A simple approach for mouse embryonic stem cells isolation and differentiation inducing embryoid body formation
Abstract
Stem cells were derived from hatched blastocyst-stage mouse embryos of the C57BL/6 strain employing a knockout serum replacement instead of the traditional fetal calf serum, thereby avoiding the use of immunosurgery. Although fetal calf serum was not good for isolation of stem cells, a combination of this serum plus knockout serum increased the expansion rate of the cell culture. The derived cells were capable of maintaining an undifferentiated state during several passages, as demonstrated by the presence of alkaline phosphatase activity, stage-specific embryonic antigen 1 (SSEA-1), and octamer binding protein 4 (Oct-4). Suspension culture in bacteriological dishes gave better results than the hanging drop method for differentiation by means of embryoid body formation. Mouse embryonic stem cells showed spontaneous differentiation into derivatives of the 3 germ layers in culture media supplemented with fetal calf serum but not with knockout serum.
Abbreviations:
-
- EBs
-
- Embryoid bodies
-
- mESCs
-
- mouse embryonic stem cells
-
- DMEM
-
- Dulbecco's Modified Eagle Medium
-
- IMDM
-
- Iscove's Modified Dulbecco's Medium
-
- PBS
-
- Phosphate Buffer Saline
-
- FCS
-
- Fetal Calf Serum
1. Introduction
Mouse embryonic stem cells (mESCs) are generally obtained from the inner cell mass (ICM) of preimplantation blastocyst-stage embryos. These cells can be grown indefinitely under in vitro conditions in an undifferentiated state in the presence of leukaemia inhibitory factor (LIF) or feeder cells. When feeder cells or differentiation-inhibiting cytokines are removed, or specific differentiation inducing substances are added to the medium, mESCs can give rise to all types of adult tissues (Baharvand and Matthaei, 2003, 2004; Rippon and Bishop, 2004). This property of pluripotency allows mESCs to produce teratomas when injected into immunodeficient mice, and to form 3-dimensional structures called embryoid bodies (EBs) when cultured in suspension in absence of anti-differentiation agents, recapitulating early developmental processes (Evans and Kaufman, 1981; Kurosawa, 2007). Expression of specific markers, like the stage-specific embryonic antigen 1 (SSEA-1), and the presence of alkaline phosphatase and telomerase constitute some of the properties of mESCs (Wobus and Boheler, 2005).
Derivation of mESCs from blastocyst-stage embryos is generally an inefficient process and the isolation of different lines differs between strains, which suggests an important genetic factor is involved (Brook and Gardner, 1997; Bryja et al., 2006; Kawase et al., 1994; Wobus and Boheler, 2005).
We have derived mESCs from hatched blastocyst of C57BL/6 strain, usually considered as non-permissive, with low success rates and relatively few lines existing at present (Baharvand and Matthaei, 2004; Bryja et al., 2006; Cheng et al., 2004). Besides, the results obtained during EB formation employing 6 different combinations of culture media are compared, as also suspension culture of mESCs in bacterial-grade dishes and culture in hanging drops are.
2. Materials and methods
Animal experiments were conducted in accordance with what is stated on the “Guide for the Care and Use of Laboratory Animals”, prepared by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council, United States of America.
2.1 Embryo collection and culture
C57BL/6 female mice were mated after superovulation. Pregnant females were sacrificed on the second day postcoitum and had their oviducts removed. The 2-cell stage embryos were flushed from the oviducts and cultured in Human Tubal Fluid (HTF) until reaching blastocyst-stage and hatching.
2.2 Obtaining of feeder cells and isolation of ESCs colonies
Murine embryonic fibroblasts (MEFs) were derived from 15.5 to 16.5 day-old fetuses of C57BL/6 strain and cultured with DMEM supplemented with 20% fetal calf serum and 1% penicillin–streptomycin. The cells were grown up to passage 4 to 5 and mitotically inactivated by γ-irradiation before use.
Hatched blastocysts were placed onto four-well culture plates covered with feeder cells in presence of ES medium composed of 80% Knockout DMEM (Ko-DMEM) with 20% Knockout Serum Replacement (Ko-SR), 2.4 mM l-Glutamine, 0.01 mM β-mercaptoethanol (BME), 1.2% Non-Essential Amino Acids (NEAA), 1.2% penicillin–streptomycin, and 1000 IU/ml of recombinant human LIF. Alternatively, ES medium was supplemented with 10% Ko-SR plus 10% FCS. After one week of culture, outgrowths of mESCs appeared. Cells were harvested for further expansion by mechanical dissociation and replated onto fresh feeder layers.
