Impact of pulmonary regurgitation and right ventricular dysfunction on oxygen uptake recovery kinetics in repaired tetralogy of Fallot
Abstract
Background:
Patients with repaired tetralogy of Fallot (ToF) featuring severe pulmonary regurgitation (PR) and/or right ventricular (RV) dysfunction have reduced exercise tolerance.
Aims:
To assess the impact of PR and of RV function on the ability to recover from exercise in ToF patients.
Methods:
61 consecutive patients aged 23.1±12.1 years underwent maximal cardiopulmonary exercise test (CPX), transthoracic echocardiography and magnetic resonance imaging. This data was compared to those of 153 matched healthy subjects.
Results:
19 patients (31%) had severe PR. RV dysfunction was noted in 19 patients (31%). Nine patients (15%) had both severe PR and RV dysfunction. Patients had lower peak oxygen uptake (VO2), VO2 slope, carbon dioxide production (VCO2) slope and O2 pulse slope (p<0.0001), especially those with severe PR and RV dysfunction (p<0.0001). Heart rate slope was similar between groups. No patient with severe PR and RV dysfunction had a predicted peak VO2>40%. CPX had a high sensitivity and specificity to identify patients with severe PR and RV dysfunction.
Conclusions:
In ToF patients, severe PR and RV dysfunction lead to delayed recovery from exercise. CPX can identify patients with severe PR and RV dysfunction and may be useful to guide the pulmonary valve replacement decision-making process.
1. Introduction
Pulmonary regurgitation (PR) is common after repair of tetralogy of Fallot (ToF) 123. It results in chronic right ventricular (RV) volume overload, ventricular dysfunction and arrhythmia 456. It has been recognized as an important cause of morbidity and even mortality eventually necessitating pulmonary valve replacement 7,8. Oxygen uptake (VO2) at peak exercise is used to grade exercise intolerance in this setting and evidence exists that VO2 is reduced in ToF patients 9, especially in those with severe PR 10. However, the ability of ToF patients to recover from exercise and the factors that may impair their recovery capacity are not completely clarified 11. Since PR and RV function are major determinants of exercise capacity in ToF patients, we hypothesized that severe PR and RV dysfunction may also play a role in the recovery phase of exercise. Therefore, we sought to assess the ability to recover from maximal exercise in patients with repaired ToF and to determine the relative role of PR and of RV function on this ability.
2. Methods
2.1. Subjects
This study was designed as a single-center cross-sectional investigation. All patients with repaired ToF (after ≥10 years from complete repair) evaluated at our outpatient clinic between January 1, 2001 and September 30, 2004 were included. The study cohort consisted of 61 patients. Patients underwent: (1) maximal cardiopulmonary exercise test (CPX) at a mean age of 23.1±12.1 years (range 14–59); (2) complete echocardiographic examination and magnetic resonance imaging (MRI) after a mean time period of 1.2±2.1 months (range 0.01–5 months) from CPX. During the same period, 153 consecutive healthy untrained subjects were referred to our centre for evaluation before participation in non-competitive physical activities. All controls underwent CPX using the same protocol adopted for patients, and their data were used as control. Before study participation, all subjects gave written informed consent. Data were collected by three different investigators (one for CPX, one for echocardiography and one for MRI) who were blinded to other tests results.
2.2. Cardiopulmonary exercise test
CPX was performed on an electronically braked ergometer cycle (Ergoline, Germany). Oxygen uptake (VO2), carbon dioxide production (VCO2) and minute ventilation (VE) were measured with a computerized breath-by-breath analyzer (V-MAX 29, Sensor-Medics, USA). Patients performed a maximal exercise test using a 1-min incremental bicycle protocol with a work rate increment of 10 W/min. Initial work load was 10 W. Criteria for test ending were considered patient exhaustion with a respiratory exchange ratio ≥1.09. A 12-lead electrocardiogram and transcutaneous oxygen saturation were also monitored throughout the study and cuff blood pressure was determined manually every 2 min. The technical details of measurement of peak VO2, VE/VCO2 slope and calculation of VO2 slope, O2 pulse slope, VCO2 slope, VE slope and heart rate (HR) slope were previously published 12 (Fig. 1). Oxygen pulse was calculated as VO2/HR. None of the patients had additional coronary artery disease, additional valvular heart disease or inability to exercise. Before exertion, a spirometric measurement was performed to assess forced vital capacity (VC) and forced expired volume in the first second (FEV1). Standard equations were used to generate predicted values for baseline spirometric and peak exercise parameters 13.
