an acute coronary syndrome (ACS) is associated with improved survival, it is unknown whether patients with AHFS and obstructive CAD benefit from revascularization in the absence of an ACS 10. In addition, it is unknown if CAD portends a poor prognosis in all patients, or whether this effect is limited to patients with left ventricular systolic dysfunction (LVSD). Approximately 50% of patients with AHFS have preserved systolic function, but these patients have been under-represented in clinical trials 11 12 13.
In spite of available medical therapies for both heart failure and CAD, the 3-6 month combined rate for death or rehospitalization in patients hospitalized with AHFS is approximately 35-50% 14,15. The correlation between coronary revascularization status and outcomes in patients with AHFS is unknown. A recent study of 2807 patients admitted with acute heart failure found that coronary revascularization was associated with improved survival to hospital discharge 16. It is possible that coronary revascularization, either prior to or during the acute hospitalization, may also improve prognosis in the post-discharge period.
OPTIMIZE-HF is a registry and performance improvement program which followed patients admitted for AHFS and tracked in-hospital outcomes in all patients and post-discharge outcomes in a pre-specified 10% sample. There were two primary aims of this study. First, we sought to determine the influence of CAD on survival both during the index hospitalization and in the early post-discharge period (60-90 days) among AHFS patients with both left ventricular systolic dysfunction (LVSD, LVEF <40%) and preserved systolic function (LVEF >= 40%). Second, we sought to examine the influence of coronary revascularization, performed prior to or during the AHFS hospitalization, on inpatient and post-discharge survival among patients with LVSD and preserved systolic function.
2. Methods
OPTIMIZE-HF enrollment took place at 259 U.S. hospitals between March 1st 2003 and December 31st 2004. The full design has been described previously 9. All regions of the United States were represented and institutions ranging from community hospitals to large tertiary medical centres participated. A total of 48,612 consecutive patients greater than 18 years of age with a primary discharge diagnosis of heart failure were enrolled. Patients admitted with a primary diagnosis of acute coronary syndrome were excluded. The case ascertainment method in use for OPTIMIZE-HF is similar to that which is utilized by the Joint Commission and certified by Medicare for national reporting of performance measure data 9. Physical exam characteristics as well as baseline demographic, diagnostic, haemodynamic, and laboratory data were obtained for all patients at the time of enrollment and throughout the hospital course. In addition, in-hospital cardiac procedures and outcomes were recorded for all patients. A pre-specified 10% sample was followed for a period of 60-90 days to track post-discharge outcomes 9. The protocol was approved by each participating centre's institutional review board or through use of a central institutional review board. Written informed consent was obtained prior to enrollment from patients who participated in the follow-up data collection. The registry coordinating centre was Outcome Sciences, Inc. (Cambridge, MA).
This analysis was confined to patients with documentation of left ventricular systolic function in-hospital. Of the 48,612 patients enrolled there were 41,267 patients (84.9%) for whom a quantification of left ventricular systolic function was recorded during the admission. Preserved systolic function was defined as LVEF >= 40%, while an LVEF <40% was considered LVSD. The presence of CAD was defined by a medical history of CAD, myocardial infarction, or coronary revascularization procedure including percutaneous coronary intervention (PCI) and coronary artery bypass surgery (CABG). An audit of a random 5% of the first 10,000 records collected showed better than 99% concordance on 53% of fields (118/223) and 95% concordance on 91% of fields (205/223). Fields with less than 95% concordance were not used in this analysis. The follow-up cohort consisted of patients with an assessment of left ventricular function who were included in the pre-specified 10% sample that was followed for 60-90 days to track post-discharge outcomes.
2.1. Statistical analysis
Continuous variables were expressed as mean±1 standard deviation (SD) or median (interquartile range) when appropriate. Discrete variables were presented as percentages. Baseline characteristics were compared among categories defined by the presence or absence of CAD, coronary revascularization, and preserved systolic function or LVSD.
