Left atrial velocity vector imaging for the detection and quantification of left ventricular diastolic function in type 2 diabetes
Abstract
Left ventricular (LV) diastolic dysfunction (DD) is diagnosed by Doppler echocardiography (DE) and Tissue Doppler imaging (TDI). Velocity vector imaging (VVI) evaluates myocardial deformation (strain). We studied left atrial (LA) deformation and volumes by VVI in relation to established Doppler-derived indices of LV diastolic function in diabetic patients.
Material:
Using DE and TDI, 87 patients (males 49%; age 60±7 years) with type 2 diabetes mellitus were classified as having no (n=60), mild (n=13) or moderate (n=14) DD.
Results:
LA volume was larger in moderate (72.3±22.4 ml) than in mild DD (58.8±16.1 ml; p=0.01) and no DD (57.9±16.0 ml; p=0.01). LA roof strain distinguished no DD from mild and moderate DD (p=0.0073). Systolic LA strain correlated to total emptying fraction (r=0.70, p<0.0001), and inversely to LA volume (r=−0.35, p=0.0009). A cross-validated analysis of no versus mild or moderate DD expressed by LA strain revealed a positive predictive value of 48% and negative of 84%.
Conclusion:
LA strain by VVI is impaired in patients with type 2 diabetes mellitus and mild or moderate LV DD. LA strain seems of value in distinguishing normal from abnormal diastolic function. VVI offers new information on regional LA function and LA volumes but has too limited discriminative power to detect early LV DD.
1. Introduction
Despite therapeutic progress the prognosis for patients with heart failure remains poor 1,2. One possibility to improve the management of such patients would be early detection and treatment of left ventricular (LV) dysfunction. Impaired diastolic myocardial function is an early sign of myocardial engagement in the diabetic patient 3 which relates to an impaired vital prognosis 4. However, there is still a lack of studies of effective therapy improving diastolic dysfunction. This limited amount of research may partly be due to deficiency or incompleteness of traditional parameters for the detection and follow-up of this condition and uncertainties in a universal definition as recently reviewed by Hatle 5 and by Paulus et al. 6.
Left ventricular diastolic dysfunction is mostly diagnosed by mitral and pulmonary vein Doppler echocardiography. A complementary method is tissue Doppler imaging (TDI), which more directly estimates myocardial tissue velocities and thus provides a relatively load independent measure of LV relaxation 7,8. The addition of maximal left atrial (LA) volume may improve diagnostic accuracy 9,10. The atrium has multiple functions, acting as a reservoir and a conduit in addition to its contractile function. Thus, there is a need for a more detailed analysis of its pathophysiological importance, and accordingly, for techniques that may supplement available technology in identifying early signs of diastolic impairment.
Velocity vector imaging (VVI) is a novel method based on two-dimensional B-mode images. The method involves tracking ultrasonic speckles permitting angle-independent measurement of tissue velocity and deformation 11. The tissue velocity is displayed as a vector projected on a two-dimensional echocardiographic (2-D echo) image where the vector shows the direction and velocity of the movement (Fig. 1). Because VVI tracks moving tissue the area or volume changes of the investigated heart chamber can be calculated automatically frame by frame. Strain, a dimensionless parameter representing object deformation, and strain rate representing deformation per unit time (expressed in s−1), have emerged as a quantitative technique for the estimation of myocardial function 12,13.
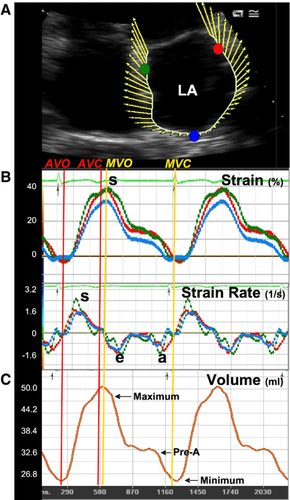
The aim of the present study was to explore the possibility of applying VVI as a method to quantify and detect early signs of LV diastolic dysfunction and LA function in patients with type 2 diabetes mellitus.
