Mesenchymal stem cells regulate the proliferation and differentiation of neural stem cells through Notch signaling
Abstract
The effects of mesenchymal stem cells (MSCs) on proliferation and cell fate determination of neural stem cells (NSCs) have been investigated. NSCs were co-cultured with MSCs or NIH3T3 cells using an in vitro transwell system. After 4 days, immunofluorescence staining showed that the number of cells positive for the cell proliferation antigen, ki-67, in neurospheres in MSCs was greater than in NIH3T3 cells. In some experiments, the top-layers of MSCs and NIH3T3 cells were removed to induce NSCs differentiation. Seven days after initiating differentiation, the levels of the neuronal marker, NSE, were higher in NSCs in MSCs co-culture group, and those of glial fibrillary acidic protein (GFAP) were lower, compared with NIH3T3 cells co-culture group. These were confirmed by immunofluorescence. The role of the Notch signaling pathway analyzed with the specific inhibitor, DAPT, and by examining the expression of Notch-related genes using RT-PCR showed that after co-culturing with MSCs for 24 h, NSCs expressed much higher levels of ki-67, Notch1, and Hes1 than did NSCs co-cultured with NIH3T3 cells. Treatment with DAPT decreased ki-67, Notch1 and Hes1 expression in NCSs, and increased Mash1 expression. The data indicate that the interactions between MSCs and NSCs promote NSCs proliferation and are involved in specifying neuronal fate, mediated in part by Notch signaling.
1. Introduction
Neural stem cells (NSCs) have the capacity for self-renewal and are able under specific conditions to differentiate into different cell types, including neurons, astrocytes, and oligodendrocytes (Reynolds and Weiss, 1992). The discovery of NSCs was revolutionary, evoking a reappraisal of the traditional view that the CNS cannot undergo regeneration. NSCs, which contribute to neurogenesis, localize in the subventricular zone (SVZ) lining the lateral ventricles and the subgranular zone of the dentate gyrus (DG) in the adult brain (Alvarez-Buylla and Lim, 2004; Gage, 2002). Neurogenic development of NSCs and survival of newly differentiated cells can contribute to self-repair after neuronal loss. This process can be activated following ischemic stroke by NSCs in the SVZ area migrating to the ischemic site and integrate into the tissue (Zhang et al., 2001; Kokaia and Lindvall, 2003; Zhang et al., 2004). However, neurogenic responses by endogenous NSCs cannot fully compensate for the neural loss observed in CNS disorders, an observation that has stimulated a search for agents that enhance neurogenesis. A number of recent studies have lent support to the idea that mesenchymal stem cell (MSCs) transplantation in vivo can stimulate this intrinsic differentiation and proliferation of NSCs after stroke in rat, and dramatically improve neurological function (Li et al., 2002; Chen et al., 2003; Deng et al., 2005). Similar results have been obtained from in vitro studies, which have shown that MSCs co-cultured with NSCs can influence NSC proliferation and differentiation (Rivera et al., 2006; Lou et al., 2003). The molecular mechanism responsible for this regulatory influence of MSC on NSCs has not been identified.
A number of extracellular signals participate in regulating the proliferation and differentiation of NSCs. Notch signaling is important in many aspects of CNS development (Louvi and Artavanis-Tsakonas, 2006). For example, Notch1 is essential for the maintenance of neural precursors, both in vivo and in vitro (Hitoshi et al., 2002). The basic helix-loop-helix gene, Hes1, which is an essential effector of Notch signaling, regulates the maintenance of neural stem cells (Selkoe and Kopan, 2003). Hes1 also functionally antagonizes differentiation genes, such as Mash1, thus controlling both cell cycle and differentiation. Notch receptors are activated by specific ligands expressed on the surface of neighboring cells, and mediate signals through cell–cell interactions. Using the transwell system to co-culture endothelial cells and NSCs, Shen et al. (2004) showed that the presence of endothelial cells increases the expression of Hes1 in NSCs, promoting NSCs proliferation and increasing the ratio of NSC-differentiated neurons. After the addition of γ-secretase inhibitor II, a Notch1 signal-blocking agent, the proliferation- and differentiation-promoting effects of endotheliocytes were inhibited, suggesting a role for the Notch signaling pathway.