The independent effect of CAD and coronary revascularization on in-hospital mortality was assessed by logistic regression analysis, and their effect expressed as ORs (95% CIs). The predictive ability of CAD history for post-discharge mortality was tested by means of survival analysis. All patients were censored at 90-day follow-up. Cumulative event-free survival curves were estimated using the Kaplan-Meier method and their differences, tested by the Peto-Peto Prentice test. The independent effect of CAD and coronary revascularization status on post-discharge mortality was assessed by Cox regression analysis. Crude and adjusted hazard ratios were presented with their respective 95% confidence intervals. To account for a potential clustering effect within hospital sites, exchangeable correlation structure within a Generalized Estimating Equations and a shared frailty were used for the logistic and Cox models respectively. For any multivariable modelling, candidate covariates were selected based on previous medical knowledge. From this initial model, a parsimonious, but highly predictive model was derived using a backward step-down selection 17. The proportionality assumption for the hazard function over time was tested by mean of the Schoenfeld residuals. The functional form of continuous variables in the log-hazard scale was examined by means of fractional polynomials and transformed when appropriate 18. A 2-sided p-value of <0.05 was considered to be statistically significant for all analyses. All analyses were performed using STATA 9.2 19.
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
3. Results
Baseline characteristics for the 41,267 patients with documentation of LVEF included in this study are listed in Table 1. CAD was known to be present in 60% of the entire cohort (n=24,738). Among patients with preserved systolic function (n=21,147, 51% of the entire cohort), 54% carried a diagnosis CAD. Among patients with LVSD (n=20,116; 49% of the entire cohort) 66% carried a diagnosis of CAD. Patients with preserved systolic function were older and more likely to be female. Diabetes was also more common in the preserved systolic function group. Patients with CAD were more likely to be Caucasian, particularly those with LVSD. Of patients with CAD, 50% (n=12,389) had undergone coronary revascularization (PCI or CABG) prior to the index hospitalization (Table 1). Of patients with preserved systolic function and CAD, 47% had under undergone prior coronary revascularization compared to 53% of those with LVSD. Patients with a previous history of coronary revascularization were more likely to be male and more likely to receive medical therapies for atherosclerosis including statins and anti-platelet agents compared to patients with CAD but without a history of coronary revascularization (Table 1).
| No CAD | CAD (CRS −) | CAD (CRS +) | ||||