2. Patients and methods
2.1. Study population
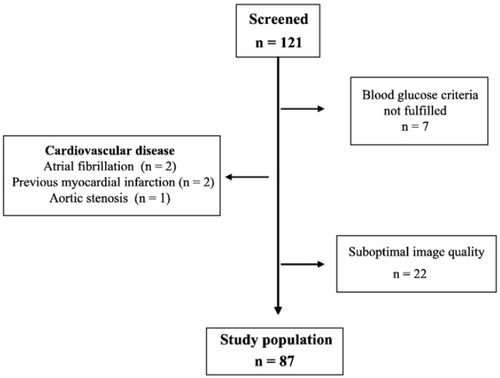
The study population comprised 121 patients with uncomplicated type 2 diabetes mellitus, treated according to current practice and screened for early diastolic dysfunction by means of transthoracic Doppler echocardiography and TDI. The patients were invited to participate in the study by advertisements in the local press and at outpatient clinics with a special interest in diabetes. In a structured nurse-based telephone interview, volunteering patients were asked about their previous disease history with a focus on cardiovascular manifestations and blood glucose lowering therapy. Those seemingly free from such diseases and without insulin therapy were invited to the research clinic for a more thorough case history, height, weight and a laboratory examination including fasting plasma glucose (FPG), glycated haemoglobin A1c (HbA1c) and serum creatinine. All investigations were performed in the morning after 12 h of fasting. Patients with well-treated hypertension were allowed to participate, whereas those with myocardial infarction and/or angina pectoris (n=2), valvular heart disease (n=1) and atrial fibrillation were excluded (n=2). Further exclusion criteria included suboptimal image quality on 2-D echo or technically inadequate studies (n=22) and normal P-glucose and HbA1c values (n=7). The final sample consisted of 87 patients (Fig. 2).
The investigational protocol was approved by the local ethics committee. All patients gave their written and oral informed consent to take part in the study. The investigation conforms to the principles outlined in the Declaration of Helsinki.
2.2. Laboratory investigations
Venous blood was sampled following 10 min of supine rest for the determination of FPG, HbA1c and serum creatinine. All samples were analysed according to routine methods at the central hospital laboratory.
2.3. Doppler echocardiography
Recordings were made with a Siemens Sequoia c512 ultrasound machine, revision 8.0 (Siemens Medical Systems, Mountain View, CA, USA) using a 4 V1c transthoracic transducer with the patient in the supine left lateral position during quiet respiration. Two-dimensional echocardiographic measurements were performed according to standards outlined by the American Society of Echocardiography 14. The following variables were measured: septal thickness and LV systolic and diastolic diameter. The maximal LA volume was calculated in the apical 4-chamber view by the single-plane area-length method 15. The outline of the atrial endocardium was traced manually at the end of ventricular systole at the point of the largest LA volume as estimated visually. The LA maximal volume was also indexed for body surface area. Significant valvular disease was excluded by Doppler.
Left ventricular systolic function was assessed by the use of Wall Motion Index (WMI) using a 17-segment model, registered both in the parasternal longitudinal and short axis views, and in the apical 4- and 2-chamber views16. The systolic function was considered normal if WMI was ≤1.1 14. Furthermore the systolic LV function was assessed as systolic velocity (S′), by TDI with the sampling volume placed in the septal part of the mitral annulus.
From the apical 4-chamber view, left ventricular diastolic function was assessed by Pulsed Doppler recordings of mitral inflow and TDI recordings of velocities in the mitral annulus. The transmitral peak E-wave velocity (cm/s) and peak A-wave velocity (cm/s) were recorded during quiet breathing. The ratio of maximal mitral flow velocities (the E/A ratio) was calculated. The longitudinal annular velocities were measured by placing the sampling gate (3 mm) at the septal part of the mitral annulus. Early (E′) and late (A′) diastolic peak annular velocities were determined and the E/E′ and E′/A′ ratios calculated.
Left ventricular diastolic function was classified into the following categories: no diastolic dysfunction (NoDD), mild dysfunction (MiDD), moderate dysfunction (MoDD) and severe dysfunction 8,17,18. NoDD was present if the deceleration time was >140 ms, E/A ratio >0.75 but <2 and E/E′ ratio<10. MiDD was present if E/A ratio was ≤0.75 and E/E′ ratio<10. MoDD presented as deceleration time >140 ms, E/A ratio>0.75 but <2 and E/E′ ratio ≥10. Severe diastolic dysfunction was defined as DT <140 ms, E/A ratio >2 and E/E′ ratio ≥10 17. Data presented are the average of three representative cardiac beats in sinus rhythm. All recordings and measurements were obtained by two experienced sonographers and stored digitally on MO discs.
