NT-proBNP response to dobutamine stress echocardiography predicts left ventricular contractile reserve in dilated cardiomyopathy
Abstract
Background:
Brain natriuretic peptide (BNP) and left ventricular (LV) inotropic reserve are major prognostic indexes in heart failure (HF).
Aims:
To investigate the relationship between N-terminal-proBNP (NT-proBNP) changes in response to dobutamine stress echocardiography (DSE) and the LV inotropic reserve, in HF patients with dilated cardiomyopathy (DC).
Methods:
We studied 41 patients with DC, LVEF 31.6±7.7%, NYHA class II–III and 15 controls. Plasma NT-proBNP levels were measured before and 60 min after three 5-min stages of dobutamine (5 to 15μg/kg/min).
Results:
Based on NT-proBNP changes in response to dobutamine, patients were categorized into two groups: In Group A circulating NT-proBNP levels fell (−16.6 ± 7.8%), and in Group B they increased (8.4±9.1%). Group A had a marked improvement in WMSI compared to Group B (32.1±9.7% vs. 18.8±15.9%, p<0.001). Multivariate analysis showed that NT-proBNP changes were an independent predictor of LV inotropic reserve (b=−0.55, p<0.001). A reduction of 21.3% in plasma NT-proBNP levels in response to dobutamine predicted an improvement in WMSI of >25% with a sensitivity of 100% and a specificity of 92.3%.
Conclusions:
NT-proBNP changes in response to dobutamine reflect improvement in LV contractility and constitute an independent predictor of LV inotropic reserve in patients with DC.
1. Introduction
Heart failure (HF) is an enormous health care problem in the industrialized countries and has a poor long term prognosis [1].
Among the various biochemical markers, atrial natriuretic peptide (ANP) and brain (BNP) natriuretic peptide have emerged as useful tools in diagnosis, monitoring therapy and evaluating outcome in patients with HF [2 3 4 5 6]. ANP is synthesized and secreted primarily in the atria, whereas BNP is produced mainly in the ventricles in response to changes in wall stretch [7]. These observations suggest that natriuretic peptides may play an important physiological or pathologic role in cardiovascular remodelling, volume homeostasis and the response to ischaemia. In patients with HF the plasma concentration of BNP is elevated and this increase is associated with a poor prognosis [8,9].
The presence of improved left ventricular function during b-stimulation (inotropic reserve) has been found to be associated with a better prognosis in patients with HF than in those patients without a contractile reserve [10 11 12]. Thus, the assessment of the presence and magnitude of inotropic reserve is of fundamental value.
Dobutamine stress echocardiography is a well established method for the detection of myocardial ischaemia or myocardial inotropic reserve in patients with HF [13,14].
Although the resting plasma concentration of BNP has been shown to be associated with left ventricular (LV) inotropic reserve in HF [15], there are currently no studies that examine whether the acute changes in plasma BNP concentration during b-adrenergic stimulation with dobutamine, can predict myocardial inotropic reserve in these patients.
We therefore studied the relationship between BNP changes during low-dose dobutamine stress echocardiography and the presence of myocardial inotropic reserve in stable patients with HF secondary to dilated cardiomyopathy.
2. Methods
2.1. Patients
We studied 41 consecutive patients enrolled from the Heart Failure Clinic of our hospital, mean age 60.9±9.7 years, 30 male and 11 women. All patients were in sinus rhythm and had an echocardiographic diagnosis of DC defined as LV end-diastolic dimension>55 mm with LVEF<45% and chronic mild to moderate HF (NYHA functional class II to III) for ≥9 months. All patients underwent left cardiac catheterization and coronary angiography before their inclusion in the study.
Exclusion criteria included: the presence of significant coronary artery disease, lumen stenosis>50% of any coronary artery or prior myocardial infarction, severe primary cardiac valve disease, hypertrophic or restrictive cardiomyopathy, long-standing or uncontrolled systemic hypertension, chronic systemic disease involving the heart muscle, drug abuse, administration of adriamycin and HIV disease.
All patients were on optimal medication including diuretics, angiotensin-converting enzyme inhibitors, digoxin and beta-blockers and had been clinically stable for at least one month. Fifteen healthy volunteers (mean age 59.2±7.7 years) with no history of cardiovascular disease, who were clinically symptom-free with normal LV function on echocardiography served as the control group.