2.3 Immunocytochemistry of mESCs colonies
To detect SSEA-1, cells were fixed in 4% paraformaldehyde (PFA), incubated overnight (ON) with a primary antibody and incubated next day with a biotinylated secondary antibody for 30 min. For Oct-4, cells were fixed and permeabilized in methanol, incubated ON with anti-Oct-4 and finally incubated with a multilink secondary antibody. Nuclei were counterstained with Hoescht 33258 and observations were made under fluorescence microscope.
2.4 EB formation, histology and immunostaining
Table 1 summarizes the six variants of EBs culture media employed.
| Culture Medium | Serum | NEAA 100X | BME | l-Glutamine 100X | Penicillin–Streptomycin 100X |
|---|---|---|---|---|---|
| DMEM high glucose | Ko-SR | 0.01 mM | 0.01 mM | 4 mM | 25 U/ml penicillin - 25 μg/ml streptomycin |
| DMEM high glucose | FCS | 0.01 mM | 0.01 mM | 4 mM | 25 U/ml penicillin - 25 μg/ml streptomycin |
| DMEM low glucose | Ko-SR | 0.01 mM | 0.01 mM | 4 mM | 25 U/ml penicillin - 25 μg/ml streptomycin |
| IMDM | FCS | 0.01 mM | 0.1 mM | – | 50 U/ml penicillin - 50 μg/ml streptomycin |
| Ko-DMEM | Ko-SR | 1.2% | 0.01 mM | 2.4 mM | 1.2% |
| Ko-DMEM | FCS | 1.2% | 0.01 mM | 2.4 mM | 1.2% |
To induce formation of EBs, two of the widely utilised methods were chosen – suspension culture in bacterial-grade dishes and culture in hanging drops. Whole colonies of mESCs were placed onto bacteriological plates and cultured in suspension for >10 days. For the hanging drop technique, colonies were trypsinized and 40–50 μl drops containing 400–1000 ESCs were placed on the lids of 100 mm-Petri dishes filled with PBS. After 5 days, cell aggregates formed in the drops were collected and transferred to bacterial-grade dishes for further culture. Some of the EBs obtained were transferred to culture dishes after 4 days to allow adherence and permit their spontaneous differentiation. The remaining EBs were harvested at different times to analyze the degree of differentiation acquired. They were fixed in 4% PFA, dehydrated, embedded in paraffin and stained with hematoxylin–eosin for morphology examination. Periodic Acid Schiff (PAS) reaction was used to detect presence of basement membrane. Primary antibodies to identify α-fetoprotein, muscle specific actin and β-III-tubulin were employed for an ON incubation of EB-derived cells attached to the culture plates.
3. Results and discussion
3.1 Acquiring mESCs
The inner cell mass of blastocyst-stage embryos were able to develop colonies of stem cells when ES medium was supplemented with Ko-SR. When this serum was employed, outgrowths of ESCs took longer to develop in comparison with cells grown in presence of FCS (data not shown), and practically no trophoblast cells were detected, facilitating the isolation of stem cells. There is no need to perform immunosurgery for isolation of the ICM because this serum prevents proliferation of trophoblast cells, making easier to obtain mESCs. In our experience, a combination of Ko-SR and FCS (50:50) achieved a higher efficiency in growing and maintaining of the good stemness throughout the passages. While Ko-SR collaborates in preserving non-differentiated cells, mESCs benefit from FCS properties, showing a rapid expansion.
3.2 Assessment of undifferentiated state
When detection of the cell surface marker SSEA-1 was performed, all cells within the colonies showed a clearly positive membrane marker (Fig. 1A), which allowed identification of numerous cell surface extensions present in some of the cells located at the edges of the colony, projecting towards the feeder cells (Fig. 1B). Since MEFs prevent ESCs from differentiating, it is assumed that they secrete proteins important in maintaining an undifferentiated state, which are taken up by the extensions mentioned above (Baharvand and Matthaei, 2003). With respect to Oct-4 expression, the cells showed the typical nuclear pattern of this transcription factor (Fig. 1C). ALP activity was detected employing a kit from Chemicon. The complete population of mESCs analyzed was positive for the expression of this enzyme (data not shown).