2.4. Velocity vector imaging (VVI)
Tissue velocity and deformation were evaluated by a new 2-D based quantitative technique applied on the routine grey-scale echocardiographic images, originally developed for analysis of LV myocardium. In short, the VVI technique uses the combination of speckle tracking (a series of unique B-mode pixel tracking algorithms), mitral annulus motion and tissue-blood border detection. An endocardial tracing of a single frame was manually derived from a routine digital cine loop, usually starting by tracing the end-systolic frame to provide the best tracking through the cardiac cycle. The periodic displacement of the pixels within this region was tracked in subsequent frames. The movement was estimated using cross-correlation with a fast Fourier transformation process. Summation of the border motion plus the relative velocity of the tissue were used to calculate the velocity vector of every point in the border. Strain and strain rate were obtained by comparing displacement of the speckles in relation to each other along the endocardial contour throughout the cardiac cycle 11.
The 2-D sequences were recorded with highest possible frame rate and stored as standard Digital Imaging and Communication in Medicine (DICOM) format images. All analyses were performed offline with Syngo VVI version 2 (Siemens Medical Solutions, Mountain View, CA, USA). The subendocardium of the left atrium was traced at the end of ventricular systole and 2 to 3 cardiac cycles were averaged to obtain a velocity vector profile of myocardial motion. Strain and strain rate measurements were performed in the myocardium of the 3 LA walls: septum, lateral wall and at the roof of the left atrium as depicted in Fig. 1. The septal and lateral points were placed approximately 1 cm above the mitral annulus. Peak systolic LA strain was measured at LV systole while LA strain rate was measured as peak systolic, peak diastolic during early LV filling and late diastolic, during atrial contraction (Fig. 1).
An LA volume curve is generated automatically with VVI software by continuous tracing of the LA myocardium and calculating LA volumes frame by frame using the single-plane Simpson method. The following volume indices have been calculated and analysed: maximal (LA vol max), minimal (LA vol min) and pre-atrial contraction volume (LA vol preA). The LA total emptying fraction (LA−EmF) was calculated as LA-EmF=(LA vol max−LA vol min)/LA vol max×100 and the LA active emptying fraction was derived as (LA vol preA−LA vol min)/LA vol preA×100.
The reproducibility of VVI measurements was determined in 15 randomly chosen patients. For intra-observer variability the same observer recalculated the VVI measurements on two occasions with one week between calculations. Inter-observer variability was assessed by comparing the results derived from two independent observers.
3. Statistical analysis
Continuous variables were summarised as mean±SD and categorical variables as counts and percentages (%). Overall differences between groups of continuous variables were assessed using the Kruskal-Wallis nonparametric one-way analysis of variance. In case of a significant overall difference (p value<0.05) pair-wise comparisons were performed by the Wilcoxon Mann-Whitney rank sum test. Because this is an exploratory study no corrections for multiple testing were performed. Overall differences between groups of categorical variables were assessed using the likelihood ratio chi-square test.
Spearman's rank correlation coefficient, which measures the monotone relation between two continuous variables, was used to calculate the associations between echocardiographic and VVI variables.
To study the classification properties of the VVI variables for LV diastolic dysfunction a discriminant analysis was performed by which the patients were allocated to one of the two groups (no or mild/moderate diastolic dysfunction) based on the echocardiographic findings. Cross-validation was used to eliminate the influence of the patients being classified. Intra- and inter-observer variability was reported as a coefficient of variation (CV). This measure of reproducibility was based on two repeated measures from each individual and quoted as a percentage according to the formula: CV (%)=(SDdiff/x)×100.
A p-value less than 0.05 was considered statistically significant. SAS for Windows XP professional platform version 9.1.3, SP 4 (SAS Institute Inc. Cary, NC, USA) was used for all statistical analyses.
4. Results
4.1. General information
The patients were divided into three groups based on diastolic function according to the criteria outlined earlier in the paper. Only one patient showed signs of severe diastolic dysfunction. This patient was nevertheless included in the MoDD group. The majority of the study population had normal diastolic function (69%). Table 1 shows pertinent baseline characteristics of the patients. There were no significant differences between the three groups except for age and FPG. Patients with MoDD were older and the FPG value was slightly, but significantly, higher in the MiDD group.
