Pulsatile hemodynamic effects of candesartan in patients with chronic heart failure: The CHARM Program
Abstract
Background:
Abnormal large artery function and increased pulsatile load are exacerbated by excess angiotensin-II acting through the AT1 receptor and contribute to the pathogenesis and progression of chronic heart failure (CHF).
Aims:
To evaluate effects of the AT1 receptor blocker candesartan (N=30) or placebo (N=34) on pulsatile hemodynamics in participants with CHF in the CHARM program.
Methods and results:
Noninvasive hemodynamics were assessed following 6 and 14 months of treatment and averaged. Using calibrated tonometry and aortic outflow Doppler, characteristic impedance was calculated as the ratio of the change in carotid pressure and aortic flow in early systole. Total arterial compliance was calculated by the diastolic area method. Brachial blood pressure, cardiac output and peripheral resistance did not differ between groups. Lower central pulse pressure in the candesartan group (57±20 vs. 67±17 mmHg, P=0.043) was accompanied by lower characteristic impedance (200±78 vs. 240±74 dyne s/cm5, P=0.039) and higher total arterial compliance (1.87±0.70 vs. 1.47±0.48 ml/mmHg, P=0.008). Similar favorable differences were seen when analyses were stratified for ejection fraction (≤0.40 vs. >0.40) and baseline angiotensin converting enzyme inhibitor use.
Conclusions:
Candesartan has a favorable effect on large artery function in patients with chronic heart failure.
1. Introduction
Pulse pressure, a crude indicator of large vessel stiffness, is an important risk factor for the development [1,2] and progression of left ventricular dysfunction [3] and chronic heart failure (CHF) [4]. Several studies have shown that arterial stiffness is increased in CHF [5,6]. There is growing evidence that abnormal arterial function may play an important role in the pathogenesis of CHF with preserved systolic function [7,8]. In light of these observations, it stands to reason that interventions that reduce the stiffness of the central arteries may be beneficial in terms of both prevention and treatment of CHF.
The renin-angiotensin-aldosterone system (RAAS) plays a key role in regulation of functional and structural properties of large arteries. Activation of the RAAS promotes myocyte hypertrophy and extracellular fibrosis and up-regulates enzymes involved in production of reactive oxygen species [9,10]. The resulting oxidative stress impairs nitric oxide availability and endothelial function and likely interferes with normal matching between arterial flow and vascular impedance. Mismatch between vascular properties and hemodynamic demand results in increased pulse pressure and pulsatile load [11], which may accelerate the progression of disease. Blockade of the RAAS through inhibition of angiotensin converting enzyme (ACE) or downstream blockade at the AT1 receptor, either alone or in combination with ACE inhibition, represents an attractive approach for reversing the adverse effects of RAAS activation on arterial properties. In support of this hypothesis, the angiotensin-II, type 1 (AT1) receptor blocker candesartan has been shown to significantly reduce pulse pressure in hypertensives [12]. The Candesartan in Heart failure Assessment of Reduction in Mortality and morbidity (CHARM) program was designed to assess the effects of candesartan on clinical events in patients with CHF and either impaired or preserved left ventricular systolic function [13]. This hemodynamic substudy was designed primarily to evaluate the effects of candesartan on direct measures of central arterial stiffness in a subgroup of the CHARM participants. A secondary aim was to evaluate modification of the effects of candesartan between patient groups based on left ventricular systolic function or baseline ACE inhibitor use.
2. Methods
2.1. Study participants
Details of the CHARM program have been published elsewhere [13]. Patients were randomized to receive either candesartan or placebo, which was force-titrated as tolerated with a target dose of 32 mg once daily. Concomitant use of conventional CHF treatments, such as beta-blockers, diuretics, digitalis, spironolactone, and, if appropriate, ACE inhibitors, was encouraged. Hemodynamic studies were performed 6 and 14 months following initiation of randomized treatment. The investigations performed in this study conform to the principles outlined in the Declaration of Helsinki. An institutional review board at each clinical center approved the study protocol and each individual gave written informed consent prior to enrollment.
