Ventricular pre-excitation and cardiac hypertrophy mimicking hypertrophic cardiomyopathy in a Turkish family with a novel PRKAG2 mutation
Abstract
Background:
Mutations in PRKAG2, the gene for the γ2 regulatory subunit of AMP-activated protein kinase, cause cardiac hypertrophy and electrophysiological abnormalities. We identified a novel mutation in PRKAG2 causing familial ventricular pre-excitation and severe cardiac hypertrophy.
Methods and results:
We studied 30 members of one family and 120 healthy controls. Molecular analysis of PRKAG2 gene revealed one missense mutation in exon 14 which was confirmed by restriction enzyme digestion. We identified a G to A transition, resulting in a Glu506Lys substitution in the PRKAG2 gene in 8 of the family members, who all had cardiac hypertrophy and ventricular pre-excitation. High incidence of right ventricular hypertrophy and left ventricular outflow tract obstruction are other prominent features of this novel PRKAG2 mutation. Family members without mutation had no cardiac disease. The 120 unrelated healthy individuals did not show this mutation.
Conclusions:
Coexistence of unexplained ventricular hypertrophy and pre-excitation should prompt the diagnosis of PRKAG2 mutations and these patients should be referred for genetic analysis. The possible alteration of AMP-activated protein kinase activity due to genetic defects in PRKAG2 may serve as a template for developing more specific therapies in the treatment of patients with this mutation.
1. Background and aims
Hypertrophic cardiomyopathy (HCM) is a genetic cardiac disorder with heterogeneous clinical course and expression 1234. Although, HCM is a disease of the cardiac sarcomere, mutations in the known contractile protein genes are not found in one third of cases. Molecular studies of HCM patients without sarcomeric protein mutations have revealed other genetic causes of cardiac hypertrophy, such as mutations in PRKAG2, the regulatory γ2 subunit of AMP-activated protein kinase (AMPK). AMPK is activated in energy deficiency states and regulates cardiac metabolism 5. To date, seven genetic mutations of the PRKAG2 gene have been identified, giving rise to a complex cardiac syndrome with the histological hallmark of profound intracellular vacuolation caused by deposition of amylopectin (glycogen) and interstitial fibrosis 6789101112. There are two important transgenic animal studies demonstrating the strong causal relationship between PRKAG2 mutations and cardiac hypertrophy and ventricular pre-excitation syndrome 13,14.
We now report a novel PRKAG2 mutation in a family with ventricular pre-excitation and severe left ventricular hypertrophy with high incidence of right ventricular hypertrophy and left ventricular outflow tract obstruction.
2. Methods
The investigation conforms with the principles outlined in the Declaration of Helsinki. The study was approved by the local ethics committee and each participant gave written informed consent after appropriate genetic counselling. The study population consisted of a proband, 29 relatives of the proband and 120 unrelated healthy controls. All study subjects were from Turkey and identified themselves as white.
Echocardiographic examinations were performed with a Vivid Five System (GE, Vingmed Ultrasound, Horten, Norway). The diagnosis of left ventricular hypertrophy was based on the demonstration of a hypertrophied, non-dilated left ventricle (diastolic wall thickness of at least 13 mm) by two-dimensional echocardiography.
Ventricular pre-excitation was diagnosed on the basis of a short PR interval (<120 ms) with widened QRS interval (>110 ms) and abnormal initial QRS vector (delta wave).
Specimens obtained from endomyocardial biopsy (the proband) were examined after staining with hematoxylin and eosin using routine protocols.
Leukocyte DNA was isolated from whole blood using standard procedures. Primers were designed from flanking intronic sequences for exons of the PRKAG2 gene. Genomic DNA fragments were amplified by polymerase chain reaction (PCR), and the products were purified using the QIAquick PCR purification kit (Qiagen). Direct sequencing reactions were performed in both the sense and antisense directions by DNA sequencing services (Genosphere Biotechnologies, France). The missense mutation detected in exon 14 was confirmed by restriction enzyme digestion.