This study was approved by the Hospital Ethics Committee and all patients gave written informed consent to participate in the study.
2.2. Echocardiographic study
All patients had a complete echocardiographic study, using a Hewlett Packard SONOS 2500 ultrasound unit (Andover Massachusetts) and a 2.5 MHz wide-angle phased-array transducer. The examinations were recorded on videotape and calculations were performed off line using the internal software of the echocardiographic device.
M-mode echocardiography recordings were obtained from the left parasternal window and all measurements were made according to the recommendations of the American Society of Echocardiography [16].
LV volumes were measured from the apical 4- and 2-chamber view using the modified Simpson's rule algorithm. Two independent investigators calculated the LV ejection fraction (LVEF). In cases where an agreement could not be reached (a difference of more than 5% between values), a third investigator reviewed the findings and a majority decision was reached. At least three measurements of each variable were averaged. Spectral Doppler recordings from the mitral inflow were obtained from the apical 4-chamber view to assess LV filling dynamics [17]. The pulsed-wave Doppler sample volume was positioned between the tips of the mitral leaflets to derive the following variables during normal breathing: (1) peak early (E) and late (A) transmitral filling wave velocities in metres per second; (2) E/A ratio; and (3) deceleration time of the E (DTE) velocity in milliseconds.
The study population was divided into normal, delayed relaxation, pseudonormal and restrictive Doppler pattern subgroups on the basis of the peak early (E) to atrial (A) transmitral filling wave velocity. E-wave deceleration time (DTE) and the response of the transmitral filling pattern to the strain phase of the Valsava manoeuvre. The subgroups were defined as follows:
- Delayed relaxation pattern (DLR): E/A ratio<1 and DTE>220 ms
- Pseudonormal pattern (PSN): E/A ratio between 1 and 2, DTE>140 ms and reverse to delayed relaxation pattern during the strain phase of the Valsava manoeuvre
- Restrictive filling pattern (RFP): E/A ratio>2 or E/A ratio between 1 and 2 with DTE<140 ms.
Diastolic dysfunction was categorized on a semi-quantitative scale as normal = 0; delayed relaxation filling pattern = 1; pseudonormal = 2; restrictive filling pattern = 3.
A colour M-mode Doppler study was also performed with the M-mode cursor placed through the centre of the mitral inflow region in the apical four-chamber view [18].
The imaging transducer was displaced towards the lateral wall if necessary, in order to position the M-mode scan line in parallel with the direction of flow observed by two-dimensional Colour Doppler. The depth was adjusted to include the entire LV from the mitral leaflets to the apex and the sweep rates were maximized if necessary.
The Early (Vp) LV filling propagation velocity was measured as the slope of the first aliasing velocity from the mitral tips to 4 cm distally into the LV. The colour velocity scale was adjusted and/or the baseline shifted as necessary to produce colour aliasing. Recordings were obtained at end-expiration to minimize misalignment due to translation.
2.3. Stress echocardiographic protocol
All patients and controls underwent a low-dose dobutamine stress echocardiography examination. Dobutamine was given intravenously in three 5-min dose increments of 5, 10 and 15 μg/kg/min. Regional wall motion was assessed according to the American Society of Echocardiography 16 segmental model [16]. In all studies, segmental wall motion, was graded semi-quantitatively as: normal = 1, hypokinetic = 2, akinetic = 3 and dyskinetic = 4.
The wall motion score index (WMSI) was derived by dividing the sum of individual segments by the number of interpretable segments. To quantify the amount of myocardium showing a contractile reserve elicited by dobutamine, myocardial viability was assessed according to a continuous parameter defined as ΔWMSI, which expresses the differences between rest and peak WMSI.
The presence of contractile reserve was defined as an improvement in segmental wall motion score by one grade in two or more dyssynergic segments, following infusion of low-dose dobutamine.
LVEF was also measured at baseline and peak stress in all patients and controls. We elected to use low-dose dobutamine for assessing myocardial inotropic reserve given that this dosage is well established in the vast majority of published studies in this field [15].
To assess the variability in interpretation, all echocardiograms were analyzed independently by two investigators. Both inter-observer and intra-observer variation was <5% for pre- and post-dobutamine echocardiography.