2.2. Hemodynamic data acquisition
Participants were studied in the supine position after approximately 10 min of rest. Supine auscultatory blood pressures were obtained by using a computer-controlled device that automatically inflated the cuff (Hokanson SC12) to a user preset maximum pressure and then precisely controlled deflation at 2 mmHg/s. This device digitized (1000 Hz) and recorded mean and oscillometric cuff pressure, ECG and a cuff microphone channel throughout the inflation and deflation sequence so that all blood pressures could be over-read by the core lab. Blood pressure was obtained 3–5 times at 2-min intervals with a goal of obtaining 3 sequential readings that agreed to within 5 mmHg for SBP and DBP. Arterial tonometry with ECG was obtained from the brachial, radial, femoral and carotid arteries using a custom transducer. This transducer has a small sensor surface area and a frequency response in the kHz range. Next, echocardiographic images of the left ventricular outflow tract were obtained from a parasternal long axis view. This was followed by duplicate acquisitions of simultaneous tonometry of the carotid artery and pulsed Doppler of the left ventricular outflow tract from an apical 5-chamber view. Finally, body surface measurements from suprasternal notch to radial, femoral and carotid recording sites were obtained. All data were digitized during the primary acquisition, transferred to CD-ROM and shipped to the core lab for analysis.
2.3. Data analysis
Tonometry waveforms were signal-averaged using the electrocardiographic QRS as the fiducial point [14]. Average systolic and diastolic cuff pressures were used to calibrate the peak and trough of the signal-averaged brachial waveform. Diastolic and mean brachial pressures were then used to calibrate carotid, radial and femoral waveforms [15]. Carotid-brachial and carotid-femoral pulse wave velocities, aortic compliance, characteristic impedance and total arterial compliance were calculated as previously described [6,16]. Characteristic impedance was estimated in the time domain as the early change in pressure divided by the corresponding change in flow, prior to return of the reflected wave. Values obtained by using a time domain approach to estimate characteristic impedance are highly correlated with frequency domain techniques, with R=0.948 to 0.994, depending on the averaging criteria used for the frequency domain estimate [6]. The foot of the carotid pressure waveform was first aligned with the foot of the aortic flow waveform [15]. Total arterial compliance was estimated by using the diastolic area method applied to the last two-thirds of diastole [17]. Carotid pressure waveforms were decomposed in the time domain into forward and reflected waves [18]. The augmentation index, a measure of the relative contribution of wave reflection to central pulse pressure, was assessed from the calibrated carotid pressure waveform [6]. Pulse pressure amplification was assessed by taking the ratio of carotid and brachial pulse pressure. As previously reported, reproducibility of measures of central aortic stiffness using our protocol in a multicenter setting is high, with intraclass correlation coefficients for repeated measures of characteristic impedance of 0.93–0.95 [6].
2.4. Statistical analysis
Baseline characteristics were tabulated according to treatment group. For the primary analysis, continuous variables were compared by using an independent-samples t-test with treatment as a grouping variable. In secondary analyses, treatment effect modification by status of left ventricular function (preserved versus impaired) or use of ACE inhibitor was assessed by using an analysis of variance with treatment and each of the above entered as grouping variables. An interaction term was also included in these models. The study was prospectively designed to evaluate differences between groups in measures of aortic stiffness, including aortic compliance, characteristic impedance and pulse wave velocity, following 6 to 14 months of treatment using an independent-samples test. Results of the 6 and 14 month evaluations were averaged. However, if the 14-month study was not obtained or was technically unacceptable, the 6-month result was carried forward. Conversely, if the 6-month study was not available, the 14-month result was used. Based on findings in a sample of 28 baseline studies [6], we estimated that a sample size of 30 participants in each treatment group provided 80% power to detect a 25% reduction in characteristic impedance or a 28% increase in total arterial compliance. Values are presented as mean±SD except in the figures, where error bars represent the SEM. A two-sided P<0.05 was considered significant.