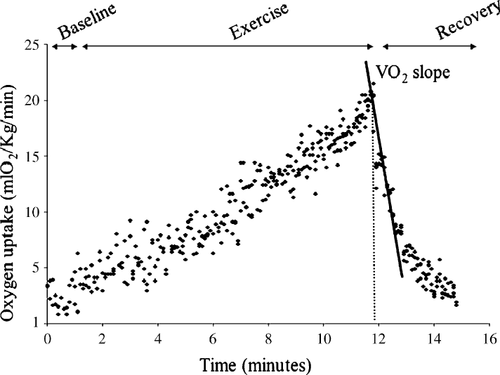
2.3. Echocardiography
Transthoracic echocardiograms were obtained using a Hewlett-Packard Sonos 5500 echocardiograph (Hewlett-Packard, Andover, Massachusetts, USA) interfaced with a multifrequency transducer. The degree of the PR was assessed by pulse-wave Doppler characteristics and color flow mapping as previously described, and it was graded as mild (no retrograde diastolic flow in pulmonary trunk with detectable regurgitant jet in the RV outflow tract), moderate (retrograde diastolic flow in main pulmonary artery) or severe (additional retrograde diastolic flow in branch pulmonary arteries) 14,15. Degree of RV outflow tract obstruction was derived from the peak velocity of the RV outflow tract obtained by continuous-wave Doppler using the modified Bernoulli equation. The mean of three consecutive beats was employed.
2.4. Magnetic Resonance Imaging (MRI)
MR imaging was performed with a 1.5-T scanner (Signa, GE Medical Systems, Milwaukee, WI). A phased-array coil (Torsopa; GE Medical Systems) was used. We used published technical details and imaging parameters of the MR pulse sequences 16. The following imaging protocol was employed: (1) three-plane localizing images; (2) breath-hold electrocardiogram (ECG)-triggered segmented k-space fast spoiled gradient recalled cine sequences in two- and four-chamber planes, followed by 12 contiguous short-axis slabs perpendicular to the long axis of the left ventricle (LV) and RV (slice thickness 6 mm, inter-slice space 2 mm); (3) ECG-triggered cine phase-contrast blood flow measurements in the main pulmonary artery and ascending aorta 17; and (4) contrast-enhanced (gadopentetate dimeglumine 0.2 mmol/kg; Magnevist, Berlex Laboratories, New Jersey, USA), three-dimensional magnetic resonance angiography sequence for anatomic evaluation of the pulmonary arteries 18.
MR images were reviewed and analyzed using a commercially available workstation (Advanced Windows 4.0, GE Medical Systems). Left and right ventricular end-diastolic (maximal) volumes and end-systolic (minimal) volumes, stroke volume and ejection fraction (FE) were measured using commercially available software (MASS, Medis, Leiden, The Netherlands) as described 19. PR fraction was defined as diastolic reversed flow expressed as a percentage of forward flow.
2.5. Statistical analysis
Normal distribution of clinical, CPX and MRI data was tested before any further analysis. Data are expressed as mean±S.D. Two-tailed, unpaired t-test was used to compare continuous variables between groups. Pearson correlation was used to assess the relation between VO2 slope and other CPX, echocardiographic and MRI variables. Receiver-operator characteristic (ROC) curves were built and were used to identify cut-off points providing the best combination of sensitivity and specificity for CPX variables associated with RV dysfunction (ejection fraction <40%) 20 in the overall study cohort and in the subgroups with and without severe PR. Cut-off values were derived from ROC curves according to the highest likelihood value. A p<0.05 was considered statistically significant.
3. Results
The demographic and clinical data of the 61 patients and 153 control subjects included in the study are summarized in Table 1. Patients were divided into two groups according to the degree of PR at echocardiography: Group I consisted in 19 patients with severe PR, whereas group II consisted of 42 patients without severe PR. Four patients in group I and eight in group II had a residual RV outflow tract obstruction, with a Doppler pressure gradient exceeding 50 mm Hg only in two patients (both without severe PR). Two patients had small residual ventricular septal defects. After repair, 19 patients (36%) underwent 26 interventional procedures (branch pulmonary artery balloon dilation in 7 patients and branch pulmonary artery stenting in 12). No patient had proximal right or left pulmonary artery stenosis at echocardiography (defined as a Doppler gradient >20 mm Hg) or at MRI. Six patients were receiving medical therapy at the time of CPX and no change in medication dosage was made in the period between CPX and other tests.