Because of their ready availability, large growth potential, and suitability for allogenic transplantation, MSCs represents a promising therapeutic option in the treatment of neural diseases, such as cerebral ischemia, with the potential of achieving functional and structural regeneration of neural tissues. MSCs are also useful and important tools for exploring NSCs proliferation and differentiation regulatory mechanisms. Building on previously published studies, we have used transwells to observe the influence of MSCs on the proliferation and differentiation of NSCs co-cultured in vitro. The Notch signal-blocking agent, γ-secretase inhibitor, N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester (DAPT), has also been used in this system to explore the role of Notch signal pathway in the regulation of these processes. In addition, we have examined changes in the expression of Notch signaling pathway components at the molecular level.
2. Materials and methods
2.1 Isolation, culture, and identification of NSCs
Cerebral cortices of BALB/C mouse embryos (E10-E14) were dissected and mechanically triturated with a pipette, as previously described (Selkoe and Kopan, 2003) with some modifications. The dissociated cell suspension was filtered through a 200-mesh sieve and collected after centrifugation. Single cells were plated at clonal density in a tissue culture bottle in serum-free culture medium: DMEM/F12 with l-glutamine, 2% B27 (Gibco), 20 ng/ml EGF and 20 ng/ml bFGF (Cytolab) added as mitogen. Plated cells were incubated at 37 °C in a humidified, 5% CO2 in air atmosphere. The expression of neuroepithelial stem cell protein (nestin) was detected using immunofluorescence.
2.2 Isolation, culture, and identification of MSCs
MSCs were generated from BALB/C mice as previously described (Meirelles and Nardi, 2003) with some modifications. Mice were killed by cervical dislocation, and femurs and tibiae were removed and cleaned of all connective tissue. BM cells, collected by flushing femurs and tibiae with alpha-Modified Eagle's Medium (a-MEM; Gibco) using a 26-gauge needle, were washed twice by centrifugation at 400g for 6 min. To initiate the MSCs culture, cells were plated in a tissue culture bottle at a 5 × 106 nucleated cells/ml in a-MEM supplemented with 10% FCS (Hyclone), 100 U/ml penicillin–100 mg/ml streptomycin, and incubated at 37 °C in a humidified, 5% CO2 atmosphere. After 48 h, non-adherent cells were removed and fresh medium was added. When the cultures approached confluence, the cells were harvested and digested with 0.125% trypsin/1 mM EDTA. The resulting suspension was expanded by plating at 105 cells/ml in bottles. The same conditions were used for subsequent passages. The expression of CD29 and CD34 was detected by immunofluorescence assay.
2.3 Co-culture model
Using a transwell cell co-culture system (EF5650T, Costar), MSCs were inoculated at 2000 cells per hole. After culturing alone for 3 days, NSCs were added to establish co-culture conditions. For co-culture, the medium was replaced with serum-free medium containing 2% B27 with no cytokines. As a control, NIH3T3 cells were used in place of MSCs and co-cultured with NSCs under the same conditions. After 4 days of co-culture, neurospheres were collected, cryosectioned and assayed by immunofluorescence for expression of ki-67. To induce NSCs differentiation, the top-layer of co-cultured cells (MSCs or NIH3T3) was removed after co-culture for 4 days. Seven days later, the differentiated cells were collected and analyzed for the expression of NSE and GFAP by Western blotting analysis and immunostaining.
To determine the role of Notch signal pathway in regulating NSCs proliferation and differentiation, 10 μM DAPT (Sigma) was added to the MSC co-culture group. Total RNA was extracted from neurospheres collected after 24 h and used for analyzing the expression of ki-67 and the Notch signal pathway molecules, Notch1, Hes1 and Mash1.
2.4 Immunofluorescence staining
After fixing for 20 min in 4% cold paraformaldehyde at room temperature, cells were incubating with 0.2% Triton in phosphate-buffered saline (PBS) for 10 min, and incubated for 2 h with primary antibody (CD29, CD34, ki-67 1:100; NSE 1 μg/ml; GFAP 1:400) at 37 °C. Cells were washed 3 times with PBS and incubated with FITC-conjugated goat anti-mouse IgG (1:100) for 1 h at 37 °C. After washing 3 times, the cells were observed under a fluorescence microscope. For quantification, a digital camera was used to capture 15 non-overlapping images (×100) of each sample. Positively stained cells were counted and compared to total cell counts. Each experiment was performed 3 times.
