Colour M-mode velocity propagation: a glance at intra-ventricular pressure gradients and early diastolic ventricular performance
Abstract
The physiology of early-diastolic filling comprises ventricular performance and fluid dynamical principles. Elastic recoil and myocardial relaxation rate determine left ventricular early diastolic performance. The integrity of left ventricular synchrony and geometry is essential to maintain the effect of their timely action on early diastolic left ventricular filling. These factors not only are prime determinants of left ventricular pressure decay during isovolumic relaxation and immediately after mitral valve opening; they also instigate the generation of a sufficient intra-ventricular pressure gradient, which enhances efficient early diastolic left ventricular filling. Accurate assessment of diastolic (dys)function by non-invasive techniques has important therapeutic and prognostic implications but remains a challenge to the cardiologist. The evaluation of left ventricular relaxation by the standard Doppler echocardiographic parameters is hindered by their preload dependency. The colour M-mode velocity propagation of early diastolic inflow (Vp) correlates with intra-ventricular pressure gradients and is a largely preload independent index of ventricular diastolic performance. In this article, the physiologic background, utility and limitations of this promising new tool for the study of early diastolic filling are reviewed.
1. Introduction
Alterations in diastolic function of the left ventricle (LV) occur in a broad range of cardiovascular diseases, often being the first and sometimes sole manifestation of the cardiac disorder. The hallmark of diastolic dysfunction is the impaired capacity to fill or maintain stroke volume without a compensatory increase in filling pressures, resulting in exertional dyspnoea and—in later stages—signs of pulmonary venous congestion 1–3. LV diastolic function is quantified in terms of indices of active relaxation and passive chamber stiffness 3. Indices that reflect changes in the oxygen, requiring active process of relaxation, generally depend on the onset, rate or extent of ventricular pressure decline and filling, and ideally do this in the entire physiologic range of haemodynamic changes in heart rate, stroke volume, pre- and afterload. Between the end of force generation (systole) and mitral valve opening, the LV pressure drops without LV filling (isovolumic relaxation). Tau, the time constant of LV-pressure decline, is generally considered as a relative heart rate and preload independent index of ventricular relaxation. After mitral valve opening, LV filling occurs with variable LV pressures (auxotonic relaxation). Measurements made during auxotonic relaxation are determined both by active relaxation and passive chamber stiffness, with the latter becoming increasingly important towards the end of ventricular filling 3. The entirety of active processes during early diastole will enhance a fast LV pressure drop during isovolumic relaxation and subsequently a fast active filling at low pressures during early auxotonic relaxation (Fig. Fig. 1). The efficiency of these active processes could be referred to as ‘early diastolic performance’.
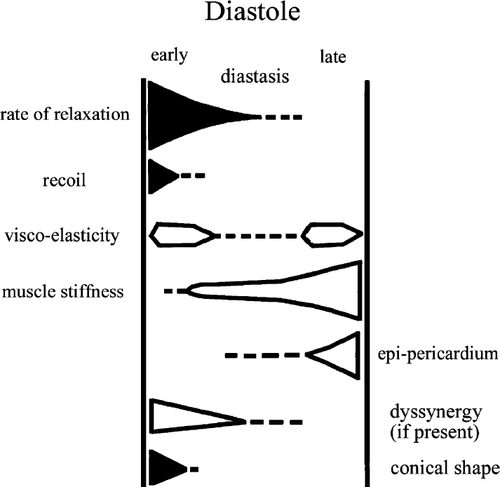
Already, in 1930, Katz speculated that normal ventricular relaxation resulted in ‘active suction’ of the blood into the chamber 4. However, only in the early 1980s, the presence of diastolic intraventricular pressure gradients (IVPGs) were confirmed 5,6. The gradients between the ventricular base (mitral valve) and apex were suggested to exert a suction effect. When subsequently it was shown that these gradients were diminished by ischemia and related to systolic function, the concept that they reflected recoil was born 7. Moreover, when Nikolic demonstrated IVPGs during early diastole in filling as well as in non-filling heart beats, the hope that IVPGs would become an index for isovolumic and early auxotonic ventricular relaxation (‘early diastolic performance’), was substantiated 8. Because pressure gradients in fluids imply fluid acceleration and fluid currents, a non-invasive index of fluid acceleration would, in theory, reflect these IVPGs. The colour M-mode velocity propagation index (Vp) has the potential to fulfil this promise. The current article reviews theories and experimental data on fluid dynamics of diastolic filling, its relation to IVPGs and to Vp. We also report on the clinical applications and some of the limitations of the technique.