| Variables | Group I (severe PR) | Group II (PR<severe) | p | Control group | p (control group vs. group I) | p (control group vs. group II) |
|---|---|---|---|---|---|---|
| Number of patients | 19 | 42 | 153 | |||
| Age at test (years) | 21.2±7.2 | 24.6±12.9 | 0.29 | 23.1±15.0 | 0.59 | 0.68 |
| Body surface area (m2) | 1.54±0.25 | 1.61±0.21 | 0.11 | 1.64±0.25 | 0.1 | 0.47 |
| Men (%) | 42% | 45% | 0.82 | 59% | 0.16 | 0.12 |
| Previous palliation (%) | 42% | 29% | 0.30 | - | ||
| Age at palliation (months) | 11.8±8.7 | 10.8±9.1 | 0.69 | - | ||
| Age at initial repair (years) | 5.0±4.2 | 3.6±4.1. | 0.22 | - | ||
| Type of repair | ||||||
| Infundibular patch/conduit (%) | 32% | 36% | 0.75 | - | ||
| Trans-annular patch (%) | 68% | 64% | 0.75 | - | ||
| Residual RVOT obstruction (%) | 22% | 19% | 0.52 | - | ||
| RVOT peak DPG (mm Hg) | 23.6±6.0 | 26.2±7.8 | 0.2 | - | ||
| NYHA class (%) | ||||||
| I | 53% | 79% | 0.07 | 100% | ||
| II | 37% | 17% | 0.10 | - | ||
| III | 10% | 4% | 0.58 | |||
| IV | 0% | 0% | 1.0 | - | ||
| Medical therapy | 16% | 7% | 0.36 | - | ||
- a Data are expressed as mean±S.D. or percentage (%), when appropriate. DPG, Doppler pressure gradient; NYHA, New York Heart Association; PR, pulmonary regurgitation; RVOT, right ventricular outflow tract.
3.1. Ventilatory function
Baseline ventilatory function (VC and FEV1) was abnormal in ToF patients, especially in those with severe PR (Table 2). Sixty-three percent of patients in group I and 51% in group II had a mildly restrictive ventilatory function.
| Variables | Group I | Group II | p | Controls | p (control group vs. group I) | p (control group vs. group II) |
|---|---|---|---|---|---|---|
| Number of patients | 19 | 42 | 153 | |||
| Ventilatory function | ||||||
| VC (l) | 2.30±1.07 | 2.9±1.18 | 0.06 | 3.73±1.1 | <0.0001 | <0.0001 |
| FEV1 (l/min) | 1.9±0.88 | 2.52±1.04 | 0.03 | 3.0±0.9 | <0.0001 | 0.003 |
| Exercise phase of CPX | ||||||
| Peak VO2 (ml O2/kg/min) | 23.1±4.4 | 27.4±7.3 | 0.02 | 34.2±9.5 | <0.0001 | <0.0001 |
| Peak O2 pulse (ml O2/kg/beat) | 6.53±2.5 | 8.91±3.4 | 0.008 | 11.3±5.1 | <0.0001 | 0.005 |
| Peak HR (beats/min) | 163.0±24.4 | 167.1±19.3 | 0.48 | 173.5±25.0 | 0.08 | 0.13 |
| Peak VE (l/min) | 32.0±12.6 | 43.5±14.7 | 0.004 | 51.5±16.8 | <0.0001 | 0.006 |
| VE/VCO2 slope | 32.5 ± 6.8 | 31.3 ± 6.5 | 0.51 | 30.5±8.4 | 0.32 | 0.57 |
| Recovery phase of CPX | ||||||
| VO2 slope (l O2/min) | 0.24±0.09 | 0.36±0.15 | 0.002 | 0.57±0.20 | <0.0001 | <0.0001 |
| O2 pulse slope (ml O2/beat/min) | 1.26±0.4 | 1.65±0.5 | 0.004 | 2.24±1.13 | 0.0002 | 0.001 |
| VCO2 slope (l CO2/min) | 0.27±0.13 | 0.39±0.15 | 0.0038 | 0.50±0.23 | <0.0001 | 0.003 |
| VE slope (l/min) | 6.9±2.4 | 10.3±3.8 | 0.0007 | 13.6±5.2 | <0.0001 | 0.0002 |
| HR slope (beats/min) | 17.9±6.6 | 18.8±6.1 | 0.48 | 20.0±4.9 | 0.1 | 0.2 |
- a Data are expressed as mean±S.D. CPX, cardiopulmonary test; FEV1, forced expired volume in the first second; HR, heart rate; VE, minute ventilation; VC, vital capacity; VCO2, CO2 minute production; VO2, oxygen uptake.