2.5 RT-PCR analysis
The expression of specific genes in NSCs co-cultured with MSCs or NIH3T3 for 24 h with or without DAPT was analyzed by RT-PCR. Total RNA was isolated using Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. cDNAs were prepared and amplified by polymerase chain reaction according to standard procedures. cDNA was synthesized from 1 μg of total RNA using SuperScript II reverse transcriptase (Invitrogen, Grand Island, NY). Notch signaling components were amplified from cDNA samples by PCR using primers (Table 1) specific for the Ki-67, Notch1, Hes1 and Mash1 genes. β-actin expression, included as a control for mRNA quality, was assayed using the same RT-PCR method. PCR products were resolved on 1% agarose gels and visualized by ethidium bromide staining.
| Gene | Primer sequence | Product size (bp) |
|---|---|---|
| ki-67 | Forward 5′-GAGCAGTTACAGGGAACCGAAG-3′ | 262 |
| Reverse 5′-CCTACTTTGGGTGAAGAGGCTG-3′ | ||
| Notch1 | Forward 5′-TTACAgCCACCATCACAgCCACACC-3′ | 379 |
| Reverse 5′-ATgCCCTCggACCAATCAgA-3′ | ||
| Hes1 | Forward 5′-AAAgACggCCTCTgAgCACA-3′ | 377 |
| Reverse 5′-TCATggCgTTgATCTgggTCA-3′ | ||
| Mash1 | Forward 5′-ACgACTTgAACTCTATggCgggTTCTC-3′ | 350 |
| Reverse 5′-gCCACTCTCAggggCCAAgACTgAAgTTAA-3′ | ||
| β-actin | Forward 5′-TCCTgTggCATCCACgAAACT-3′ | 314 |
| Reverse 5′-gAAgCATTTgCggTggACgAT-3′ |
2.6 Western blot analysis
Total protein was extracted from samples of NSCs cultured in the various media. Equal amounts of protein from cell lysates were separated by SDS-polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes. The membranes were blocked with 1% (wt/vol) BSA in PBS containing 0.1% Tween 20 and blotted with antibodies for NSE (0.4 μg/ml) and GFAP (1:4000). Immunoreactive bands were visualized with horseradish peroxidase–conjugated secondary antibodies (Sigma) and ECL reagents (Pierce).
2.7 Statistical analysis
Quantitative results are expressed as means ± standard deviations (SD). Statistical analyses were performed using a two-tailed unpaired t test. Probability values <0.05 were considered statistically significant.
3. Results
3.1 The effect of mesenchymal stem cells on NSCs proliferation
All cells used in co-culture experiments exhibited characteristics typical of NSCs and MSCs. Third-passage MSCs exhibited a fibroblast-like shape, and ∼80% expressed CD29 instead of CD34 by immunofluorescence staining (Fig. 1). NSCs proliferated and aggregated into neurospheres containing large numbers of cells expressing the NSCs marker, nestin (Fig. 2).

Morphology and immunofluorescence staining of MSCs. MSCs show a fibroblast-like shape. Immunofluorescence shows that >80% of MSCs expressed CD29 (B), while no cells expressed CD34 (A). Bar = 50 μm.
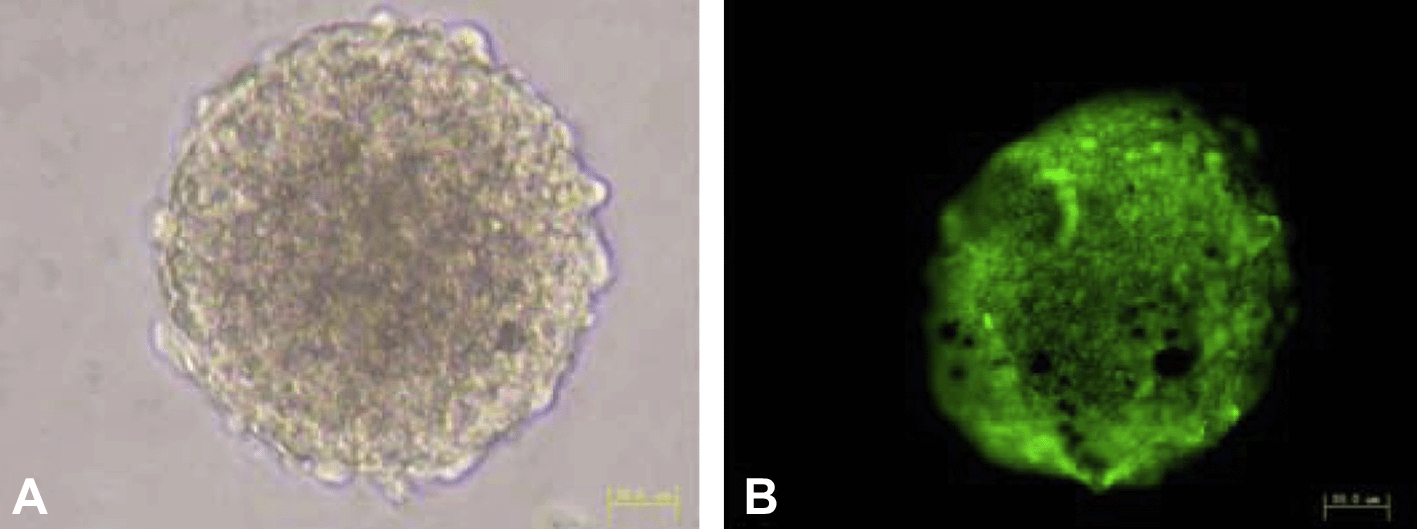
Morphology and immunofluorescence staining of NSCs. NSCs proliferated and aggregated into neurospheres (A). Immunofluorescence shows that a large number of cells express nestin (B). Bar = 50 μm.