|---|---|---|---|---|---|---|
| n=16,529 | n=12,349 | n=12,389 | ||||
| PSF | LVSD | PSF | LVSD | PSF | LVSD | |
| n=9741 | n=6788 | n=6079 | n=6270 | n=5329 | n=7060 | |
| Demographics | ||||||
| Mean age, years (SD) | 74 (15) | 66 (17) | 77 (12) | 73 (13) | 75 (10) | 72 (11) |
| Male (%) | 32.6 | 54.8 | 36.5 | 57.6 | 50.4 | 70.7 |
| Age >65 (%) | 74.4 | 53.6 | 83.4 | 74.5 | 82.2 | 75.4 |
| Caucasian (%) | 71.1 | 59.4 | 79.4 | 72.7 | 83.8 | 80.3 |
| African American (%) | 20.1 | 32.0 | 12.6 | 19.7 | 8.7 | 11.4 |
| History | ||||||
| Myocardial infarction (%) | N/A | N/A | 33.2 | 45.5 | 32.0 | 41.2 |
| Diabetes (%) | 36.2 | 30.9 | 44.9 | 41.0 | 52.3 | 47.3 |
| Smoking (%) | 14.7 | 24.2 | 12.9 | 20.0 | 12.2 | 17.7 |
| Hypertension (%) | 75.1 | 64.6 | 76.9 | 68.2 | 76.1 | 65.3 |
| Implantable cardioverter defibrillator (%) | 0.7 | 5.3 | 1.3 | 8.9 | 2.7 | 13.7 |
| Atrial arrhythmia (%) | 32.7 | 26.5 | 34.1 | 29.3 | 31.8 | 29.8 |
| Admission data | ||||||
| Serum creatinine >2.0 (%) | 16.3 | 14.3 | 21.4 | 20.5 | 22.2 | 21.8 |
| Mean systolic blood pressure, mm Hg (SD) | 151 (34) | 139 (33) | 148 (34) | 135 (30) | 147 (32) | 133 (29) |
| Mean heart rate, beats per minute (SD) | 87 (22) | 95 (23) | 84(21) | 88 (21) | 81 (19) | 84 (20) |
| Elevated troponin (%) | 16.0 | 22.7 | 22.5 | 28.0 | 18.9 | 24.0 |
| Mean B-type natriuretic peptide level (ng/dl) (SD) | 904 (1048) | 1598 (446) | 1039 (1128) | 1730 (1549) | 1036 (1096) | 1591 (1383) |
| Admit meds | ||||||
| Diuretic (%) | 59.3 | 58.8 | 66.6 | 70.2 | 68.2 | 72.9 |
| Beta blocker (%) | 42.0 | 43.3 | 55.3 | 59.1 | 64.4 | 64.5 |
| ACE inhibitor (%) | 32.6 | 39.1 | 37.8 | 47.0 | 40.1 | 47.7 |
| ARB (%) | 11.2 | 9.9 | 12.9 | 11.2 | 14.7 | 11.5 |
| Aldost antagonist (%) | 4.3 | 9.2 | 5.0 | 9.8 | 5.1 | 11.2 |
| Digoxin (%) | 15.4 | 26.3 | 18.1 | 32.4 | 18.0 | 31.9 |
| Aspirin (%) | 27.8 | 27.8 | 42.2 | 45.2 | 52.7 | 53.3 |
| Statin (%) | 19.1 | 16.1 | 33.5 | 33.8 | 52.2 | 49.3 |
| Discharge meds | ||||||
| Diuretic (%) | 76.2 | 78.9 | 77.3 | 80.1 | 77.9 | 80.0 |
| Beta blocker (%) | 53.2 | 71.2 | 63.4 | 73.1 | 69.8 | 74.9 |
| ACE inhibitor (%) | 47.0 | 66.3 | 48.0 | 61.1 | 48.3 | 58.5 |
| ARB (%) | 12.3 | 10.5 | 13.4 | 11.2 | 14.8 | 11.4 |
| Aldosterone antagonist (%) | 7.8 | 19.6 | 8.4 | 16.0 | 8.9 | 18.2 |
| Digoxin (%) | 19.0 | 39.0 | 18.7 | 37.0 | 18.5 | 36.9 |
| Aspirin (%) | 37.0 | 42.1 | 48.4 | 52.3 | 56.3 | 57.2 |
| Statin (%) | 20.3 | 20.8 | 35.0 | 36.1 | 51.7 | 51.3 |
- a CAD = coronary artery disease, CRS = coronary revascularization status, PSF = preserved systolic function, LVSD = left ventricular systolic dysfunction, ARB = angiotensin receptor blocker.
There were 949 patients (2.3%) who underwent a coronary revascularization procedure during the index hospitalization (541 PCI, 417 CABG, and 9 both). Of these, 378 (39.8%) had preserved systolic function and 571 (60.2%) had LVSD (Table 2).