2. Colour M-mode Doppler: data acquisition and processing
Whereas spectral Doppler analyses blood velocities of individual blood particles at a given point in space, colour M-mode Doppler is a pulsed Doppler technique in which mean velocities are colour encoded and displayed in time (on the horizontal axis) and depth along the entire scan line (on the vertical axis). Acquisition is made in the four-chamber view, usually by trans-thoracic apical imaging, although acquisition during TEE has also been reported 9. In order to visualize the direction of the inflowing blood, a large colour box is placed from the mitral valve to the apex. Care should be taken for the scan line to go through the centre of the mitral valve and along the central part of the blood column (staying within the 60% central part of the mitral orifice and limiting misalignment to ≤20° is acceptable) 10,11. Depending on the technique, gain is set at subsaturation levels and the Nyquist range limit is adapted to ±75% of the spectral E velocity to obtain overflow (‘aliasing’). Switching to M-mode and with the chart recorder set at a sweep rate of 100 mm/s, an M-mode spatio-temporal velocity map with the shape of a ‘flame’ is displayed (Fig. Fig. 2). A typical colour M-mode map has a temporal resolution of approximately 5 ms, a spatial resolution (depth) of approximately 1 mm and a velocity resolution of approximately 4 cm/s, according to the Nyquist limit.
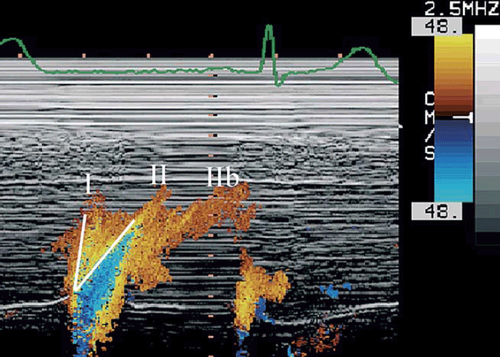
By digital processing, for each pixel of the displayed colour M-mode map, time, depth and numerical velocity can be determined. The Euler equation offers the unique opportunity to calculate instantaneous pressure gradients along the inflow tract in a non-invasive way 11. In other words, the magnitude, direction and duration of the generated pressure gradients along the ventricular inflow tract is expressed and can be decoded from the depicted data. In this way, the colour M-mode map also represents a spatio-temporal pressure map. Extraction of the IVPGs from the displayed ‘flames’, however, is too laborious for daily clinical use.
Therefore, for clinical practice, the spatio-temporal velocity information is expressed as distance to time ratios (cm/s or ms/cm). This is commonly referred to as the ‘flow propagation velocity’ (FPV) because it has the dimensions of velocity and because for long it has been interpreted as the distance the flow-wave (E-wave) propagates into the left ventricle as a function of time. Three alternative methods have been proposed for determining FPV.