NSCs and MSCs were co-cultured using the transwell system; co-cultures with NIH3T3 cells were included as a control. After 4 days of co-culture, neurospheres were collected, cryosectioned and examined by immunofluorescence for expression of the antigen, ki-67, in proliferating cells (Fig. 3; left panel). Results showed that the number of ki-67-positive cells in the neurospheres from MSCs was substantially higher than from NIH3T3 cells (Fig. 3; right panel), providing clear evidence of a proliferation-promoting effect of MSCs on NSCs. Because the cells were physically separated in the co-culture system, the promoting effect of MSCs was probably due to secreted cytokines.
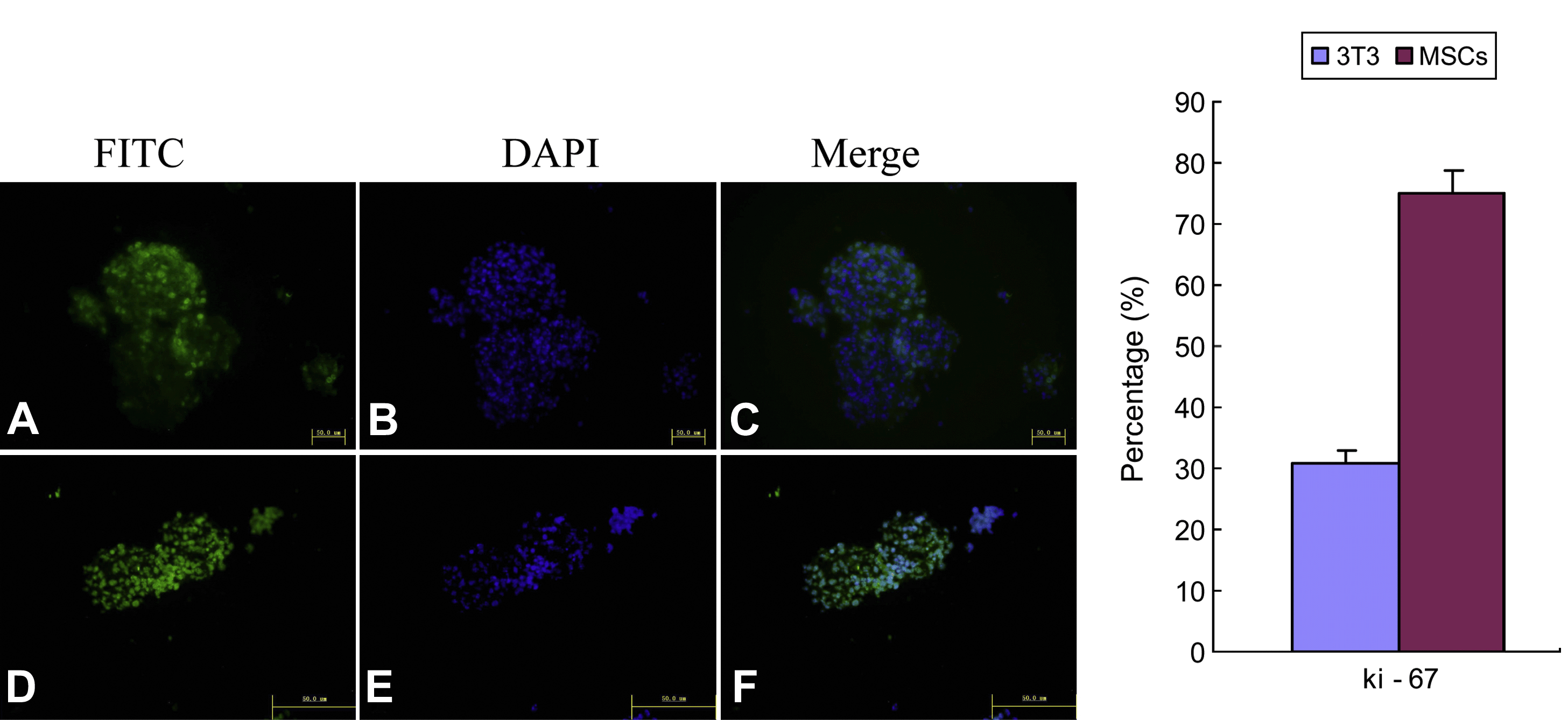
Expression of ki-67 in NSCs co-cultured with MSCs or NIH3T3 for 4 days. Representative image (at left) shows that the number of ki-67-positive cells in neurospheres from the MSCs co-culture group (D) was clearly higher than in the NIH3T3 group (A). Summary data (at right) shows percentage of positive ki-67 cells in each group. Bar = 50 μm.