3. Results
3.1. Genetic results
G1606>A transition in exon 14 of PRKAG2 was identified and detected in 8 affected family members (family DK). The mutation replaces glutamic acid (E) at amino acid residue 506 with lysine (K) [Glu506Lys] (Genbank accession number: AJ249976). The substituted glutamic acid is invariant across species and isoforms. The Glu506Lys missense mutation affects a residue in the fourth CBS (cystathionine β-synthase) domain with unknown function, although thought to be regulatory. This missense mutation creates a restriction enzyme recognition site for DraI. The affected individuals are heterozygous for a G to A transition at position 1606. Restriction enzyme digestion with DraI of DNA from 30 family members provided confirmation of the mutation (Fig. 1A). All 8 patients with mutation in PRKAG2 had cardiac hypertrophy and ventricular pre-excitation. Family members without mutation had no cardiac disease. 120 unrelated healthy individuals did not show this mutation.
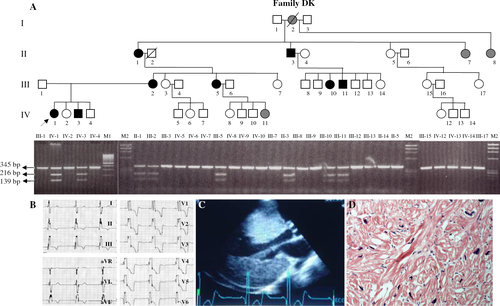
3.2. Clinical results
Three generations of a family (family DK) with 8 affected individuals, diagnosed as having ventricular hypertrophy with normal systolic function and ventricular pre-excitation were studied. The ECG, 2D echocardiographic image and endomyocardial biopsy specimens of the proband are demonstrated in Fig. 1. Recent echocardiography showed severe concentric left ventricular hypertrophy, right ventricular hypertrophy, left ventricle outflow tract obstruction with maximal resting gradient of 85 mmHg, and severe mitral regurgitation. The plasma N-terminal pro-B type natriuretic peptide level of the proband was extremely high: 32822 pg/ml. The clinical details of the proband and other affected family members are shown in Table 1.
| Patient | Age/sex | Symptoms | MWT (mm) | LVO | RVH | Pre-excitation |
|---|---|---|---|---|---|---|
| II-1 | 54/f | NYHA class III | 32 | Yes | Yes | Yes |
| Failed ASA | ||||||
| II-3 50/m | NYHA class IV, syncope | 37 | Yes | Yes | Yes | (Previous ECG) |
| AV block, DDDR pace | ||||||
| III-2 | 37/f | Asymptomatic | 33 | No | Yes | Yes |
| III-5 | 33/f | NYHA class III | 35 | Yes | Yes | Yes |
| III-10 | 20/f | NYHA class II | 35 | Yes | Yes | Yes |
| III-11 | 18/m | Asymptomatic | 29 | No | Yes | Yes |
| IV-1 | 19/f | NYHA class III | 47 | Yes | Yes | Yes |
| IV-3 | 18/m | Asymptomatic | 15 | No | No | Yes |
- a NYHA; New York Heart Association, MWT; left ventricular maximal wall thickness, LVO; left ventricle outflow tract obstruction, RVH; right ventricular hypertrophy, ASA; alcohol septal ablation.
4. Conclusions
We identified a novel PRKAG2 mutation (Glu506Lys) causing familial ventricular pre-excitation and ventricular hypertrophy mimicking HCM. This mutation does not seem to be associated with malign clinical manifestations such as family history of sudden death, ventricular and supra-ventricular arrhythmias and ventricular dilatation, but these data require support from long term follow up. A high incidence of right ventricular hypertrophy and left ventricular outflow tract obstruction are other prominent features of this mutation. The severity of the left ventricular hypertrophy and the resultant small left ventricular cavity may explain the frequency of left ventricular outflow tract obstruction.
Differentiating glycogen storage cardiomyopathy and hypertrophic cardiomyopathy is of great importance because the clinical features and outcomes of these two diseases are very different although they have a similar phenotype.
Coexistence of unexplained ventricular hypertrophy and pre-excitation should suggest the possibility of PRKAG2 mutations and these patients should be referred for genetic analysis.
The ability to perform quick and accurate molecular genetic diagnosis is of great importance for future generations of this large family. In addition, the possible alteration of AMP-activated protein kinase activity due to genetic defects in the PRKAG2 may serve as a template for developing more specific therapies for the treatment of patients with this mutation. The progressive nature of ventricular hypertrophy in PRKAG2 mutations resulting from continuous glycogen deposition may be stopped or even reversed by therapies directed towards altered AMPK activity at the tissue level.