2.4. Blood sampling
Before the dobutamine stress test and 60 min after the test, fasting blood samples were collected from an antecubital vein after a 20-min supine rest. Samples were collected into pyrogen-free vacuum blood collection tubes (Greiner labortechnik, Kremsmunster, Austria) with EDTA as anticoagulant. Tubes were centrifuged within 2 h of collection at 3000 rpm and 4 °C for 15 min to obtain plasma or serum, which was then separated in multiple aliquots and stored at −70 °C prior to assay.
2.5. Enzyme immunoassays (ELISA)
Plasma levels of NT-proBNP (detection limit 5 fmol/ml) were measured by ELISA according to the manufacturer's instructions (Biomedica, Wien, Austria). Intra- and inter-assay coefficients of variation for all enzyme immunoassays were <5% and <10% respectively.
2.6. Statistical analysis
Summary data are expressed as mean±standard deviation. Correlations between continuous variables were assessed using the Pearson correlation coefficient. For ordinal data the Spearman rank correlation was used. Group comparisons were made using the unpaired Student's t test or the Mann-Whitney U test, as appropriate.
Receiver operator characteristic curves were analyzed to evaluate the sensitivity vs. specificity of NT-proBNP changes in response to dobutamine echocardiography at different diagnostic thresholds to predict the presence of a WMSI improvement>25% in response to dobutamine.
Multiple stepwise linear regression analysis was used to assess correlations between WMSI changes in response to dobutamine and other parameters that were found to relate to the univariate analysis. p<0.05 was considered significant. All tests were 2-sided and analyses were performed using a commercially available statistical package (SPSS for Windows 11.0).
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
3. Results
Baseline clinical characteristics for the patients and the controls are listed in Table 1. There were no significant differences in age or sex between patients and controls. The majority of patients were on b-blockers and ACE inhibitors.
| Patients (n=41) | Controls (n=15) | p | |
|---|---|---|---|
| Age (years) | 60.9±9.7 | 59.2±7.7 | 0.55 |
| Male/female | 30/11 | 11/4 | 0.95 |
| NYHA class | 2.37±0.49 | 1 | <0.001 |
| HR (b/min) | 73.2±12.0 | 71.9±5.3 | 0.18 |
| SBP (mm Hg) | 118±12.5 | 117.3±8.4 | 0.64 |
| EF (%) | 31.6±7.7 | 64.7±4.6 | <0.001 |
| EDV (ml) | 160.5±56.8 | 67.5±8.4 | <0.001 |
| ESV (ml) | 110.4±52.2 | 23.6±3.1 | <0.001 |
| E/A | 1.01±0.38 | 1.09±0.19 | 0.67 |
| E/Vp | 22.2±8.3 | 6.9±2.3 | <0.001 |
| DTE (ms) | 211±40.5 | 188.3±12.1 | 0.004 |
| WMSI | 2.1±0.22 | 1 | <0.001 |
| NT-proBNP (ng/l) | 644±299 | 160±29.1 | <0.001 |
| Diuretics | 25 (60%) | - | |
| ACE inhibitors | 32(78%) | - | |
| b-blockers | 33 (80%) | - | |
| ARBs | 7 (17%) | - | |
| Digoxin | 4 (9.7%) | - | |
| Amiodarone | 3 (7.3%) | - |
- a Abbreviations: HR= heart rate, SBP= systolic blood pressure, LVEF= left ventricular ejection fraction, EDV= end-diastolic volume, ESV= end-systolic volume, E/A= the early to late transmitral PW-Doppler velocity ratio, Vp = early colour M-mode propagation wave through the LV, DTE = deceleration E-wave time, ARBs = angiotensin receptors blockers, NT-proBNP = N-terminal pro-brain natriuretic peptide.
The plasma concentration of NT-proBNP was significantly higher in patients with DC compared to controls (644±299 vs. 160±29.1 ng/l, p<0.001), LVEF was significantly lower and WMSI was greater. Low-dose dobutamine stress echocardiography was performed in all patients and controls without significant side effects. A slight increase in heart rate and systolic blood pressure was noted in both groups. Overall, in patients LVEF increased, WMSI decreased and NT-proBNP decreased (−8.09±14.5%) (Table 2). According to the individual NT-proBNP response after 60 min of dobutamine stress echo, two groups were identified. Group A included 27 patients in whom NT-proBNP levels decreased (−16.6±7.8%) and Group B included 14 patients in whom NT-proBNP levels increased (8.4±9.1%). There were no significant differences between these two groups in terms of age, NYHA class, or in resting WMSI and NT-proBNP plasma concentration at baseline (Table 3).