| Variable | All | Diastolic dysfunction | p overall | ||
|---|---|---|---|---|---|
| No | Mild | Moderate | |||
| n=87 | n=60 | n=13 | n=14 | ||
| Age [years] | 60.3±7.2 | 58.8±7.5 | 63.1±5.9 | 63.7±5.6* | 0.035 |
| Males | 43 (49) | 32 (53) | 4 (31) | 7 (50) | 0.33 |
| Weight [kg] | 83.1±17.4 | 82.6±17.2 | 77.4±12.8 | 90.1±20.8 | 0.074 |
| Height [cm] | 170.5±8.2 | 170.9±7.6 | 167.1±10.7 | 171.8±8.2 | 0.19 |
| Body mass index [kg/m2] | 28±5 | 28±5 | 27±4 | 30±7 | 0.38 |
| Diabetes duration [years] | 4.4±3.7 | 4.1±3.4 | 6.1±5.5 | 4.1±2.6 | 0.63 |
| Hypertension | 36 (42) | 22 (38) | 7 (54) | 7 (50) | 0.47 |
| Laboratory findings | |||||
| HbA1c [%] | 6.0±1.4 | 6.0±1.6 | 6.1±1.0 | 6.1±0.9 | 0.21 |
| F-plasma glucose [mmol/L] | 8.1±2.8 | 8.1±3.2 | 8.5±1.0** | 8.0±1.9 | 0.031 |
| S-creatinine [μmol/L] | 71.2±12.9 | 72.3±13.5 | 67.5±13.3 | 72.8±10.2 | 0.35 |
| Glucose lowering treatment | |||||
| Lifestyle only | 46 (54) | 32 (55) | 6 (46) | 8 (57) | 0.81 |
| Sulphonylurea | 17 (20) | 11 (19) | 3 (23) | 3 (21) | 0.93 |
| Metformin | 35 (41) | 22 (38) | 6 (46) | 7 (50) | 0.66 |
| Thiazolidinediones | 1 (1) | 1 (2) | 0 | 0 | NA |
| Pharmacological treatment1 | |||||
| β-blocker | 12 (14) | 5 (9) | 3 (23) | 4 (28) | 0.11 |
| ACEI/ARB | 27 (32) | 15 (25) | 6 (46) | 6 (43) | 0.23 |
| Calcium channel blocker | 9 (11) | 5 (9) | 1 (8) | 3 (21) | 0.41 |
| Statins | 22 (26) | 13 (22) | 6 (46) | 3 (21) | 0.22 |
| Diuretics | 12 (14) | 6 (10) | 2 (15) | 4 (29) | 0.25 |
- a ACEI — Angiotensin-converting enzyme inhibitors, ARB — Angiotensin II receptor blocker, NA — not applicable.
- 1 Information complete in 85 (96%) of the patients.
- * Significant difference between NoDD and MoDD, p-value=0.039.
- ** Significant difference between NoDD and MiDD, p-value=0.01.
4.2. Echocardiographic data
Doppler-echocardiographic data are presented in Table 2. Left ventricular diastolic and systolic dimensions did not differ between the three groups, whereas there were significant differences in mitral inflow and TDI parameters in accordance with the three classification criteria. Of these, the E/E′ ratio showed a graded increase, whereas the E/A ratio and deceleration time demonstrated a biphasic pattern with increasing diastolic dysfunction. Of the classification independent measures, LA volume and LA volume index were significantly larger in the MoDD than in the NoDD group. Septal S′ velocity was significantly lower in the MiDD and MoDD groups than in the NoDD group despite similar WMI (data not shown) in all groups.
| Variables | Diastolic dysfunction | p value=overall | p values= | ||||
|---|---|---|---|---|---|---|---|
| No | Mild | Moderate | No/mild | No/moderate | Mild/moderate | ||
| n=60 | n=13 | n=14 | |||||
| Included in the classificationa | |||||||
| E — velocity (cm/s) | 73±15 | 58±8 | 86±14 | NA | |||
| A — velocity (cm/s) | 65±13 | 85±14 | 83±17 | NA | |||
| Deceleration time (ms) | 176±27 | 197±45 | 168±22 | NA | |||
| E/A — ratio | 1.2±0.3 | 0.7±0.1 | 1.1±0.4 | NA | |||
| E′ — velocity (cm/s) | 10.0±1.8 | 7.3±1.4 | 7.8±1.1 | NA | |||
| E/E′ — ratio | 7.4±1.4 | 8.2±1.8 | 11.1±1.2 | NA | |||
| Further variables | |||||||
| Left ventricular dimension | |||||||
| End diastole (mm) | 46±4 | 44±5 | 48±7 | 0.080 | |||
| End systole (mm) | 30±4 | 26±8 | 30±6 | 0.41 | |||
| Interventricular septal thickness (mm) | 10.6±1.5 | 11.3±1.5 | 11.6±1.7 | 0.064 | |||
| Left atrial volume (ml) | 57.9±16.0 | 58.8±16.1 | 72.3±22.4 | 0.015 | 0.94 | 0.004 | 0.090 |
| Left atrial volume index (ml/m2) | 29.5±6.0 | 30.0±6.9 | 35.7±9.7 | 0.071 | |||
| A′ — velocity (cm/s) | 12.0±1.8 | 11.0±1.4 | 11.0±2.5 | 0.52 | |||
| S′ — velocity (cm/s) | 8.5±1.0 | 8.0±1.5 | 7.7±1.1 | 0.012 | 0.035 | 0.019 | 0.75 |
- a Values presented are mean and SD if not otherwise stated.