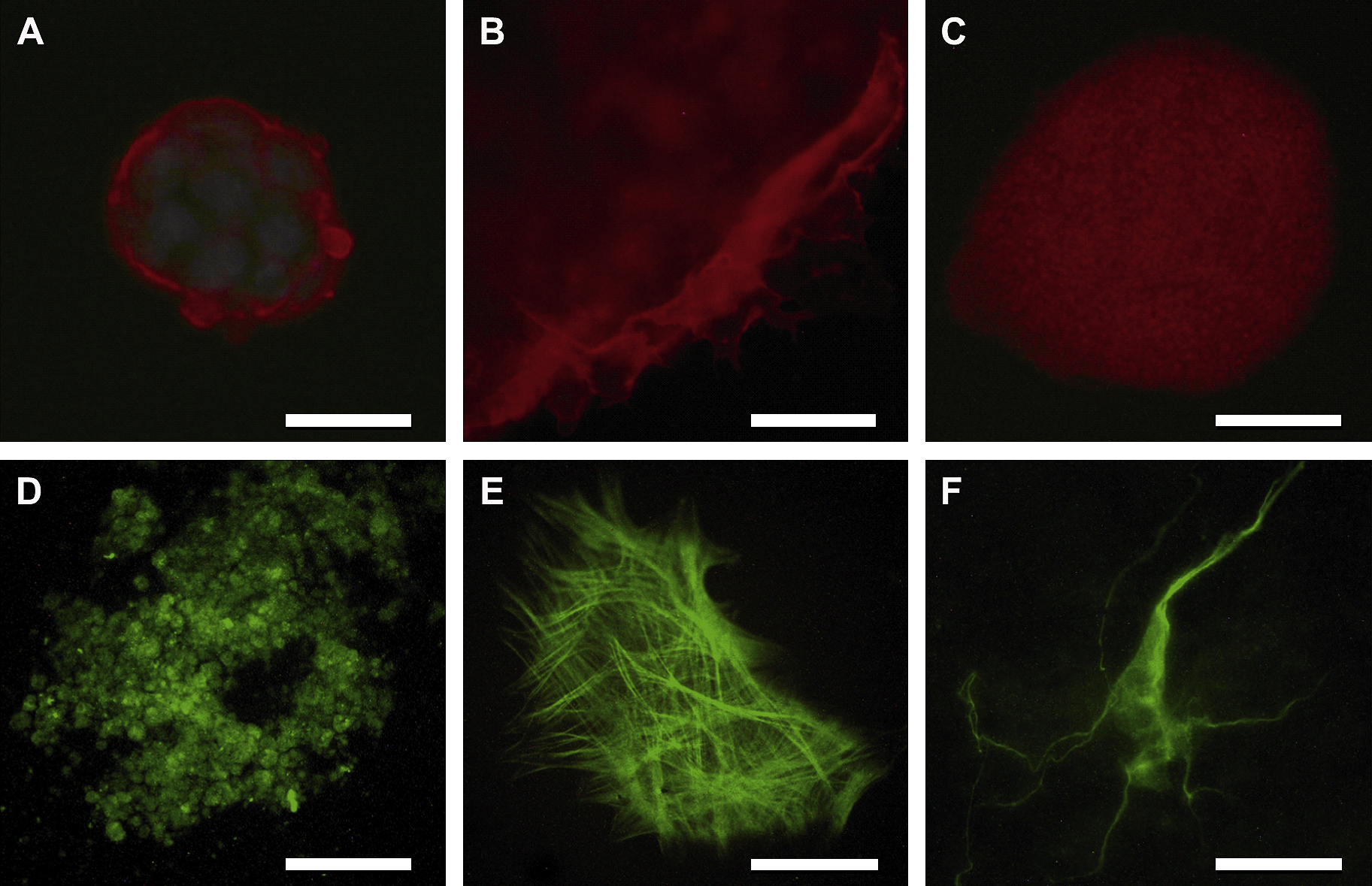
Immunostaining of mESC cultured in Ko-SR and spontaneously differentiated cells grown in presence of FCS. (A) Cell surface staining with SSEA-1 (red) and nuclei counterstained with Hoescht (blue); (B) Extensions emitted by cells located at the edges of the colony; (C) Nuclear staining pattern of Oct-4; (D) (E) (F) endodermal, mesodermal and ectodermal cell derivatives, respectively. Scale bar: 100 μm.