3. Results
On-treatment hemodynamic studies were performed in 69 individuals at 8 centers. There were 64 participants with at least one study judged to be technically acceptable by the core lab prior to unblinding of treatment codes (34 assigned to placebo and 30 assigned to candesartan). Of these 64 cases, 39 had a 6-month study, 56 had a 14-month study and 31 had both. Characteristics of the study sample are presented in Table 1, which demonstrates a balanced randomization. As compared to the main trial [13], substudy participants were younger and less likely to be female. Medication usage tended to be higher in the substudy participants as compared to the main trial. Just over half of the participants had preserved left ventricular systolic function with an ejection fraction greater than 0.40 and just over half were taking ACE inhibitors at the time of randomization (Table 1).
| Variable | Placebo | Candesartan |
|---|---|---|
| N | 34 | 30 |
| Female, N (%) | 7 (21) | 5 (17) |
| Age (years) | 61±12 | 60±10 |
| Height (cm) | 174±12 | 174±11 |
| Weight (kg) | 87.0±28.2 | 88.7±19.4 |
| Body-mass index (kg/m2) | 28.2±6.5 | 29.2±6.0 |
| Ejection fraction (−) | 0.40±0.14 | 0.41±0.15 |
| Ejection fraction >0.40, N (%) | 15 (44) | 15 (50) |
| Treatment duration (months) | 13.3±2.9 | 13.8±3.4 |
| History of, N (%): | ||
| Hypertension | 23 (68) | 22 (73) |
| Diabetes | 12 (35) | 13 (43) |
| Prior myocardial infarction | 19 (56) | 18 (60) |
| Medications, N (%): | ||
| ACE inhibitor | 19 (56) | 15 (50) |
| Digoxin | 21 (62) | 14 (47) |
| Beta adrenergic blocker | 18 (53) | 18 (60) |
| Diuretic | 28 (82) | 24 (80) |
| Lipid lowering agent | 18 (53) | 20 (67) |
| Aspirin | 25 (74) | 19 (63) |
- a ACE, angiotensin converting enzyme.
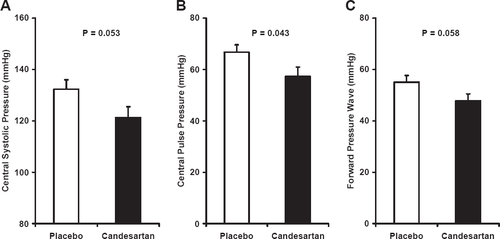
Results of hemodynamic studies performed at the time of the on-treatment visit are presented in Table 2. There were no significant differences in brachial blood pressure although pulse pressure tended to be lower in the candesartan group. There were no differences in measures of steady-flow load, including mean arterial pressure, cardiac output and peripheral resistance, and there were no differences in augmentation index or pulse wave velocities between treatment groups (Table 2). However, central pulse pressure was lower in the candesartan group (fig. Fig. 1). This reduction in central pulse pressure was accompanied by consistent trends toward reductions in central systolic pressure and the amplitude of the forward pressure wave (fig. Fig. 1). Lack of a difference in augmentation index or peak flow (Table 2) coupled with strong trends toward an increase in carotid-brachial pressure amplification and a reduction in central forward wave amplitude suggest that proximal aortic stiffness was reduced by treatment with candesartan. Consistent with this hypothesis, which was the main hypothesis of the study, there was a reduction in characteristic impedance (fig. Fig. 2A), which evaluates the most proximal segment of the aorta, and a strong trend toward an increase in aortic compliance (fig. Fig. 2B), which represents the averaged properties of the full aorta. In addition, total arterial compliance, which is affected by changes in properties of the aorta, peripheral arteries and small vessels, was significantly greater in patients treated with candesartan (fig. Fig. 2C).
| Variable | Placebo | Candesartan | P |
|---|---|---|---|
| Brachial blood pressures (mmHg) | |||
| Systolic pressure | 131±21 | 123±21 | 0.095 |
| Diastolic pressure | 65±11 | 64±9 | 0.569 |
| Mean arterial pressure | 90±14 | 85±13 | 0.202 |
| Pulse pressure | 66±17 | 59±17 | 0.088 |
| Heart rate (min−1) | 67±13 | 65±9 | 0.340 |
| Cardiac output (ml/s) | 68±17 | 74±21 | 0.258 |
| Peak flow (ml/s) | 310±69 | 323±68 | 0.456 |
| Stroke volume (ml) | 63±16 | 68±15 | 0.202 |
| Peripheral resistance (DSC) | 1883±561 | 1661±477 | 0.095 |
| Augmentation index (%) | 8.3±19.3 | 8.6±13.3 | 0.951 |
| Amplification (ratio) | 1.00±0.12 | 1.05±0.13 | 0.075 |
| Carotid-brachial PWV (m/s) | 8.0±2.0 | 8.3±2.4 | 0.516 |
| Carotid-femoral PWV (m/s) | 10.0±2.9 | 9.6±3.4 | 0.623 |
- a DSC, dyne s/cm5; PWV, pulse wave velocity.