- b NA — not applicable.
- a Within the definition.
4.3. VVI measurement variability
Coefficients of variation for inter-observer variability for LA strain at the roof, and in the septal and lateral wall were 10.1, 11.8 and 11.4% respectively. CV for intra-observer variability for the same variables was slightly lower 9.4 (roof), 10.3 (septal) and 10.6% (lateral). Inter-observer variability for LA volumes as measured by VVI ranged from 8.8% for maximal to 11.6% for minimal LA volume. The CV for intra-observer variability for these variables ranged from 8.3 (maximal LA volume) to 9.8% (minimal LA volume).
4.4. LA deformation and volumes by VVI
Data from VVI are summarised in Table 3. Maximal LA volume as measured by VVI correlated well with that measured by the single-plane area-length method (r=0.89; p<0.0001). Maximal LA strain at LV end-systole was highest among patients with NoDD, with no significant further decrease with increasing severity of diastolic dysfunction. LA systolic strain rate did not differ between the diastolic function groups. In contrast, LA strain rate at atrial contraction was lower in MoDD group in comparison with the NoDD and MiDD groups, suggesting decreased left atrial contractile function. The maximal and minimal LA volumes and the LA pre-atrial contraction volume were larger in the MoDD group than in NoDD and MiDD groups but the overall group comparison revealed a significant difference only for pre-A volume. The total LA emptying fraction was lowest in patients with MoDD while the LA active emptying fraction showed a biphasic response (Table 3), with highest values in patients with impaired LV relaxation (MiDD).
| Variable | Diastolic dysfunction | p-values overall | p value | ||||
|---|---|---|---|---|---|---|---|
| No | Mild | Moderate | No/mild | No/moderate | Mild/moderate | ||
| n=60 | n=13 | n=14 | |||||
| Left atrial volume (ml) | |||||||
| Maximum | 62.7±15.0 | 63.7±13.1 | 74.9±19.7 | 0.061 | |||
| Pre A | 42.9±13.7 | 47.7±11.8 | 54.5±16.7 | 0.033 | 0.21 | 0.018 | 0.24 |
| Minimum | 31.1±10.6 | 30.6±8.2 | 40.1±14.3 | 0.059 | |||
| Total emptying fraction (%) | 51.2±8.5 | 52.1±5.8 | 45.9±6.5 | 0.044 | 0.72 | 0.028 | 0.021 |
| Active emptying fraction (%) | 27.8±7.4 | 35.6±7.1 | 26.2±7.3 | 0.0040 | 0.0033 | 0.55 | 0.0054 |
| LA strain at left ventricular systole (%) | |||||||
| Septum | 29.9±9.3 | 25.9±7.4 | 23.7±4.9 | 0.037 | 0.14 | 0.023 | 0.66 |
| Roof | 29.2±7.2 | 23.9±8.2 | 23.7±4.9 | 0.0073 | 0.039 | 0.0095 | 0.81 |
| Lateral | 30.9±8.7 | 26.7±7.2 | 25.0±4.7 | 0.029 | 0.14 | 0.017 | 0.55 |
| Mean | 30.0±7.6 | 25.5±6.9 | 24.1±4.4 | 0.0094 | 0.089 | 0.0065 | 0.59 |
| LA strain rate systole mean (s−1) | 1.2±0.4 | 1.9±3.3 | 1.1±0.3 | 0.16 | |||
| LA E strain rate diastole mean (s−1) | −1.2±0.6 | −0.8±0.3 | −0.9±0.4 | 0.037 | 0.020 | 0.16 | 0.58 |
| LA A strain rate diastole mean (s−1) | −1.0±0.5 | −1.1±0.5 | −0.7±0.3 | 0.016 | 0.42 | 0.012 | 0.019 |
4.5. Relation between LA deformation and parameters of diastolic function
There was a relatively strong positive correlation between the mean systolic LA strain and LA-EmF (r=0.70; p<0.0001). Systolic LA strain in the septal, lateral and roof positions correlated significantly with LA-EmF (r=0.64, r=0.58, r=0.63; p<0.0001 respectively). Moreover, there were inverse relationships between LA strain and LA volumes (strongest for the minimal LA volume). Left atrial volume pre-A showed a slightly higher correlation with E/E′ (r=0. 28, p=0.0081) as compared with LA maximal volume (r=0.21, p=0.050).