| Patients (n=41) | Controls (n=15) | p value | |
|---|---|---|---|
| ΔHR (%) | 12.8±4.3 | 16.5±3.5 | 0.004 |
| ΔSBP (%) | 8.9±4 | 8.1±3.9 | 0.53 |
| ΔWMSI (%) | −27.6±13.6 | - | |
| ΔLVEF (%) | 42.6±35.5 | 25.8±7.09 | <0.001 |
| ΔDTE (%) | 26.3±22.6 | −3.1±5,7 | 0.11 |
| ΔE/A (%) | 23.6±66.5 | 3.2±9.8 | 0.03 |
| ΔE/Vp (%) | −10±28.6 | 8.2±14 | <0.001 |
| ΔNT-proBNP (%) | −8.09±14.5 | 4.6±12.1 | 0.004 |
- a Abbreviations: HR = heart rate, SBP = systolic blood pressure, LVEF = left ventricular ejection fraction, WMSI = wall motion score index, E/A = early to late transmitral PW-Doppler velocity ratio, Vp = early colour M-mode propagation wave through the LV, DTE = deceleration E-wave time, Δ = dobutamine-induced changes, NT-proBNP = N-terminal pro-brain natriuretic peptide.
| Group A (n=27) | Group B (n=14) | p | |
|---|---|---|---|
| Age (years) | 62.8±8.7 | 57.2±10.7 | 0.07 |
| HR (b/min) | 71.5±9.5 | 77.5±18.6 | 0.10 |
| SBP (mm Hg) | 118.3±15.1 | 120.1±15.1 | 0.67 |
| WMSI | 2.1±0.18 | 2.1±0.28 | 0.41 |
| LVEF (%) | 32.6±7.4 | 30.9±9.1 | 0.55 |
| DTE (ms) | 224±39.5 | 184±28 | 0.002 |
| E/A | 0.92±0.40 | 1.24±0.27 | 0.003 |
| E/Vp | 20.8±6.6 | 25±10.6 | 0.12 |
| NT-proBNP(ng/l) | 640±304 | 651±302 | 0.91 |
- a Abbreviations: HR = heart rate, SBP = systolic blood pressure, WMSI = wall motion score index, LVEF = left ventricular ejection fraction, E/A = early to late transmitral PW-Doppler velocity ratio, Vp = early colour M-mode propagation wave through the LV, NT-proBNP = N-terminal pro-brain natriuretic peptide.
Table 4 shows the dobutamine-induced changes in the echocardiographic and NT-proBNP profiles of patients in Groups A and B. Patients whose natriuretic peptide levels decreased in response to b-stimulation (Group A) exhibited a significant LV inotropic reserve, expressed as improvements in WMSI and LVEF, compared to patients whose NT-proBNP levels increased.
| Group A (n=27) | Group B (n=14) | |
|---|---|---|
| ΔHR (%) | 13.1±3 | 12.2±5.9 |
| ΔSBP (%) | 9.3±3.9 | 7.8±3.8 |
| ΔWMSI | −32.1±9.7 | −18.8±15.9 |
| ΔEF (%) | 52.2±34.4 | 24±30.7 |
| ΔE/A (%) | 20.8±50 | 37.1±86.4 |
| ΔDTE (%) | 4.3±25.4 | −7.5±13.3 |
| ΔE/Vp (%) | −20.8±17.4 | 10.7±34.6 |
| ΔNT-proBNP (%) | −16.6±7.8 | 8.4±9.1 |
- a Abbreviations: Δ = dobutamine-induced changes, HR = heart rate, SBP = systolic blood pressure, LVEF = left ventricular ejection fraction, E/A = early to late transmitral PW-Doppler velocity ratio, Vp = early colour M-mode propagation wave through the LV, WMSI = wall motion score index, NT-proBNP = N-terminal pro-brain natriuretic peptide.