| No CAD | CAD (CRS −) | CAD (CRS +) | ||||
|---|---|---|---|---|---|---|
| n=16,529 | n=12,349 | n=12,389 | ||||
| In-hospital procedures | PSF | LVSD | PSF | LVSD | PSF | LVSD |
| n=9741 | n=6788 | n=6079 | n=6270 | n=5329 | n=7060 | |
| Coronary artery bypass grafting | N/A | N/A | N/A | N/A | 2.9 | 3.7 |
| Percutaneous coronary intervention | N/A | N/A | N/A | N/A | 4.3 | 4.4 |
| Valve surgery | 0.3 | 0.3 | 0.2 | 0.3 | 0.8 | 0.6 |
| Coronary angiography | 5.8 | 15.0 | 6.2 | 10.2 | 11.0 | 13.9 |
| Mechanical ventilation | 2.5 | 3.3 | 2.8 | 3.6 | 3.9 | 4.2 |
| Cardiac resynchronization therapy | 0.4 | 3.1 | 0.4 | 3.8 | 0.7 | 6.0 |
| Implantable cardioverter defibrillator | 0.2 | 3.2 | 0.1 | 3.8 | 0.7 | 5.9 |
- a CAD = coronary artery disease, CRS = coronary revascularization status, PSF = preserved systolic function, LVSD = left ventricular systolic dysfunction.
3.1. Outcomes by CAD and LV function
When examining the entire cohort, CAD was associated with higher in-hospital (3.7% vs. 2.9%, OR 1.29, 95% CI 1.14-1.46, p<0.0001) and post-discharge mortality (9.2% vs. 6.9%, HR 1.46, 95% CI 1.14-1.85, p=0.002) compared to patients without CAD before adjustment for baseline variables (Tables 3 and 4). Among patients with LVSD, CAD was associated with significantly increased rates of in-hospital (OR 1.35, 95% CI 1.15-1.57) and post-discharge mortality (1.57, 95% CI 1.11-2.22) compared to patients without CAD. Among patients with preserved systolic function, CAD was associated with similar in hospital mortality (OR 1.14, 95% CI 0.95-1.37) and post-discharge mortality (1.32, 95% CI 0.94-1.86) when compared to the no-CAD cohort.
| CAD | No CAD | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | |
|---|---|---|---|---|
| n=24,738 | n=16,529 | p-value | p-value | |
| All patients | 907 (3.7) | 474 (2.9) | 1.29 (1.14-1.46) | 1.14 (1.00-1.31) |
| 0.0000 | 0.051 | |||
| Preserved systolic function | 345 (3.0) | 259 (2.7) | 1.14 (0.95-1.37) | 1.13 (0.94-1.36) |
| 0.153 | 0.197 | |||
| Left ventricular systolic dysfunction | 562 (4.2) | 215 (3.2) | 1.35 (1.15-1.57) | 1.16 (0.96-1.40) |
| <0.0001 | 0.122 |
- a The GEE-logistic regression model was adjusted by age, race, smoking status, history of CVA, history of hyperlipidaemia, history of peripheral vascular disease, history of acute renal failure, history of liver disease, history of chronic obstructive pulmonary disease, LVSD status, admission beta-blockers, admission ACE inhibitors, admission statins, CRT and ICD placement during the index hospitalization, admission heart rate, admission systolic blood pressure, admission diastolic blood pressure, admission haemoglobin, admission serum sodium and admission creatinine.
| CAD | No CAD | CAD | ||
|---|---|---|---|---|
| n=2910 | n=1961 | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | |
| p-value | p-value | |||
| All patients | 210 (9.2)a | 101 (6.9)a | 1.46 (1.14-1.85) | 1.37 (1.03-1.81) |
| 0.002 | 0.028 | |||
| Preserved systolic function | 82 (8.1)a | 56 (6.3)a | 1.32 (0.94-1.86) | 1.39 (0.95-2.03) |
| 0.112 | 0.090 | |||
| Left ventricular systolic dysfunction | 128 (9.4)a | 45 (6.3)a | 1.57 (1.11-2.22) | 1.33 (0.89-1.99) |
| 0.010 | 0.158 | |||
- a The Cox model was adjusted by age, length of stay, discharge creatinine, history of depression, angiographic procedure performed, mechanical ventilation, discharge ACE inhibitors, discharge digoxin, discharge diuretics, discharge lipid-lowering medications, discharge heart rate, discharge systolic blood pressure and admission serum sodium.
- a Kaplan-Meier survival rates.