(1) Stugaard et al. measured the time delay (TD) between onset of maximal flow velocity at the mitral valve tips and at 3.7 cm towards the apex 12. The TD can be indexed for the mitral to apex distance (TDn in ms/cm). This technique requires off-line post processing using dedicated software to decode the colour of each pixel into numerical values. It depends on two individual pixels, and the reliance on accurate measurement of apical flow constitutes its primary limitation. When apical flow velocities are low, approaching noise levels, or when a bifurcated pattern is observed, the choice of the apical peak velocity, therefore becomes more cumbersome 13. (2) Brun et al. measured slopes of the isovelocity lines formed by transition of no colour to colour (black to red) 14. The technique was later modified by Garcia et al. who traced the slope of the first ‘aliasing’ velocity (red to blue) from the mitral valve plane to 4 cm distal in the LV (Vp in cm/s). The latter technique only requires on-line adaptation of the Nyquist range limit and/or baseline to obtain ‘aliasing’ 15. Both techniques rely on a large number of pixels instead of only two. Moreover, measurements can be performed on-line. (3) The Takatsuji method of FPV calculation depends on off-line, progressive scaling down of the Nyquist range by specific software. The slope between the site of the highest velocity at the mitral valve, connected with the point were ‘aliasing’ becomes apparent when the Nyquist range is further scaled down by 30%—regardless of the depth of this point—is defined as Vp 16.
It should be stressed that this ‘flow propagation velocity’ is not always an actual measurement of fluid propagation from base to apex but is an artificial connection of points of maximal observed velocity. These points can represent different fluid elements that are almost simultaneously accelerated by local pressure gradients at different depths along the inflow tract 12,13 (see next chapter). Therefore, simply ‘velocity propagation’ is somewhat more accurate. For the remainder we will refer to it as Vp irrespective of the acquisition method.
3. Pressure and flow propagation: fluiddynamics and (patho)physiology
Left ventricular inflow during diastole is a complex, three-dimensional event that has been incompletely described and understood at this time. Moreover, the concepts of fluid and velocity propagation are challenging.
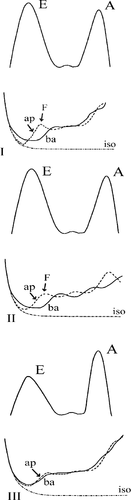
During the isovolumic relaxation and immediately at the opening of the mitral valve, blood already in the atrium and the ventricle moves as a hardly or non-compressible column of fluid (phase I). In other words, displacement of blood particles at one end of the column causes displacement of blood particles at the other end of the column with hardly any delay (columnar flow). Pressure gradients and hence blood velocities formed in this phase will almost immediately be conducted throughout the ventricle, according to the Moens–Kortew, e.g. equation (pressure wave). Consequently, the propagation of this pressure wave is very fast, exceeding the velocity of the individual blood particles themselves. Reduced compliance causes an increase in pressure wave propagation while the velocities of the blood particles decreases, consistent with the principles of acoustic conduction. When the pressure wave approaches the apex, the actual pressure near the apex is formed by a superposition of the apical pressure drop due to relaxation and the pressure rise due to apical filling 17,18. In ventricles with normal relaxation, the influence of relaxation is initially more prominent than the influence of filling 18. At the onset of filling, the pressure can still drop in regions of the ventricle, even after the pressure wave has passed by. However, when the pressure wave reaches the apex, apical pressure will abruptly rise due to the reflection of this wave at the apex (F-wave), aborting columnar filling (Fig. Fig. 3).
In a second phase, the blood particles themselves are transported into and throughout the ventricle, with the formation of a filling or a ‘flow’ wave (phase II). The propagation of this flow wave into the ventricle will be governed by the principles of convective conduction. As a consequence, the propagation velocity of the flow wave can never exceed the velocity of the fastest blood particles themselves. If the propagation of the entire filling wave is slower than the velocity of the fastest blood particles, some form of redistribution of the blood within the ventricle has to occur. Vortex formation is the most admitted form of redistribution. In vivo 19, in vitro 20, as well as computer models 17,21 of normal ventricles, confirmed both columnar flow with fast propagation of a pressure wave in phase I and the formation of a ring vortex in phase II. Vortex centre and flow wave are simultaneously conducted throughout the ventricle 21. In the Colour M-mode map, pressure (phase I) and flow/vortex (phase II) propagation can sometimes be recognized as forked flames 12,13,15,19,22 (Fig. Fig. 2).