3.2. Exercise phase of CPX
Exercise capacity was reduced in ToF patients (Table 2), especially in those with severe PR (group I). Peak VO2 was 42.3±17.7% of predicted in group I, 65.5±16.5% in group II and 94.0±19.3% in the control group. Peak HR was similar in patients and in controls.
3.3. Recovery phase of CPX
Recovery capacity was lower in patients compared to controls (Table 2), especially in patients with severe PR (group I), whereas HR slope was similar in the three groups.
3.4. MRI
As expected, group I patients had higher PR fraction and PR volume. They also had higher RV volumes and inferior LV end-diastolic (LVEDV) volume than group II patients, whereas LV end-systolic (LVESV) volume, RV and LV ejection fractions were similar (Table 3). Group I patients had a slightly higher prevalence of RV dysfunction (47% vs. 24%, p=0.08) and of LV dysfunction (LV ejection fraction <50%, 32% vs. 17%, p=0.31) than group II patients. Five patients in group I (26%) and six patients in group II (14%) had both RV and LV systolic dysfunction. Left ventricular ejection fraction correlated with RV ejection fraction (r=0.56, p=0.003). Despite a similar RV stroke volume index, effective RV stroke volume index was inferior in group I than in group II patients.
| Variable | Group I (severe PR) | Group II (PR<severe) | p |
|---|---|---|---|
| Number of patients | 19 | 42 | |
| RVEDV (ml/m2) | 134±29 | 108±33 | 0.004 |
| RVESV (ml/m2) | 78±19 | 57±13 | <0.0001 |
| RV ejection fraction (%) | 42±10 | 47±11 | 0.06 |
| RV stroke volume index (ml/m2) | 56±11 | 51±9 | 0.06 |
| Effective RV stroke volume index (ml/m2) | 35±6 | 42±5 | 0.0001 |
| LVEDV (ml/m2) | 75±12 | 89±13 | 0.0002 |
| LVESV (ml/m2) | 36±9 | 38±10 | 0.46 |
| LV stroke volume index (ml/m2) | 39±8 | 51±9 | <0.0001 |
| LV ejection fraction (%) | 52±10 | 57±11 | 0.09 |
| PR fraction (%) | 37±12 | 18±11 | <0.0001 |
| PR volume (ml/m2) | 21±4 | 9±3 | <0.0001 |
- a Data are expressed as mean±S.D. LV, left ventricle; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; PR, pulmonary regurgitation; RV, right ventricle; RVEDV, right ventricular end-diastolic volume; RVESV, right ventricular end-systolic volume; ToF, tetralogy of Fallot.
3.5. Role of RV dysfunction in patients with severe PR
Nine patients in group I had a RV ejection fraction <40%. Group I patients with RV dysfunction had both a reduced exercise capacity and a delayed recovery from exercise when compared to group I patients without RV dysfunction, despite a similar age at repair (5.3±4.6 vs. 4.8±4.4 years, p=0.81) and age at test (21.5±6.1 vs. 20.9±5.9 years, p=0.83). Cardiopulmonary exercise test had a high sensitivity and specificity to predict the presence of RV dysfunction in patients with severe PR (Fig. 2 and Table 4).