After adjustment for baseline variables, CAD remained a significant predictor of post-discharge mortality in the entire cohort (HR 1.37, 95% CI 1.03-1.81) but not in the LVSD or preserved systolic function subgroups when analyzed separately. There was a trend toward increased in-hospital mortality that did not reach statistical significance (OR 1.14, 95% CI 1.00-1.31) (Table 3).
3.2. Outcomes by coronary revascularization status
When examining the influence of coronary revascularization status on survival, there was a significantly increased risk of in-hospital (3.9% vs. 2.9%, OR 1.38, 95% CI 1.20-1.59, p<0.001) and post-discharge mortality (10.6% vs. 6.9% HR 1.75, 95% CI 1.34-2.28, p<0.001) in the no revascularization cohort compared to patients without CAD, before adjustment for baseline variables (Tables 5 and 6). This was true both in patients with preserved systolic function (1.25, 95% CI 1.02-1.51 for in-hospital mortality, and 1.69, 95% CI 1.17-2.44 for post-discharge mortality) and those with LVSD (1.46, 95% CI 1.31-1.75 for in-hospital mortality, and 1.79, 95% CI 1.22-2.62 for post-discharge mortality) (Tables 5 and 6).
| No CAD | CRS − | CRS + | Crude OR (95% CI) CRS− vs. no CAD | Adjusted OR (95% CI) CRS − vs. no CAD | Crude OR (95% CI) CRS + vs. no CAD | Adjusted OR (95% CI) CRS + vs. no CAD | |
|---|---|---|---|---|---|---|---|
| n=16,529 | n=12,349 | n=12,389 | |||||
| All patients | 474 (2.9) | 485 (3.9) | 422 (3.4) | 1.38 (1.20-1.59); p=0.0001 | 1.16 (1.00-1.35); p=0.051 | 1.19 (1.03-1.39); p=0.021 | 1.12 (0.96-1.32); p=0.161 |
| Preserved systolic function | 259 (2.7) | 200 (3.3) | 145 (2.7) | 1.25 (1.03-1.51); p=0.026 | 1.16 (0.94-1.43); p=0.160 | 1.02* (0.80-1.31); p=0.848 | 1.08 (0.86-1.37); p=0.494 |
| Left ventricular systolic dysfunction | 215 (3.2) | 285 (4.6) | 277 (3.9) | 1.46 (1.21-1.75); p=0.0001 | 1.17 (0.95-1.44); p=0.150 | 1.25 (1.06-1.48); p=0.010 | 1.15 (0.93-1.42); p=0.196 |
- a The GEE-logistic regression model was adjusted by age, race, smoking status, history of cerebral vascular accident, history of hyperlipidaemia, history of peripheral vascular disease, history of acute renal failure, history of liver disease, history of chronic obstructive pulmonary disease, LVSD status, admission beta-blockers, admission ACE inhibitors, admission statins, CRT and ICD placement during the index hospitalization, admission heart rate, admission systolic blood pressure, admission diastolic blood pressure, admission haemoglobin, admission serum sodium and admission creatinine. CRS = coronary revascularization status.
| No CAD | CAD (CRS −) | CAD (CRS +) | Crude HR (95% CI) CRS− vs. no CAD | Crude HR (95% CI) CRS + vs. no CAD | Adjusted HR (95% CI) CRS − vs. no CAD | Adjusted HR (95% CI) CRS + vs. no CAD | |
|---|---|---|---|---|---|---|---|
| n=1961 | n=1416 | n=1494 | |||||
| All patients n, (%) | 101 (6.9)* | 126 (10.6)* | 84 (7.8)* | 1.75 (1.34-2.28); p=0.0001 | 1.14 (0.84-1.53); p=0.400 | 1.56 (1.15-2.11); p=0.004 | 1.12 (0.79-1.59); p=0.529 |
| Preserved systolic function n, (%) | 56 (6.3)* | 58 (10.5)* | 24 (5.3)* | 1.69 (1.17-2.44); p=0.006 | 0.83 (0.51-1.35); p=0.444 | 1.58 (1.05, 2.39); p=0.029 | 1.06 (0.62-1.80); 0p=0.843 |
| Left ventricular systolic dysfunction n, (%) | 45 (6.3)* | 68 (10.4)* | 60 (8.6)* | 1.79 (1.22-2.62); p=0.003 | 1.32 (0.89-1.96); p=0.173 | 1.52 (0.98-2.35); p=0.061 | 1.13 (0.71-1.80); p=0.599 |
- * Kaplan-Meier Survival Rates.