According to the most recent knowledge, the two major principles of fluid dynamics predicting the characteristics of fluid propagation in the left ventricle during the entire diastole are pressure gradient and vortex formation. Both of them are modified by left ventricular geometry and synchrony of wall relaxation.
3.1. Pressure gradient formation
Any acceleration and deceleration of fluid implies the formation of local pressure gradients. Studies by Courtois from the late eighties 7, as well as more recent studies 11,23,24 demonstrated that in normal ventricles during early diastole, dynamic intraventricular pressure gradients (IVPGs) are created with lower pressures at the apex compared to the cardiac base. These IVPGs diminish or disappear in case of ventricular ischemia because ischemia diminishes the rate and extent of wall relaxation 7,12,18,24. Courtois et al. speculated the apex to be a source of early diastolic recoil, creating suction forces that actively aspire blood into the LV-cavity 7. Nikolic et al. demonstrated that, besides recoil, ventricular shape-changes determine the magnitude of pressure gradients during early diastole, and do this irrespective of whether blood flow occurred or not 8. However, the same pattern of intra-ventricular apex to base gradients in early diastole has been reproduced using a numerical, computed simulation of left ventricular inflow which incorporated relaxation but did not incorporate elastic recoil 17 (Fig. Fig. 3). Recoil (and thus active aspiration) is, therefore not required but will enhance pressure gradient formation 8,24.
3.2. Vortex formation
The creation of a vortex indicates transition from the fast columnar filling to a slower form of flow propagation with a more complex pattern of pressure gradients and redistribution of blood flow 17,18. Originally, vorticity (turbulence) was believed to be solely created by shear between inflowing blood and stationary blood. However, vortex formation by shear only would take much longer than the duration of diastole 25, which does not correspond to the observed formation of a ring vortex in early diastole 17,19–21. Vorticity, therefore likely results from the acceleration of blood along the inflow tract, which represents a force applied on quiescent blood already in the ventricle. The ring vortex will first appear at the cardiac base, at the level of the mitral valve tips, with subsequent propagation towards the apex during the deceleration phase of transmitral flow 19,21,26. Contrarily to the effect on the pressure wave, reduced compliance is believed to slow down the vortex (flow) propagation 17,21. Consistent with the ‘inside out’ rotation of a ring vortex compared to the central flow column, reversed E-waves have been reported to appear earlier in the left ventricular outflow tract than at the apex 19. Previously, these reversed E-wave velocities were believed to result from the reflection of the pressure wave at the apex, and therefore to depend on relaxation and compliance 27.
3.3. Dyssynchrony of relaxation
Delayed outward or paradoxical inward movement of some segments annihilates part of the pressure lowering effect of the outward moving segments 28,29, altering the vector and magnitude of the instantaneous IVPGs. Moreover, any segment with a delayed relaxation is still in a state of relative contraction and represents an area of reduced compliance.
3.4. Left ventricular geometry
Magnitude and vector of pressure gradient formation will depend on ventricular geometry: in small ellipsoidal cavities, apex to base negative gradient formation is enhanced 8,18,24. Blood redistribution by vortex formation and propagation requires that the ventricular diameter sufficiently exceeds the mitral diameter 26. If the ability for velocity redistribution is limited, the velocity of the flow wave will approximate the velocity of the fastest particles. Inversely, in a dilated ventricle with a more spherical shape, a larger vortex will be created, taking more time to detach from the mitral valve and to propagate towards the apex 30.
Because IVPGs can be calculated from the velocity profile depicted by colour M-mode (r=0.96) 11, the velocity profile will in turn correspond to the instantaneous pressure gradients. Although the value of Vp per se does not play a role in calculation of the IVPGs, Steine et al. showed a strong correlation of Vp to IVPGs (r=0.94), even stronger after logarithmic transformation (r=0.98; P<0.001) 18. Recent data demonstrated that IVPGs correlated to end-systolic volume and the time constant of relaxation tau 24. As a consequence, the aforementioned mechanisms predict propagation velocities to be correlated with tau (by relaxation rate and non-uniformity 29), ventricular shape, dimension and/or relative mitral orifice diameter (by recoil and/or vortex formation), while being relatively independent of loading conditions.