Moreover, when we categorized our patients according to their diastolic filling pattern, we found that patients with normal and mild diastolic dysfunction at rest (delayed relaxation diastolic filling pattern, 24 patients) exhibited higher inotropic reserve (LVEF change 54.2±35.2 vs. 26.2±29.6, p=0.01 and WMSI change 33.8±8.7% vs. 18.8±14.6%, p=0.001) and greater reduction in NT-proBNP levels (16.4±9.07% vs. 3.6±12.6%, p<0.001) compared to those with advanced diastolic dysfunction (pseudonormal or restrictive filling pattern, 17 patients).
We found a correlation between the baseline plasma concentration of NT-proBNP and the resting WMSI (r=0.32, p=0.03), LVEF (r=−0.30, p=0.04), NYHA class (r=0.32 p=0.03), DTE (r=−0.34 p=0.04), E/A ratio (r=0.48, p=0.001) and E/Vp ratio (r=0.37, p=0.02).
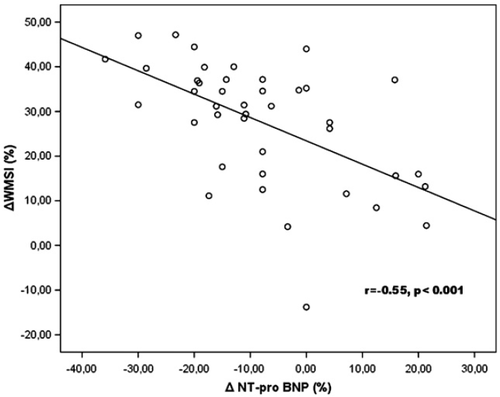
The WMSI changes in response to dobutamine correlated with the changes in NT-proBNP (r=0.55, p<0.001) (Fig. 1), stress NT-proBNP values (r=−0.39, p=0.01), LVEF changes (r=−0.52, p<0.001) and resting DTE (r=0.46, p=0.005). NT-proBNP changes correlated with indices of LV diastolic function such as DTE changes (r=0.30, p=0.05) and E/Vp changes (r=0.45, p=0.003). Multivariate analysis showed that the NT-proBNP changes (b=0.52, p=0.001, 95% CI=0.22–0.80) after dobutamine infusion were the most independent predictor of LV inotropic reserve. A ROC analysis revealed that a 21.3% reduction in NT-proBNP under dobutamine stress predicted an improvement of WMSI>25% with a sensitivity of 100% and specificity of 92.3%.
When we added the LV diastolic filling pattern into the multivariate analysis [which included age, resting and stress LV diastolic pattern, WMSI and changes in WMSI and NT-proBNP], we found that the LV diastolic pattern at rest [b=−0.47, p<0.001, 95% CI=(−17.01)−(−4.4)] and the WMSI changes in response to dobutamine [b=−0.31, p=0.02, 95% CI=(−30.7)−(−1.9)] were the most independent predictors of NT-proBNP changes in response to dobutamine stress echocardiography.
4. Discussion
This is the first study to examine the relationship between plasma NT-proBNP changes in response to dobutamine and the presence of LV contractile reserve in patients with HF due to DC. We found that the patients in whom NT-proBNP plasma levels dropped during dobutamine stress exhibited a greater increase in myocardial inotropic reserve compared to those patients in whom NT-proBNP plasma levels increased.
A growing body of evidence suggests that evaluation of BNP plasma concentration is useful for monitoring therapy, provides prognostic information and appears to have clinical utility mainly in excluding the diagnosis of HF. [19 20 21].
NT-proBNP, which represents the N-terminal fragment of pro-BNP, the high molecular weight precursor of biologically active BNP, circulates in high concentrations in plasma, has a relative long half-life, is stable in whole blood and is more likely to reflect an “average” peptide concentration than BNP levels which can change relatively rapidly in response to exercise or ischaemia [22 23 24].