- b The Cox model was adjusted by age, length of stay, discharge creatinine, history of depression, angiographic procedure performed, mechanical ventilation, discharge ACE inhibitors, discharge digoxin, discharge diuretics, discharge lipid-lowering medications, discharge heart rate, discharge systolic blood pressure and admission serum sodium.
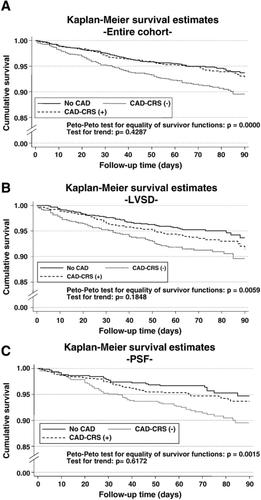
After adjustment for differences among the groups, in-hospital mortality was similar among all patients regardless of coronary revascularization status. Post-discharge, patients with preserved systolic function and CAD with a history of coronary revascularization had similar mortality compared to the group without known CAD (HR 1.06l 95% CI 0.62-1.80). However, the absence of coronary revascularization was associated with increased post-discharge mortality (HR 1.58, 95% CI 1.05-2.39). Among patients with LVSD, revascularized patients had similar post-discharge mortality compared to patients without CAD (HR 1.13, 95% CI 0.71-1.80). There was a trend towards increased post-discharge mortality in patients with LVSD and no previous revascularization (HR 1.52, 95% CI 0.98-2.35) (Fig. 1, Table 6).
4. Discussion
Multiple studies have suggested that CAD is a marker for increased mortality and repeat hospitalization among patients with heart failure 6,7,20. The prognosis of heart failure with LVSD is directly related to the extent and severity of CAD, and previous studies have suggested a systematic evaluation for ischaemia is necessary to accurately determine the aetiology of de novo heart failure 4,20. Diagnosing CAD in AHFS patients may lead to the early implementation of life-saving therapies that usually are not recommended in AHFS patients without CAD such as anti-platelet agents, statins, and coronary revascularization.
The contribution of coronary revascularization to outcomes in patients with AHFS has not been well studied. Although patients with LVSD have traditionally been regarded as more likely to benefit from revascularization, patients with acute heart failure and preserved systolic function have post-discharge event rates similar to those with LVSD 11. Acute heart failure identifies a particularly high risk subset of patients, and the investigation and treatment of CAD in this population may be particularly important to improve outcomes. The traditional classification of heart failure as having an “ischaemic” or “non-ischaemic” aetiology fails to account for the multi-factorial nature of the disease process and the dynamic nature of CAD which is evident in many patients with AHFS. Accordingly, in this analysis we chose to examine outcomes based on the presence or absence of CAD and revascularization status rather than clinically determined aetiology.