3.2 The effect of mesenchymal stem cells on NSCs differentiation
After 4 days of co-culture, the top-layer of cells (MSCs or NIH3T3 cells) was removed to induce NSCs differentiation. Seven days later, NSCs were observed microscopically for evidence of morphological changes indicating differentiation. The number of smaller, neuron-like cells with refractile bodies was greater in the MSCs co-culture group than in the NIH3T3 group. In contrast, the number of larger, polymorphic astrocytes was dramatically reduced in the MSCs group. Immunofluorescence staining showed that the cytoplasm of the smaller cells stained strongly for the neuronal marker, NSE, while that of the larger cells was strongly positive for GFAP staining. Consistent with these results, there were more NSE-positive cells in the MSCs co-culture group than in the NIH3T3 group, and fewer GFAP-positive cells (Fig. 4). These results were confirmed by Western blot analysis, which showed that of the levels of NSE were higher in the MSCs co-culture group and those of GFAP were lower compared with the NIH3T3 co-culture group (Fig. 5). Collectively, these results suggest that MSCs could indeed induce neuronal differentiation of NSCs.
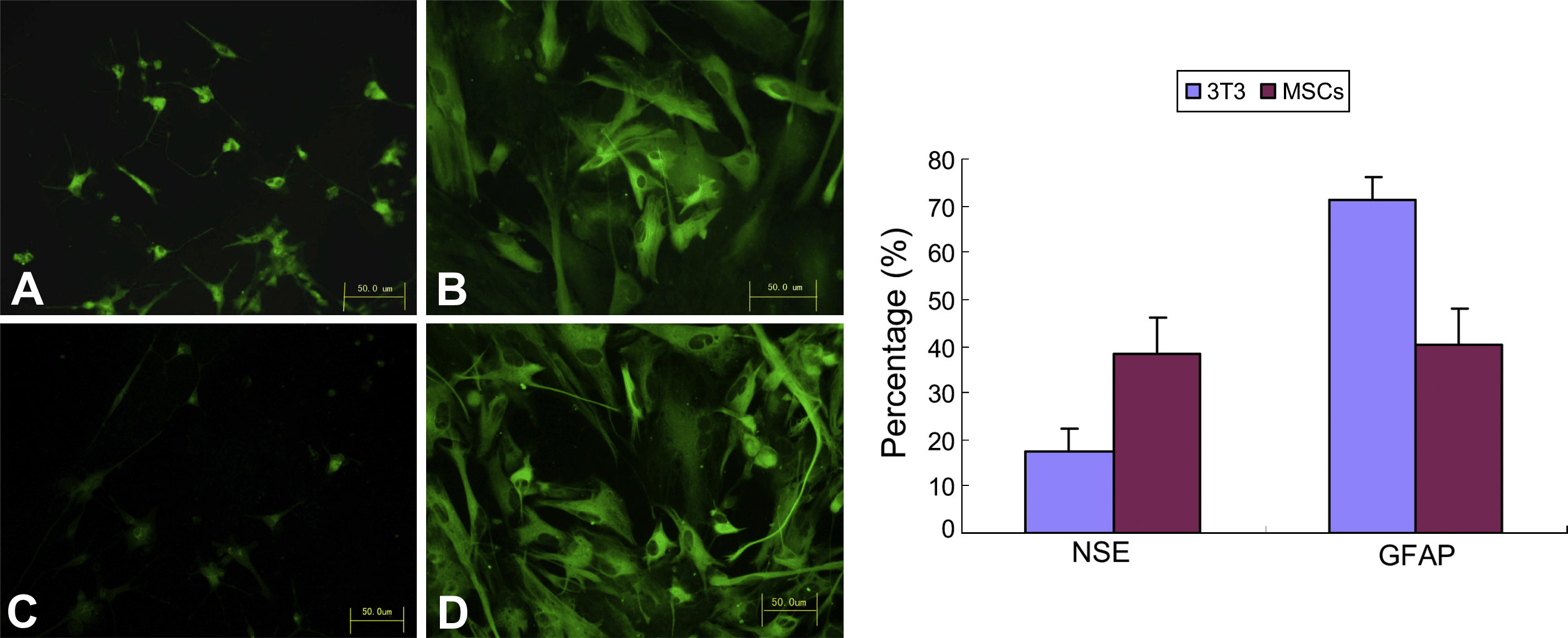
Immunofluorescence analysis of NSCs neuronal differentiation. (A) Indicates NSE-positive cells in the MSCs co-culture group and (C) indicates NSE-positive cells in the NIH3T3 co-culture group; (B) indicates GFAP-positive cells in the MSCs co-culture group and (D) indicates GFAP-positive cells in the NIH3T3 co-culture group. Seven days after induction of differentiation, the percentage of NSE-positive cells (right panel) was significantly higher in the MSCs co-culture group than in the NIH3T3 group, while the percentage of GFAP-positive cells was significantly lower in MSCs (p < 0.05). Bar = 50 μm.