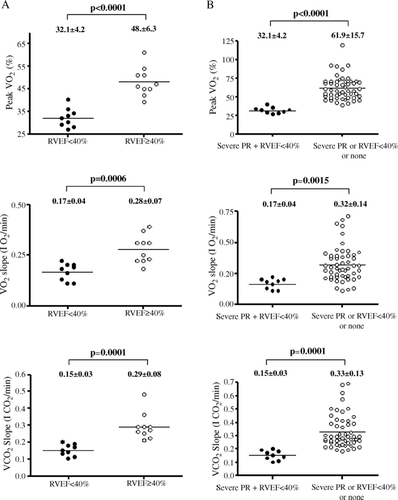
| Sensitivity (%) | CI (%) | Specificity (%) | CI (%) | AUC | |
|---|---|---|---|---|---|
| Cut-off values for RV dysfunction in ToF patients with severe PR (n=19) | |||||
| Predicted peak VO2<40% | 90.0 | 55.5–99.7 | 88.9 | 51.7–99.7 | 0.944 |
| VO2 slope<0.21 l O2/min | 90.0 | 55.5–99.7 | 87.5 | 47.3–100.0 | 0.943 |
| VCO2 slope<0.19 l CO2/min | 100 | 69.1–100 | 100 | 67.0–100.0 | 1.000 |
| Cut-off values for RV dysfunction in ToF patients without severe PR (n=42) | |||||
| Peak VO2<57% | 81.2 | 71–96.5 | 84.0 | 47.0–97.5 | 0.845 |
| VO2 slope<0.25 l O2/min | 84.4 | 67.2–94.7 | 80.3 | 44.4–97.5 | 0.901 |
| VCO2 slope<0.23 l CO2/min | 87.5 | 71.0–96.5 | 90.0 | 55.5–99.7 | 0.910 |
| Cut-off values for RV dysfunction and severe PR in overall study cohort (n=61) | |||||
| Peak VO2<40% | 100.0 | 93.1–100 | 88.9 | 51.7–99.7 | 0.998 |
| VO2 slope<0.20 l O2/min | 82.7 | 69.7–91.8 | 88.9 | 51.7–99.7 | 0.904 |
| VCO2 slope<0.19 l CO2/min | 94.2 | 84.0–98.8 | 89.8 | 51.7–99.6 | 0.950 |
3.6. Role of RV dysfunction in patients without severe PR
To assess the impact of isolated RV dysfunction on cardiopulmonary function, a subgroup analysis of group II patients was performed. Ten patients in group II had RV dysfunction (RV ejection fraction range from 29% to 38%). They had reduced peak VO2 (53±8% vs. 69±15%, p=0.0034), VO2 slope (0.19±0.07 vs. 0.38±0.14 l O2/min, p=0.0002) and VCO2 slope (0.21±0.05 vs. 0.37±0.14 l CO2/min, p=0.0007) when compared to patients without RV dysfunction, whereas residual RVOT pressure gradient (29±7 vs. 24±9 mm Hg, p=0.11) and HR slope (14±10.0 vs. 19±10 beats/min, p=0.19) were similar. They also had an older age at repair (4.9±3.5 vs. 2.2±2.4, p=0.008). Sensitivity and specificity of CPX to predict the presence of RV dysfunction in patients without severe PR are shown in Table 4.
3.7. Identification of patients with severe PR and RV dysfunction
In the overall study cohort, nine patients had both severe PR and RV dysfunction. They had significantly lower exercise capacity and prolonged recovery from exercise when compared to patients without the combination of both severe PR and RV dysfunction (p<0.0001; Fig. 2). Cardiopulmonary exercise test had good sensitivity and specificity to identify patients with severe PR and RV dysfunction in the overall study cohort (Table 4).
3.8. Determinants of VO2 slope
As expected, in ToF patients, VO2 slope during recovery correlated with peak VO2 (r=+0.84, p=0.0003). We also observed that VO2 slope correlated with O2 pulse slope (r=+0.85, p<0.0001), VCO2 slope (r=+0.97, p<0.0001), VE slope (r=+0.86, p<0.0001), HR slope (r=+0.64, p=0.01), VC (r=+0.84, p<0.0001) and FEV1 (r=+0.81, p<0.0001), whereas no correlation was found with age at test (r=+0.24, p=0.64) and RV (r=+0.58, p=0.13) or LV ejection fraction (r=+0.60, p=0.12). Recovery capacity was also associated with RV end-diastolic volume (r=−0.80, p<0.0001) and PR fraction (r=−0.78, p=0.0005).
4. Discussion
Since ToF patients with severe PR and RV dysfunction may significantly benefit from surgical or interventional relief of RV volume overload 3,8, identification of such patients is critical. However, despite the number of reports showing in ToF patients with severe PR an abnormal response to exercise, the possible role of CPX as a tool to guide the difficult decision-making process in these patients has never been evaluated. The main finding of the present study is that CPX has a high sensitivity and specificity to identify ToF patients with severe PR and RV dysfunction, and cut-off values are provided that combine the best sensitivity and specificity.
There is no conclusive explanation for a higher sensitivity and specificity of CPX to identify RV dysfunction in patients with severe PR than in patients without. We can hypothesize that the behavior of cardiac output and of stroke volume during exercise may be different in patients with severe PR from that of patients without for at least two reasons: (1) for a given increase of RV function during exercise, the increase of cardiac output and of stroke volume would be inferior, and therefore relatively more inappropriate, in patients with severe PR than in patients without; (2) in patients with severe PR+RV dysfunction, the increase of cardiac function during and immediately after exercise may not be of the same extent of that of patients without severe PR and RV dysfunction. The final result may reasonably be that the negative impact of RV dysfunction on recovery capacity is much more prominent in patients with associated severe PR than in patients without.