This analysis confirms that CAD is an important predictor of mortality in AHFS and extends this finding to a large well defined cohort of patients drawn from all regions of the United States and all types of hospitals 6,7. Most striking is that patients with AHFS and CAD who survive hospitalization have death rates in the early discharge period roughly twice as high as during the index hospitalization, and that coronary revascularization is independently associated with an improved prognosis. Most of the patients in the OPTIMIZE-HF registry underwent revascularization prior to admission, which suggests that prior revascularization may have a protective effect. It is also possible that revascularization procedures identify a healthier subset of patients with an overall improved prognosis, and selection bias cannot be ruled out in this type of observational analysis. Revascularized patients were more likely to be taking aspirin, statins, and beta-blockers at the time of admission. There was higher use of cardiac resynchronization therapy and implantable cardioverter defibrillator therapy among patients with LVSD who underwent revascularization. However, patients with and without revascularization were similar in age and had similar rates of smoking and hypertension. Revascularized patients had higher rates of diabetes mellitus. Revascularization was independently associated with improved post discharge outcomes even after adjustment for multiple prognostic and treatment variables. It should also be noted that there were significantly more female and African-American patients who did not undergo coronary revascularization. Gender and racial biases against coronary angiography and revascularization in women and African-Americans, respectively, have been demonstrated in other studies, even in the setting of acute myocardial infarction 21 22 23.
Coronary revascularization has previously been associated with improved survival in patients with heart failure in several different clinical situations, including early post-MI and chronic ambulatory heart failure 24 25 26. However, these studies were limited by their observational nature and/or subgroup analysis, and reflect the lack of clinical trial data addressing revascularization strategies in patients with heart failure. Our observational analysis also supports a favourable effect of coronary revascularization among acute heart failure patients, but the cause-effect relationship remains unclear. In patients with heart failure and LVSD, improved mortality after revascularization may be due to the reduction in the amount of jeopardized myocardium, resulting in improved LVEF and ventricular remodelling 27,28. The exact cause of death in many patients with heart failure is not clinically apparent, however autopsy data have demonstrated a high incidence of acute coronary syndromes and suggested that out-of hospital deaths are frequently misclassified as due to arrhythmia or pump failure 29,30. It is possible that a proportion of the early post-discharge deaths in patients with CAD in our study are due to ischaemic events, and coronary revascularization may lower the risk of death in these patients by reducing the amount of jeopardized myocardium. This mechanism has been previously invoked as a possible explanation for why post-CABG diabetic patients are less likely to die after an MI compared to diabetic patients who received balloon-only angioplasty. A more complete revascularization may provide protection from mortality due to an ischaemic event 31. In fact, coronary revascularization was associated with a decreased risk of sudden cardiac death in the Coronary Artery Surgery Study (CASS) registry 32.
In our study, the risk of death in the early post-discharge period was highest in patients with CAD and no history of coronary revascularization, and was similar in patients with preserved systolic function or LVSD. In patients with preserved systolic function, the aetiology of heart failure likely involves an interaction between hypertension, CAD and diabetes in many patients. Diabetes was more common in patients with preserved systolic function, and increasing HbA1c levels have been shown to produce functional abnormalities leading to diastolic dysfunction independent of CAD 33,34. In patients with preserved systolic function and poorly controlled hypertension, obstructive CAD may exacerbate subendocardial ischaemia, resulting in increased left ventricular end-diastolic pressure, decreased coronary flow and pulmonary congestion 35,36. In one study of 46 consecutive patients admitted with acute pulmonary oedema, the majority were found to have CAD with preserved systolic function 37. Ambulatory patients with heart failure and preserved systolic function are more likely to be hospitalized for unstable angina than patients with HF and LVSD 38. It is possible that revascularization may increase coronary perfusion in patients with preserved systolic function, leading to decreased mortality by the prevention of acute coronary syndromes and worsening heart failure.
The utilization of revascularization procedures also has important implications for medical therapy in patients with acute heart failure. In the OPTIMIZE-HF population, coronary revascularization performed prior to or during the acute hospitalization resulted in increase use of antiplatelet agents and statins, which may have influenced mortality in our study. Discharge statin use was an independent predictor of mortality, and this variable was included in the Cox regression model.