3.3 In vitro differentiation
EBs formed in the presence of medium supplemented with Ko-SR failed to develop into well-defined structures, as they looked like lax cell aggregates with irregular and non-defined edges, i.e. lacking any change in their undifferentiated state. However, those EBs grown in medium with FCS showed a significant increase in volume, and the resulting EBs displayed distinct degrees of differentiation (Fig. 2E and F). This discrepancy in growth rate and differentiation status may be due to the intrinsic properties of both sera employed, as found by Cheng et al. (2004). FCS contains pro-differentiation factors useful in differentiation protocols. Many of the EBs gave rise to cystic structures of perfectly delimitated borders (Fig. 2A), and some produced yolk sac-like structures (Fig. 2B). After 4 days of culture, an external layer of primitive endoderm was seen. Below primitive endoderm cells, Reichert-like basement membrane deposits were identified by PAS reaction (Fig. 2C).

Morphology and histological staining of EBs. (A) Cystic EB; (B) EB showing yolk sac-like structure; (C) PAS staining showing Reichert-like basement membrane (arrow) (X100); (D) EB showing apoptotic areas along with peripheral and centric cavities; (E) Epithelium (arrow) lying over a basement membrane (X100); (F) Rosette formation resembling the early neural tube (X100). Scale bar: 150 μm.
During the first days of culture in suspension, solid rounded structures of uniform aspect possessing big rounded to oval nuclei with scarce cytoplasm were found. Immunohistochemistry showed positive SSEA-1 cells, indicating no differentiation (data not shown). When culture was prolonged, some of the EBs were decrease in cell number, with the presence of simple cuboidal epithelia resting over a Reichert-like basement membrane (Fig. 2E) and numerous foci of cells with apoptotic changes, mainly in the peripheral and central region (Fig. 2D). Some apoptosis-related genes are involved in the differentiation of ES cells into EBs, as well as in the formation of the 3 germ layers (Choi et al., 2005). Since no specific assay to detect apoptosis was used (e.g. TUNEL assay), we cannot be sure that such alterations were truly apoptotic areas. However, this is probably due to the characteristic aspect of the changes observed.
The immunocytochemistry of the cells which grew out of EBs adhered to culture dishes showed positive results for α-fetoprotein, muscle actin and β-III-tubulin, indicating presence of endodermal, mesodermal and ectodermal derivatives, respectively (Fig. 1D–F).
Although many researchers have adopted the hanging drop method as an initial differentiation strategy for mESCs, we failed to obtain EBs using this technique because cells were unable to group and to form 3-dimensional aggregates. Generation of EBs in suspension culture employing whole mESCs colonies has some disadvantages, such as heterogeneous sizes with consequent variations in the differentiation status and a number of apoptotic cells located within them (Choi et al., 2005). However, we suspect that the proximity established among cells when growing could be an important factor favouring the differentiation process, since cell to cell contact plays a major role in the early stage of embryonic development (Choi et al., 2005).
In conclusion, our results show that Ko-SR is more appropriate than FCS in deriving mESCs due to its qualities of maintaining the stemness of ES cells. Combination with FCS could also be beneficial to increase growth rates while conserving the undifferentiated status, thus confirming similar results previously obtained by others (Bryja et al., 2006; Lee et al., 2006; Tanimoto et al., 2008). With respect to EB formation, suspension culture in bacteriological dishes turned out to be a better option, and culture in the presence of FCS seems to be more appropriate because of the intrinsic factors of this serum. Even though some researchers could obtain differentiated cells in serum-free medium (Bettiol et al., 2007; Taha and Valojerdi, 2008), they agree that small amounts of FCS are indeed necessary to favour differentiation.
Acknowledgements
This study was supported by the Fundación para el Desarrollo de Ciencias Básicas (FUCIBA) and the Department of Gynaecology and Fertility of the Hospital Italiano de Buenos Aires. We thank Marcelo Ielpi, Pablo Silvestri and Maximiliano D′Adamo for the technical assistance, as well as Sung Ho Hyon and Isabel Milicay for language help and writing assistance.