The only previous study dealing with exercise recovery in ToF patients 11 showed that the prolonged recovery kinetics of VO2 is related to an abnormal baro-reflex sensitivity and to elevated pulmonary artery pressure. The present study is the first to demonstrate the adverse impact of PR and of RV dysfunction on recovery of VO2 in ToF patients. Furthermore, our data suggest that a prolonged recovery of O2 pulse slope (an indirect marker of cardiac stroke volume) due to severe PR (with subsequent RV dilation and adverse ventricular interaction) and to RV dysfunction is an important determinant of prolonged recovery of VO2 after exercise. This interpretation is in keeping with the observation of prolonged recovery of cardiac output and stroke volume after exercise in a smaller group of patients 21.
The delayed recovery of VO2 and ventilation after exercise in patients with chronic congestive heart failure has been explained by a delay in the recovery of energy stores in the muscle 22, although other factors, such as skeletal muscle metabolic abnormalities, sustained hyperpnoea, CO2 retention, prolonged recovery of cardiac output and increased cost of breathing 12,23 may contribute to the characteristic response. Our data indicate that ToF patients, especially those with severe PR and/or RV dysfunction, show the same anomalies.
As previously reported in other conditions 12, we could not find any difference in the speed of recovery of HR between the three groups, suggesting that the mechanisms that regulate heart rate during recovery may be different from those during exercise.
With severe PR, the effective RV stroke volume is decreased because a large amount of blood regurgitates into the RV during diastole, leading to RV volume overload and dilation. Consistently, VO2 slope was closely associated to both PR fraction and RV end-diastolic volume. When severe RV overload acts chronically, RV dilation is followed by right and left ventricular dysfunction, as suggested by the association noted between RV and LV ejection fractions.
The anomalous recovery from exercise also affected the ventilation, with increased CO2 retention. The explanation for this finding remains unknown. Possible mechanisms may be an abnormal CO2 retention due to impaired lung gas diffusion 26 and an abnormal baseline spirometric function. The correlation between prolonged CO2 retention and prolonged recovery of VO2 may indeed suggests that the post-exercise decline toward resting values of VO2 and of cardiac output 21 is delayed to enhance CO2 elimination, as previously described in adults with congestive heart failure 24,25.
Consistent with previous studies 20, we were unable to see any difference in RV ejection fraction between group I and group II patients. We also observed a similar prevalence of trans-annular patch repair in patients with vs. without severe PR similarly. Congruently, a recent study demonstrated large, but similar amounts of PR in patients who received a trans-annular patch or an infundibular patch repair 27.
4.1. Methodological considerations
Timely identification of ToF patients with severe PR and RV dysfunction is critical to perform pulmonary valve replacement before irreversible RV dysfunction ensues. Although cardiac MRI represents the gold standard to evaluate RV dimension and function and to assess the degree of PR, it is still not currently available and/or reliable in many centers. In contrast, transthoracic echocardiography and exercise testing are widely available, and the assessment of repaired ToF still widely relies on these methodologies. Accordingly, we decided to assess how well an integrated approach using these two methodologies would identify patients with an indication to pulmonary valve replacement according to available MRI criteria 7.
5. Study limitations
The population studied may not be representative of the entire spectrum of ToF patients. In particular, the population studied does not represent the result of more recent surgical approaches (in terms of surgical timing and operative techniques). The results of the present study need therefore to be interpreted according to patient baseline characteristics. Even if MRI angiography did not show significant stenosis of the main pulmonary arteries, the presence of distal pulmonary artery branch stenosis could not be systematically excluded. Elevated RV pressure can affect VO2 recovery kinetic 11. In the present study, RV pressure was not included in the analysis since RV pressure data were not available for all patients (cardiac catheterization not performed or velocity of tricuspid regurgitant jet not measurable).
6. Conclusions
Cardiopulmonary exercise test can identify patients with severe PR and RV dysfunction and, with the use of proposed cut-off values, could be useful to guide the pulmonary valve replacement decision-making process. In ToF patients, severe PR, RV dysfunction and especially their combination negatively impact not only maximal response to exercise but also the ability to recover from exercise.