4. Haemodynamic determinants of velocity propagation: data from clinical and experimental studies
4.1. Preload independence of Vp
The poor correlation between peak mitral inflow velocity (E) and Vp of the early filling wave is generally agreed on. When, despite worsening relaxation, E increases due to increasing filling pressures (pseudo-normalization), Vp does not rise 16. This indirectly suggests preload independence. In meticulously controlled invasive canine studies, significant preload alteration by balloon occlusion of the inferior vena cava or rapid saline infusion in baseline state 12 as well as under varying inotropic and lusitropic conditions with dobutamine or esmolol 31, did not induce significant change in propagation velocity. Recently, preload independence has also been demonstrated in human patients with and without left ventricular systolic dysfunction, and moreover, in healthy individuals 31–33.
4.2. Relation to the time constant of relaxation
The isovolumetric time constant of relaxation (tau) is considered to be the gold standard for defining the rate of relaxation during the isovolumic relaxation, which in turn determines myocardial relaxation at the onset of ventricular filling. Changes in diastolic relaxation rate can be reversibly induced by ischemia and beta-blockers that will prolong tau, or use of beta-mimetics that will foreshorten tau 12,31,34. In dogs, Stugaard et al. showed a significant reduction of Vp in parallel with an increase in tau during induction of diastolic failure with regional 12 or global 18 ischemia as well as with high dose propranolol 34. Similar highly significant correlations between Vp and changes in tau during esmolol and dobutamine infusion were obtained by Garcia et al. in dogs 31.
Invasive studies in humans also show a consistent high inverse correlation between tau and the propagation rate Vp. Takatsuji et al. 16 found a strong inverse relation in 40 patients undergoing cardiac catheterisation (r=−0.82; P<0.001), similar to values reported by Garcia (r=−0.86; P<0.001) 31, and Brun (r=−0.74; P<0.0001) 14
4.3. Relation to diastolic volume
In an invasive study in which LV end-systolic volume was significantly (P<0.001) reduced, the acute changes in diastolic volume, without significant alterations in ventricular geometry, did not induce significant change in tau or Vp, and multivariate analysis failed to demonstrate any relation between diastolic volumes and Vp 31. In a general population, an ambivalent relation is expected: when small LV volumes reflect the degree of left ventricular hypertrophy, a weak inverse relation has been described 31. When left ventricular systolic dysfunction prevails, ventricular dilatation becomes prevalent and the relation becomes positive. The end-systolic volume index, as a parameter for elastic recoil, becomes a main determinant of Vp in such a population 35. Nevertheless, most authors have reported a weak but significant inverse relation between diastolic volumes and Vp when comparing hypertrophic (HCM) and dilated cardiomyopathy (DCM) to represent diseases at both ends of the pathological spectrum 36, as well as within a general population of patients 37. These observations are in keeping with the principles of IVPGs-generation and vortex propagation 18,30.
4.4. Importance of geometry and dyssynergy
The importance of left ventricular shape on Vp has only very recently been demonstrated by Barbier et al., showing a significant positive correlation between Vp and the long/short axis ratio 37. Their observation that Vp was higher in slim-lined cavities with low end-systolic volume is supported by the previously mentioned findings of Nikolic 8 on IVPGs, and of Shortland and Baccani 26,30 on vortex formation. Moreover, Barbier et al. demonstrated that the degree of wall motion dyssynergy was an important independent determinant of Vp, in particular, when left ventricular systolic function was little or not affected.