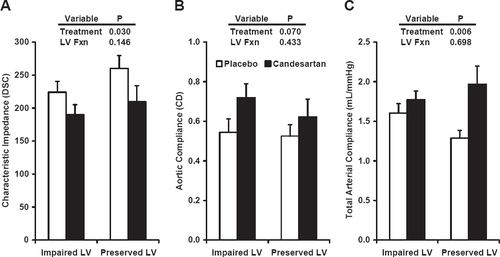
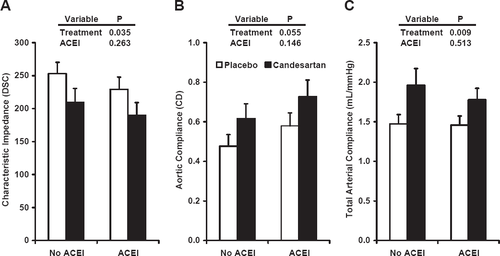
In order to determine whether treatment effect was influenced by the status of left ventricular function, we evaluated central hemodynamic variables with participants grouped according to the pre-specified ejection fraction cutoff of 0.40 [13] and found no significant interaction between treatment assignment and ventricular function, although there was a trend toward an interaction for total arterial compliance (P=0.088) (fig. Fig. 3). We found no evidence of an interaction between treatment effect and concomitant use of ACE inhibitors (fig. Fig. 4).
4. Discussion
This study evaluated the effects of candesartan on pulsatile load in individuals with CHF. The results demonstrated a favorable reduction in pulsatile load as evidenced by a reduction in characteristic impedance and an increase in total arterial compliance in patients assigned to candesartan therapy. Similar favorable changes in pulsatile load were observed following treatment with candesartan regardless of whether participants were treated with concomitant ACE inhibitors or not and in those with impaired or preserved left ventricular systolic function. These findings suggest that treatment with candesartan had a favorable direct effect on proximal aortic properties and provide a mechanism for the reduction in pulse pressure that was observed in the main CHARM program. Our results also demonstrate that changes in brachial blood pressure following an intervention that improves large artery function may underestimate changes in central pressure and pulsatile load on the heart and central vessels. Thus, treatment with candesartan reduced pulsatile load on the heart, which we have shown previously to be markedly abnormal in CHF [6]. Candesartan had a lesser effect on mean load, which was minimally abnormal in these treated CHF patients. Since increased pulsatile load is associated with development and progression of CHF [1,4], we speculate that the observed reduction in pulsatile load on the heart may have contributed to the 12% reduction in cardiovascular death and 16% reduction in cardiovascular death or hospitalization for CHF that was observed in the overall CHARM program [13]. Thus, AT1 receptor blockade with candesartan offers an opportunity to reduce pulsatile load on the heart in patients with CHF.
Prior studies have evaluated various components of pulsatile load in CHF. One study showed a marked increase in characteristic impedance in CHF [5], whereas others found no difference compared to controls [192021]. Utilizing data from a subset of the CHARM program, as compared to a group of patients with comparable cardiac risk factors but no history of CHF, we previously demonstrated that characteristic impedance was significantly elevated in CHF [6]. Controversy in the literature regarding the status of characteristic impedance in CHF may be attributable to differences in patient selection and data analysis (see Ref. [6] for a detailed discussion). Recent studies that specifically evaluated individuals with CHF and preserved left ventricular function have demonstrated increased central aortic stiffness [7,8]. Prior studies of segmental arterial properties have shown increased stiffness of the carotid [22], brachial [23] and radial [24] arteries in CHF. Abnormal endothelial modulation of arterial stiffness has been demonstrated in the femoral artery of CHF patients [25]. Thus, a component of abnormal arterial stiffness observed in CHF patients may be dynamic and amenable to improvement following interventions that improve endothelial function. For example, exercise improves endothelial function in CHF [26] and increases total arterial compliance [27]. Our study shows that treatment with candesartan is associated with a reduction in characteristic impedance, which is a measure of proximal aortic stiffness, and an increase in total arterial compliance.