A cross-validated discriminant analysis of normal versus mild/moderate diastolic dysfunction as expressed by LA strain alone revealed a misclassification rate of 33% with a sensitivity of 74% (20 of 27) and a specificity of 63% (38 of 60). To answer the question regarding what proportion of patients with abnormal (normal) LA strain has mild/moderate (no) DD as assessed by echocardiography the positive (negative) predictive value was calculated. Of 42 patients with abnormal LA strain 20 had mild/moderate dysfunction (positive predictive value=48%) and of the 45 patients with normal LA strain 38 had NoDD (negative predictive value=84%).
5. Discussion
To our knowledge this is the first study to demonstrate that patients with uncomplicated type 2 diabetes mellitus and early signs of diastolic dysfunction have a lower systolic LA strain as revealed by the speckle-tracking echocardiographic method VVI. This technique is feasible for the quantification of regional LA function and LA volume analyses, with acceptable measurement variability. Furthermore, there was a significant correlation between LA strain and volume generated by this technique. Left atrial VVI can be useful in the analysis of the LV diastolic function but LA strain only has limited value as a single tool for screening purposes because of a low positive predictive value.
The present study population comprised patients with type 2 diabetes mellitus without any previous history of cardiovascular disease. Patients with hypertension were not excluded. Because hypertension was equally distributed between the groups and not severe and because patients with LV hypertrophy were excluded, it seems plausible that diastolic dysfunction is not explained by hypertension.
Within the context of a clinical trial, the patients were all screened for signs of diastolic dysfunction by traditional DE and TDI methods for measurement of mitral annular E' 17,18. It is of clinical relevance to find novel, accurate and easy to apply techniques for such screening considering that diastolic dysfunction is an early manifestation of myocardial involvement in patients with type 2 diabetes mellitus. It has indeed been suggested that early detection and treatment may stop or at least retard progression towards more severe diabetes-related cardiomyopathy and clinical signs of heart failure, as recently reviewed in the European guidelines on diabetes, pre-diabetes and cardiovascular diseases 19.
No single parameter or Doppler pattern can be used in isolation to accurately assess diastolic function 8. Furthermore, parameters such as E/A ratio are age-dependent 20. Thus, in the present study diastolic function was classified using a combination of diastolic variables Doppler echocardiography and TDI, according to previously established criteria 17,18.
Traditional diastolic parameters reflect the combined influence of impairment in LV relaxation (MiDD group in our study) and impairment in LV relaxation with elevation in filling pressures (MoDD in our study), which may cause atrial enlargement. Doppler indices reflect filling pressures at one point in time, whereas increased LA size may better reflect the cumulative burden of LV diastolic dysfunction and elevated pressures. Accordingly, LA end-systolic enlargement has been shown to be a marker of cardiovascular risk 21 and systolic 22 and diastolic dysfunction 10.
The left atrium serves both as a reservoir and as a conduit for the passage of blood from the pulmonary veins to the left ventricle, and as a contracting chamber optimising LV filling. The size of the LA varies during the cardiac cycle 23,24 and phasic LA volumes may be used to describe the LA function 25. When measured by VVI, the left atrium pre-A volume and the total and active emptying fraction were better related to measures of diastolic dysfunction and indirect measures of LV filling pressures than the maximal and minimal atrial volume.