4.5. Relation to heart rate and ejection fraction (EF)
The clinically observed correlations seem to correspond to inherent physiological effects of heart rate or impaired systolic function on relaxation rate. Multiple regression analysis has failed to allocate heart rate as an independent variable of Vp in humans 35. A strong correlation with heart rate is only observed when changes in heart rate are due to beta-adrenergic stimulation or inhibition 31, whereas atrial pacing induces only a non-significant increase in flow propagation 12.
In the presence of LV systolic dysfunction, a moderate positive correlation between EF and Vp has been described 15,38. However, when end-systolic volume index was taken into account, EF was not an independent variable anymore 35. In fact, the apparent relation between ejection fraction and Vp can be explained by coupling of the systolic and diastolic myocardial performance 3,24,29,39, which implies that ventricles with impaired systolic function have impaired relaxation and often have an increased end-systolic volume index. As a consequence, low Vp will be common in this population, but similarly common if relaxation is impaired due to other causes, not affecting EF. This explains why most studies failed to point out ejection fraction as an independent determinant of Vp 14,22,31,35.
5. Clinical use of colour M-mode Doppler
From the colour M-mode velocity map of the early filling wave, qualitative and quantitative information can be extracted.
5.1. Qualitative information
In normal adults, little loss in blood velocities occurs between the basal ventricle and the apical region. In particular in young adults, maximal velocities, at which ‘aliasing’ occurs, can even originate beyond the basal portion of the ventricle as the blood is accelerated throughout the ventricular volume. The colour M-mode flame appears as a coherent bundle in which the apical region is reached before early flow at the mitral valve level has stopped. The slope of the displayed filling wave is steep. With impaired relaxation, the slope of the first filling wave is less steep, velocity loss makes that maximal velocities reside at the level of the mitral tips and mostly do not reach the apical region anymore. With further reduction in diastolic relaxation and elevation of filling pressures due to reduced compliance, the slope of the early filling wave will stay low, the wave usually gets blurry due to low flow velocities in the apical region which can continue after early mitral inflow has stopped or even after mitral valve closure. This delay in apical filling represents a shift from a filling pattern dominated by column motion to a pattern mainly dominated by convection (indicating redistribution) 18 (Fig. Fig. 4).
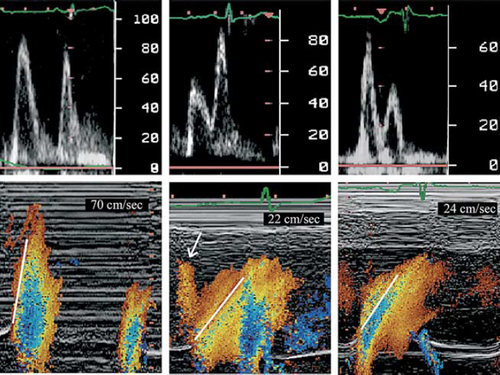
5.2. Quantitative information
5.2.1. Estimation of diastolic performance
Brun et al. have been the first to show that velocity propagation is related to wall relaxation 14, and a number of colleagues confirmed that Vp decreases in various disease states known to alter diastolic relaxation. In fact, the progressive decrease of Vp runs in parallel with the increase of the isovolumic relaxation constant tau, irrespective of rising filling pressures 16. Accordingly, the impaired relaxation and recoil induced by ischemia, either local during PTCA 12 or global during diffuse ischemia 34, or following myocardial infarction 22,40, has been demonstrated to significantly reduce Vp. Nishihara et al. have confirmed that the strong correlation between tau and Vp is also valid in hypertrophic cardiomyopathy (HCM) 41, although the low values of Vp itself in this pathology (mean 32 cm/s) have been reproduced by some 38,42, but not all authors 37. In general, the reported normal and cut-off values are strikingly similar. Using his acquisition method, Garcia has proposed a cut-off value of 55 cm/s and 45 cm/s to define impaired relaxation in the young, respectively, mid-aged adults 43.