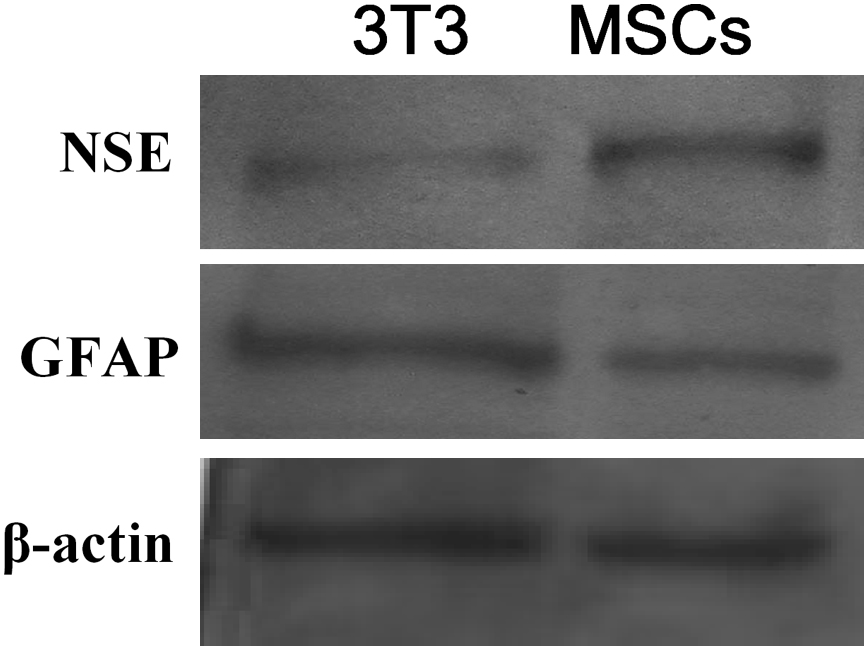
Western blot analysis of NSC neuronal differentiation. Seven days after induction of NSC differentiation, NSE expression was higher and GFAP expression was lower in the MSCs co-culture group compared with the NIH3T3 group.
3.3 Regulation of NSC proliferation and differentiation by MSCs through the notch signal pathway
To verify a role for the Notch signaling pathway in the regulation of NSC proliferation and differentiation by MSCs, NSCs with MSCs or NIH3T3 were co-cultured in the presence and absence of the Notch signaling pathway inhibitor, DAPT (10 μM). After 24 h of co-culture in the absence of inhibitor, RT-PCR results showed that the expression of ki-67, Notch1 and Hes1 in NSCs was much higher in the MSCs co-culture group than in the NIH3T3 group. When DAPT was added, the expression of Notch1 and Hes1 dropped, as did that of the ki-67 cell proliferation antigen; in contrast, Mash1 expression increased (Fig. 6). These results are consistent with the interpretation that the activation of Notch1 and Hes1 genes can promote the proliferation of NSCs, and that neuronal differentiation of NSCs reflects the down-regulation of Hes1 expression and resulting up-regulation of Mash1 expression. Collectively, these results suggest that the Notch signaling pathway mediates MSCs-dependent regulation of NSC proliferation and differentiation.