We found comparable effects of AT1 receptor blockade with candesartan on pulsatile load regardless of whether participants were taking ACE inhibitors at baseline or not. Prior studies have shown incremental benefit of AT1 receptor blockade added to ACE inhibition on conventional hemodynamics and neurohormone levels [28,29]. This added benefit may reflect failure of conventional ACE inhibitors to fully suppress the RAAS in CHF. Lack of complete suppression of the RAAS has been attributed to loss of competitive inhibition by ACE inhibitors following compensatory up-regulation of angiotensin-I production and ACE levels [30]. There are also alternative pathways for angiotensin-II generation from angiotensin-I, which may not be affected by ACE inhibitors [31]. AT1 receptor blockade provides a logical downstream intervention that eliminates activity at the AT1 receptor regardless of the source of angiotensin-II.
We found a reduction in characteristic impedance and an accompanying increase in total arterial compliance but no change in augmentation index or regional pulse wave velocities. We speculate that this dissociation between changes in characteristic impedance and pulse wave velocity may involve an increase in aortic diameter since characteristic impedance is markedly more sensitive to changes in diameter than pulse wave velocity [6]. However, we did not measure the diameter of the aortic root directly so we cannot confirm this hypothesis. In order to interpret the change in total arterial compliance in an arterial system with distributed compliance properties and finite propagation velocities, it is necessary to measure the various potential regional contributors to total compliance, as we have done. The change in total arterial compliance, which is even more sensitive to aortic diameter than characteristic impedance, was predominantly attributable to changes in proximal aortic properties, as indicated by the decrease in characteristic impedance, with no change in regional pulse wave velocities. The lack of change in augmentation index is consistent with the lack of a significant change in regional pulse wave velocities or peripheral resistance. In order to assess the validity of averaging on-treatment hemodynamic variables, we evaluated change in characteristic impedance and total arterial compliance between 6 and 14 months in the 31 participants who had results from both time points. We found no effect of time (P=0.640 for characteristic impedance and P=0.137 for total arterial compliance) and no time-by-treatment interaction (P=0.374 for characteristic impedance and P=0.113 for total arterial compliance). Lack of a time-treatment interaction between 6 and 14 months is consistent with a relatively prompt effect of candesartan on proximal aortic properties, occurring prior to the 6-month study. A similarly prompt reduction in aortic characteristic impedance has been reported in hypertensive individuals treated for 12 weeks with the vasopeptidase inhibitor omapatrilat [32]. Thus, the prior study and our present results indicate that aortic properties are amenable to modification following relatively short-term treatment. However, we caution that our power to detect a time-treatment interaction was low in the subset of participants who had studies performed at both 6 and 14 months. Therefore, we cannot exclude the possibility of additional effects with longer treatment.
There are important limitations of our study that must be considered. In this mechanistic study, statistical power would have been enhanced if change from pretreatment baseline had been assessed. However, the prolonged setup and training that was required for sites participating in this substudy limited our ability to obtain baseline and 6-month studies in most of our study participants. With this limitation in mind, the trial was prospectively designed with a primary goal of evaluating independent differences in pulsatile hemodynamics between groups at follow-up. The patient population was heterogeneous and our sample represents a small fraction of the overall CHARM cohort, making randomization imbalances a concern. However, we found no differences in baseline characteristics between treatment groups in the participants in our study. The sample size is small and the significance level is marginal for several of the key variables. However, the findings were internally consistent when evaluated in preplanned subgroups and across several relatively independent measures of arterial stiffness, including pulse pressure from clinical blood pressures assessed in the full cohort in the CHARM program.
In summary, we have shown that AT1 receptor blockade with candesartan in patients with CHF was associated with improved pulsatile hemodynamics, including lower characteristic impedance of the aorta and higher total arterial compliance, which are major determinants of pulse pressure. Benefit was comparable in individuals with preserved or impaired left ventricular systolic function and in those treated or not treated with concomitant ACE inhibition. This favorable reduction in pulsatile load may have contributed to the clinical benefit that was observed in the CHARM program [13].
5 Acknowledgements
This study was funded by a grant from AstraZeneca.
Additional study investigators: Michael R. Zile, Charleston, SC; James Fang, Boston, MA; Robert McKelvie, Hamilton, ON; Gervasio A. Lamas, Miami, FL.