5.2.2. Estimation of filling pressures and prognosis
Combined indices of 1/Vp, as a preload independent surrogate for tau, with preload and relaxation-dependent mitral inflow parameters, provide close prediction of mean pulmonary wedge (PCWP) pressure 15,33,44,45. In fact, diastolic relaxation rate and mean atrial pressure are analytically related to the isovolumetric relaxation time (IVRT) 46, and are major determinants of the early transmitral filling velocity (E). In a direct comparison, the combination of IVRT and Vp turned out to be more accurate 45. With an r-value of 0.89 (P<0.0001), the reported accuracy of this combination is very reasonable:
However, E/Vp is the most widely used index. In a heterogeneous group comprising normal, ischemic, hypertrophic and dilated hearts, PCWP was calculated as follows:
Correlation between E/Vp and PCWP outperforms correlations with maximal E (r=0.62) and E/A (r=0.52), independent of ejection fraction. Nagueh et al. have confirmed that the same formula is valid in HCM 42,47. The best previous parameter for assessing elevated filling pressures in HCM, the difference in duration of the mitral A wave and the pulmonary reversed flow, has proven to be less accurate. The E/Vp ratio has also been applied to assess filling pressures in atrial fibrillation with reasonable accuracy: averaged over three heart beats E/Vp≥1.4 yields a 100% specificity and 72% sensitivity in predicting a PCWP>15 mmHg 48. It favourably corresponds to NYHA-class and elevated values of brain natriuretic peptide in this group 49. The clinical importance of this index has particularly been stressed by its relation to symptoms and prognosis following an acute myocardial infarction 38,50. An E/Vp ratio ≥1.5 is a strong predictor of in hospital heart failure and of 1–3 months mortality (P<0.0001), outperforming any of the conventional systolic parameters (wall motion index and ejection fraction).
6. Limitations of Vp as a preload independent index for relaxation
6.1. Technical limitations
Reliability and reproducibility of the three different acquisition and reading methods constitute a major limitation for the application of Vp. In a direct comparison of the three methods, large differences in obtained numerical values and in inter- and intra-observer reproducibility of Vp were generated 13. The method described by Garcia 15, is advocated because of the acceptable and superior reproducibility compared to the other methods 13. Reported intra-and inter-observer variability varies from <10%; in the lower (pathological) ranges of Vp and up to 20% in the ‘higher’ (normal) range of Vp and for TEE acquisitions 15,31,32,35,37,44,48. Moreover, it does not require dedicated software, and therefore is the most feasible technique for rapid, on-line measurements.
6.2. Physiologic restrictions
All conditions associated with altered intra-ventricular flow patterns, giving rise to significant turbulence or significant local pressure gradient formation constitute a limitation to the application of the technique: significant aortic regurgitation, mitral stenosis, artificial mitral valves and intra-cavitary obstructions. Merging of early and late inflow due to tachycardia or atrio-ventricular conduction delay could lead to erroneous measurements. Marked variations on both transmitral inflow and Vp recordings can be seen during atrial fibrillation and in some patients during respiration. In these patients, it should be recommended to average more than three cardiac cycles.
6.3. Unanswered questions
6.3.1. Physiologic limitations of preload independency
Although this was never indicated by invasively controlled studies, recent studies suggest that preload dependency might be valid within certain physiologic limits only. In fact, controversy still exists whether excessive preload changes, as seen during hemodialysis, per se affect Vp 51,52 or not 53. We have to bear in mind that in certain conditions and above certain limits preload stretch can affect ino- and lusitropic state 54. Excessive pre- and afterload 29 alterations will likely affect relaxation itself. In the light of the current inconsistent data, further invasive studies are required to establish how preload dependent the technique is in a broad range of patients with cardiac disease.