Left atrial function remains poorly understood, which is mainly due to the lack of appropriate objective non-invasive measures. Recently, several investigators applied TDI techniques to quantify regional LA myocardial velocities, strain and strain rate in healthy subjects 13,24,26, after myocardial infarction 27 and following cardioversion 28. Still the main inevitable limitation of Doppler-based techniques is the angle dependency of the technique. To overcome this problem new angle-independent ultrasound techniques, based on speckle-tracking analyses of 2-D images were developed and validated in experimental and animal studies 11,29. Although this new approach was designed mainly for the assessment of regional LV myocardial function it has been used for the evaluation of left atrial deformation and function comparing patients with idiopathic and ischaemic cardiomyopathy treated with biventricular pacing 30. We applied VVI to explore the possibility of improving the diagnosis of early LV diastolic dysfunction beyond that accomplished by traditional Doppler echocardiography and TDI.
During the LA reservoir period, maximal positive LA strain occurs at the end of LV systole, representing a measure of the maximal stretching of the left atrium. Quantification of longitudinal myocardial LA deformation with TDI was reported by Sirbu et al. 13 to be feasible in a clinical setting assessing regional strain and strain rate profiles in the LA wall in 40 young, healthy individuals. In an evaluation of LA function in patients with acute myocardial infarction and a control population it was concluded that strain may quantify LA function relatively independently of LV function thereby providing new insights into LA function 27. These findings are supported by the present results that LA strain is related to different levels of LV diastolic dysfunction. Systolic strain was evenly distributed along the LA wall, with no significant difference between the three measurement points (see Table 3). Moreover, in contrast to the biphasic response of the other diastolic parameters (E/A ratio and deceleration time), LA strain decreased linearly with increasing severity of LV diastolic dysfunction. Therefore, LA strain seems the most promising variable for the expression of the intrinsic LA function when investigating patients with different stages of diastolic dysfunction. Finally the assessment of characteristics in the LA roof necessitates an angle-independent method making VVI of more value compared with TDI, a technique providing only longitudinal information. In addition, VVI simplified the calculation of LA volumes in comparison with traditional methods because of automated endocardial border tracking through the cardiac cycle, resulting in the LA volume curve generated from volume measurements at every frame. Despite these advantages VVI did not prove to be a useful, single tool for the detection of mild/moderate diastolic dysfunction in type 2 diabetes mellitus. As revealed by discriminant validation, there is a large overlap between VVI and what traditional methods classify as normal and pathological diastolic function. It may be that the negative predictive value of 84% can be accepted as reasonable but the very low positive predictive capacity (48%) makes VVI too insensitive as a screening tool.
6. Study limitations
It has to be acknowledged that this study recruited only diabetic patients. Thus, the present findings may not generalise to other patient groups. The lack of an age-matched control group may be seen as a further limitation. However, two thirds of the patients had a normal LV function according to established criteria. It is therefore unlikely that the addition of a separate control group would have changed the general pattern in this investigation. BNP was not analysed in this study, which may be seen as a limitation.
Another limitation is that the patients in this study mostly had mild to moderate diastolic dysfunction. Thus the benefit of VVI when characterising patients in the early phase of disease remains to be further explored. Whether VVI may be a better tool for the classification of patients with more severe myocardial involvement is another open question in need of further exploration.
Two-dimensional speckle-tracking algorithms are dependent on image quality and technically adequate stored digital images. Furthermore, endocardial border definition in the left atrium may be difficult and image quality may vary through the cardiac cycle. We excluded patients who, for these reasons had recordings that could not be adequately interpreted. Accordingly the results are representative of a population among whom it is possible to use the VVI technique.
7. Conclusions
Left atrial deformation measured as regional and overall systolic strain is impaired in patients with uncomplicated type 2 diabetes mellitus and mild to moderate LV diastolic dysfunction. Strain measurements seemed of value in distinguishing patients with normal from those with abnormal diastolic function. Although, VVI has a too limited discriminative power as a single measure to detect early LV diastolic dysfunction in patients with type 2 diabetes mellitus, it does offer new information on regional LA function and LA volumes and may replace some of the traditional Doppler echocardiography measurements.
Acknowledgements
This study was supported by AFA Insurance and by unconditional research grants from Aventis US. None of these providers of research funds had any role in the design and conduct of the study, collection management, analyses and interpretation of the data, and preparation, review or approval of the manuscript. The authors gratefully acknowledge thoughtful comments on the manuscript concerning statistical assistance from Associate Professor John Öhrvik. We thank Mattias Lidin RN, Margareta Ring BMA and Pernilla Jacobsson BMA for their skilled handling of the patients and data.