In the absence of large randomized trials of revascularization in the AHFS population, retrospective analyses of registries such as OPTIMIZE-HF offer insight into the treatment of CAD in this high risk group. The findings of this study are provocative, particularly when considering that misclassification of CAD vs. no-CAD may have resulted in increased homogeneity of the subgroups which would be expected to weaken the effect of coronary revascularization status on outcomes. It is likely that many patients with CAD and AHFS are often clinically misclassified as “non-ischaemic”, and therefore may never become candidates for coronary revascularization. Our analysis suggests that an assessment of CAD to identify candidates for revascularization may be warranted in patients with AHFS.
4.1. Limitations
The non-randomized nature of this retrospective study increases the likelihood that unmeasured variables may have differed between the groups that partially account for the association between CAD, revascularization and survival. Patients with CAD and no history of revascularization may represent a higher risk group due to a greater extent and severity of CAD where medical management was the only available treatment. It is also possible that revascularization is a marker for better overall treatment. In our study, revascularized patients were more likely to be taking aspirin, statins, and beta-blockers at the time of admission. Revascularized patients with LVSD were also more likely to receive implantable cardiac defibrillators and cardiac resynchronization pacing therapy.
Although OPTIMIZE-HF represents an opportunity to study AHFS patients in a real-world setting, there are other limitations to consider. Data were collected by medical chart review and are dependent upon the accuracy and completeness of documentation and abstraction. In addition, the participating hospitals in the OPTIMIZE-HF registry are self-selected and these findings may therefore differ from the overall population of AHFS patients. The follow-up cohort in OPTIMIZE-HF represented only 10% of the initial study sample, however these patients had similar characteristics to the overall hospital cohort and are therefore likely representative of the entire group 39,40. The type of revascularization procedure, CABG or PCI, was not analyzed and therefore the relative merits of each, as well as the severity of underlying CAD and completeness of revascularization, cannot be ascertained from this study. NYHA functional class was not recorded consistently in the OPTIMIZE-HF registry, and quality of life data was also not available. It is therefore unknown if revascularization was associated with any symptomatic improvement in this population.
The data from OPTIMIZE-HF come from US centres only and may or may not be able to be generalized to HF patients in other regions of the world. Prior studies have suggested there are significant regional differences in patient populations, use of coronary revascularization, use of medications, and intensity of patient follow-up.
4.2. Conclusions
Hospitalization for AHFS identifies a group of patients with high risk of subsequent short term mortality, particularly in the early post-discharge period. CAD is common in AHFS and appeared to be associated with even worse in-hospital and post-discharge mortality. In OPTIMIZE-HF, coronary revascularization status appeared to be associated with improved early survival in AHF patients regardless of baseline left ventricular systolic function.
Funding sources
GlaxoSmithKline funded the OPTIMIZE-HF registry under the guidance of the OPTIMIZE-HF steering committee and funded data collection and management by Outcome Sciences Inc, Cambridge, Mass,
Financial disclosures
Joseph S. Rossi, MD, James D. Flaherty, MD, and Charles J. Davidson, MD have no disclosures to report. Gregg C. Fonarow, MD, reported that he has received research grants, honorarium and has served as a consultant for GlaxoSmithKline. William T. Abraham, MD, reported that he has received research grants, honorarium and has served as a consultant for GlaxoSmithKline. Nancy M. Albert, PhD, RN, reported that she is a consultant for GlaxoSmithKline. Wendy Gattis Stough, PharmD, reported that she has received research grants, honorarium and has served as a consultant for GlaxoSmithKline. Mihai Gheorghiade, MD, reported that he has received research grants, honorarium and has served as a consultant for GlaxoSmithKline. Barry H. Greenberg, MD, reported that he has received research grants, honorarium and has served as a consultant for GlaxoSmithKline. Christopher M. O'Connor, MD, reported that he has received research grants, honorarium and has served as a consultant for GlaxoSmithKline. Clyde W. Yancy, MD, reported that he has received research grants, honorarium and has served as a consultant for GlaxoSmithKline. James B. Young, MD, reported that he has received research grants, honorarium and has served as a consultant for GlaxoSmithKline. Eduardo Nunez, MD, was an employee of GlaxoSmithKline.