6.3.2. Pseudo-normalization in hypertrofic hearts
Pseudo-normalization of Vp can be defined as the encounter of high Vp values in conditions in which impaired relaxation and/or high filling pressures are present. The concerns about pseudo-normalization arise from observational data 37,55 and from the aforementioned fluid-dynamical concepts. Wall thickening in primary and secondary HCM will result in a small ventricle with an elongated shape, in which pressure gradient formation can be enhanced and vortex formation inhibited. Recently, reported velocity propagation values 37 support the existence of pseudo-normalization although the available literature has poorly addressed the topic and shows contradictory data 36,37,41,42. In our opinion, Vp correctly expresses IVPGs 56 but the dependency on geometry of the relation between absolute values of Vp and of IVPGs as well as the extent to which the IVPGs predict late diastolic filling, in particular in hypertrophic and restrictive cardiac disease, is insufficiently clarified. In fact, in a subset of patients with a preserved systolic function (EF>60%) and a restrictive filling pattern, unexpectedly high values of Vp (>60 cm/s) have been described 55,57. In accordance, we recommend not to use Vp or E/Vp in this subset of patients (for example, post cardiac transplantation).
7. From global diastolic filling to myocardial relaxation or back=
Our continuing quest for better diagnostic tools to determine diastolic left ventricular function should primarily be inspired by the bearing diastolic dysfunction has on symptoms and prognosis. Another but secondary aim would be to provide means for earlier diagnosis of global or regional myocardial disease.
In an attempt to overcome the principle limitation of the standard (=blood flow) Doppler technique's preload dependency 58, the current evolution is characterized by a shift towards techniques that determine myocardial relaxation in a direct and largely preload independent way 43. This review demonstrates that colour M-mode Vp is a novel means to determine global ventricular recoil and relaxation, independent of loading conditions, over a wider—probably not the complete—range of clinical conditions. It remains an indirect representation of ventricular relaxation but on the other hand it also offers a glance at different intrinsic LV properties that determine LV filling, such as geometry and wall motion synergy. Recently, tissue Doppler (TDI) 59 and strain rate (SRI) 60 imaging have been introduced. Their accuracy to directly measure myocardial relaxation in a non-invasive way has not been equalled before. They also represent promising tools for the early diagnosis of myocardial disease 61. However, they depict local relaxation, which does not necessarily assess global ventricular relaxation 62. Moreover, the link to filling pressures, symptoms and prognosis requires a step backwards from myocardial relaxation to global ventricular relaxation and filling (geometry/synchronicity), and eventually towards the preload dependent filling parameters. The combined information of mitral inflow E with Vp (E/Vp) 15, and with tissue Doppler Em (E/Em) 63 provides fair estimates of filling pressures and also constitutes an important symptomatic and prognostic index 38,39,50. Few authors have reported on the comparison between Em and Vp 33,38,42,45,57. Em and Vp appear to be similarly affected by myocardial disease 38,42, but also show a similar paradoxical increase in case of constrictive pericarditis 57. In general, Vp and E/Vp performs well in normal and dilated ventricles and in the presence of coronary artery disease, irrespective of ventricular dilatation 33,45. In particular, in hypertrophic and restrictive cardiomyopathy, its value is debated; TDI appears to be more accurate and has higher reproducibility 41.
8. Conclusions
Colour M-mode provides unique information on left ventricular filling dynamics and pressure gradients in a non-invasive way, previously not available. The velocity propagation Vp is an index that strongly relates to left ventricular myocardial relaxation and recoil as proven by its correlation to the invasive relaxation constant tau and the left ventricular end systolic volume index. It is a promising tool to address the cumbrous problem of pseudo-normalization of the transmitral Doppler flow pattern since Vp is preload independent, and accurate estimates of filling pressures using the ratio E/Vp can be made.
There is a technical learning curve to its incorporation in clinical practice. Its technical reliability is rather mediocre, there is not yet a consistent method of measuring Vp and the various techniques reported are not interchangeable. However, accurate determination of Vp is a promising variable for the comprehensive assessment of diastolic function. Future investigations should address these technical issues and determine whether pseudo-normalization of Vp in ventricles with low compliance, small ellipsoidal cavities, and preserved systolic function truly exists.