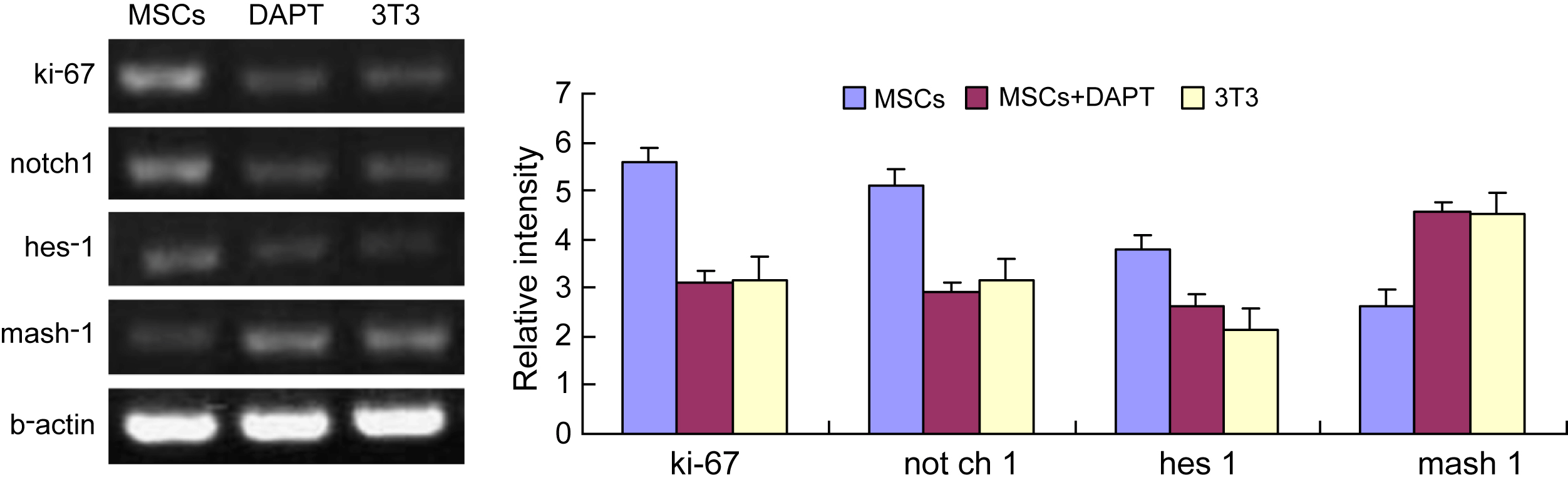
RT-PCR analysis of Notch- and proliferation-related genes. Left panel shows representative image ethidium bromide-stained agarose gels. Densitometric analysis of PCR product intensities expressed relative to β-actin, is presented at right. The expression of ki-67, Notch1, and Hes1 in NSCs was much higher in MSCs co-culture group than in NIH3T3 cells co-culture group, while the expression of Mash1 is lower. Addition of 10 μM DAPT to the medium decreased ki-67, Notch1 and Hes1 expression, and increased Mash1 expression.
4. Discussion
NSCs co-cultured with MSCs exhibited a much higher fraction of ki-67-positive cells than did those in the NIH3T3 group after 4 days, indicating that MSCs are capable of stimulating NSCs proliferation. Since MSCs and NSCs were not in physical contact with one another in the co-culture system, although soluble factors could pass freely through the separating membrane, the results suggest that stimulation of NSCs proliferation is mediated by factors secreted by MSCs. After co-culturing NSCs with MSCs for 4 days, the top-layer containing MSCs (or NIH3T3 cells) was removed to induce NSCs differentiation, and 7 days after initiating NSCs differentiation, more NSCs in the MSCs co-culture group had differentiated into neuron-like cells than in the controls NIH3T3, as indicated by the higher frequency of cells staining positive for the neuronal marker, NSE. These results were supported by Western blot analysis.