Several recent studies have suggested that the presence of LV contractile reserve assessed by dobutamine stress echocardiography reflects improved prognosis in DC patients, in addition it may be associated with a subsequent improvement in LVEF and may also be correlated with peak VO2 and five year mortality in these patients [12 14 25 26 27]. Although the effects of dynamic exercise on plasma concentrations of BNP in patients with HF have been examined previously, there are no studies to evaluate the effects of dobutamine on BNP levels in these patients. Kato et al. [12,14,28] showed that the BNP exercise ratio (exercise plasma BNP response normalized with exercise workload) was augmented in patients with LV dysfunction and became progressively higher in HF patients during dynamic exercise. Matsumoto et al. [29] also showed an increase in plasma levels of BNP and ANP during a bicycle exercise test in patients with DC. These changes in BNP concentrations reflect intra-cardiac pressure overload during exercise, the LV elevated end-diastolic pressure may be an important stimulus for BNP secretion. The decrease in plasma BNP concentration we found after low-dose dobutamine is likely to have been due to acute LV haemodynamic improvement and falling LV end-diastolic pressures. As Kazanegra et al. [30] have shown that there is a significant correlation between the drop in pulmonary capillary wedge pressure from baseline and the decrease in BNP plasma levels during treatment of patients with decompensated HF. Furthermore, the relationship we found between NT-proBNP changes and improvement in indices of LV diastolic function such as DTE, or E/Vp, indices reflecting LV end-diastolic pressures, suggests that the improvement in LV filling induced by dobutamine is a major indicator of the decrease in NT-proBNP synthesis in our patients. This relationship between the presence of inotropic reserve and the increase in DTE in response to dobutamine stress echocardiography has also been reported previously in patients with ischaemic cardiomyopathy [31]. BNP is secreted rapidly, mainly by the cardiac ventricles in the presence of cardiac pressure or volume overload. Kinnunen et al. [32] have shown that stretching of hypertrophied ventricles produces a rapid (from 1–5 min) increase in immunoreactive BNP secretion, while Nakagawa et al. [33] demonstrated that BNPmRNA increases immediately within 15 min of endothelin-1 infusion in a model of cardiac hypertrophy using cultured neonatal rat ventricular cardiocytes.
We decided to measure NT-proBNP at rest and 60 min after the end of the stress, given that the estimated in vivo half-life of NT-proBNP ranges from 25 min [as shown in a recent study [34]], to over 60 min. The improved renal flow due to dobutamine administration could also remove or degrade the peptide even faster, thus allowing us to adequately measure the reduction of the peptide production resulting from an improvement in LV haemodynamics.
We found that NT-proBNP change was the most significant predictor of LV inotropic reserve, as expressed by the increased LVEF and reduced WMSI. A cut-off value of 15.8% reduction in NT-proBNP predicts an improvement in WMSI greater than 25%. We chose the cut-off value of 0.25 improvement in WMSI since this value has been found to indicate patients with low vs. high risk of death in ischaemic HF [35]. Although resting plasma concentrations of NT-proBNP correlated with resting WMSI, no significant relationship with the changes in WMSI in response to dobutamine was noted. In this regard, our findings in patients with DC differ from the findings of a previous study [15], in ischaemic patients, but the presence of ischaemia may have influenced these results. The myocardial responsiveness of dobutamine in DC has been evaluated in combination with ANP changes. Stangl [36] found that a significant decrease in ANP was observed in response to dobutamine, which paralleled the decrease in haemodynamic parameters such as pulmonary artery and pulmonary wedge pressure. We did not evaluate ANP changes given that NT-proBNP represents LV functional status more accurately.
4.1. Limitations and clinical implications
We assessed NT-proBNP changes 60 min after the infusion of dobutamine, therefore we do not have serial measurements of the changes in peptide levels. In addition, we did not evaluate the effects of medications on plasma NT-proBNP levels. However, the majority of our patients were on b-blockers and ACE inhibitors, while digitalis was given in equal percentage in both groups.
Since we did not perform invasive haemodynamic measurements, we could not study the relationship between haemodynamic parameters and b-stimulation induced plasma NT-proBNP changes. Additional studies are necessary to define this relationship more accurately.
Although intra-coronary infusion of dobutamine is a more precise method for assessment of inotropic reserve (it minimizes peripheral effects of dobutamine on cardiac loading), intravenous infusion is less invasive, safer and clinically practical.
From the current study the precise interrelation between NT-proBNP changes and inotropic reserve cannot be derived, although both indices point in the same direction. However, the combination of NT-proBNP changes and myocardial inotropic reserve during dobutamine stress may help to identify HF patients at high risk for adverse prognosis.
5. Conclusion
In summary, a rapid reduction in NT-proBNP plasma concentrations during the infusion of dobutamine in patients with DC reflects improvement in myocardial contractility. The NT-proBNP changes in response to dobutamine are an independent predictor of LV myocardial contractile reserve and these changes could be an additional prognostic index in the risk stratification of patients with HF due to DC.