These results are in accord with previous reports that MSCs are capable of influencing the differentiation of NSCs. For example, Lou et al. (2003), using MSC-conditioned media or direct inoculation of NSCs onto the surface of MSCs, found that either method could induce NSCs neuronal differentiation. Bai et al. (2007), using MSC-conditioned media or co-culture NSCs with MSCs or astrocytes, found that MSCs have a direct and pronounced effect on the genesis of neurons and oligodendrocytes from NSCs, and indicated the effect is mediated by MSCs specific soluble signals. Rivera et al. (2006, 2008) reported that instead of inducing neuronal differentiation, factors secreted by MSCs promoted differentiation of NSCs into oligodendrocytes and inhibited astrocytic differentiation. It is possible that some differences reflect the fact that the environmental factors during culturing affect the outcome. For example, Bai et al. (2007) reported that MSCs affect differentiation of NSCs differently depending on how long the media is conditioned. Rivera et al. (2006, 2008) used serum-containing media for culturing and differentiation-induction experiments. Our study employed serum-free media. All the studies demonstrated the fact that MSCs are capable of regulating the fate of NSCs.
A network of multiple signaling systems and transcriptional regulators controls the proliferation and differentiation of NSCs (de la Pompa et al., 1997; Kageyama and Nakanishi, 1997; Conti et al., 2001; Panchision et al., 2000). The Notch signaling pathway is prominently involved in regulating the proliferation and differentiation of NSCs (Androutsellis-Theotokis et al., 2006; Grandbarbe et al., 2003; Chojnacki et al., 2003). Activated Notch translocates into nucleus where it stimulates expression of Hes genes of the HLH family. In vitro experiments have shown that neurospheres express high levels of the gene, Hes1 (Palm et al., 2000). Originally, it was proposed that Hes1 activation promotes NSCs proliferation, but inhibition of Hes1 expression promotes NSCs neuronal differentiation (Kabos et al., 2002). Other research has shown an inverse relationship between Hes1 and Mash1 expression: down-regulation of Hes1 results in up-regulation of Mash1 (Baek et al., 2006). Shen et al. (2004) discovered that endotheliocytes, co-cultured with NSCs in a transwell system, could increase the expression of Hes1 in NSCs and promote NSCs proliferation, an effect that was blunted in the presence of the Notch1 signaling blocker, γ-secretase inhibitor II. Furthermore, they found that endotheliocytes could induce NSCs neuronal differentiation; the proportion of NSCs that differentiated into neurons decreased when Notch1 activity was inhibited. Our results show that the expression of Notch1 and Hes1 increased in NSCs co-cultured with MSCs for 24 h compared to NSCs co-cultured with NIH3T3 cells. Further, after inhibition of Notch signaling with DAPT (10 μM), Notch1 and Hes1 expression in NSCs decreased. Importantly, these changes were associated with a decrease in the expression of the cell proliferation antigen, ki-67, demonstrating that the Notch signal pathway plays an important role in the regulation of NSCs proliferation by MSCs.
MSCs are multipotential stem cells, capable of differentiating into several types of cells, including osteoblasts, adipocytes and chondrocytes, under specific experimental conditions. Recently, the potential of MSCs to develop into neural lineages in vivo, such as neurons and astrocytes, has been reported (Celil and Campbell, 2005). MSCs thus represent an unlimited source of autologous cells for transplantation in stroke, one that possess no ethical or immune-rejection concerns. There is evidence showing that transplanted MSCs lead to improved recovery of neural functional in rats with cerebral ischemia, although the underlying mechanism remains unclear. The functional benefits derived from MSCs transplantation may reflect the promotion of brain plasticity, rather than replacement of existing cells by integration of MSCs into the cerebral tissue (Mahmood et al., 2001). Specifically, after MSCs transplantation, proliferation of neural precursor cells in the subventricular zone is much more evident than in un-transplanted controls, suggesting that the transplanted MSCs mobilize the proliferative potential of intrinsic neural precursors, stimulating a self-repairing process that leads to improvement of certain neural functions.
By using an in vitro transwell system we found that MSCs increased the expression of the cell proliferation marker, ki-67, in co-cultured NSCs, and increased the number of NSE-positive cells following induction of NSCs differentiation. We also showed that the expression of Notch1 and Hes1 in NSCs was higher in the MSCs/NSCs co-culture group than in the NIH3T3/NSCs co-culture group. Furthermore, blocking the Notch signal pathway with DAPT led to a dramatic decrease in the expression of ki-67 in NSCs co-cultured with MSCs. Collectively, these results demonstrate that the interaction between MSCs and NSCs, mediated at least partially by Notch signaling, promotes NSCs proliferation and specifies the neuron fate of NSCs.
Acknowledgments
This research was supported by National Natural Science Foundation of China (No. 30560156) and Natural Science Foundation of Jiangxi province (No. 0540087).




