The management of hyperuricemia and gout in patients with heart failure
1. Introduction
Gout is a clinical syndrome resulting from the deposition of urate crystals in joints causing inflammation, intense pain, and even disability to patients. Patients with chronic heart failure frequently present with hyperuricemia. In these patients, the management of gout can raise specific problems. Cardiovascular risk factors such as hypertension and hyperlipidemia are associated with elevated urate levels and are frequently present. Chronic renal failure, which often complicates the pharmacotherapy of heart failure, is also associated with hyperuricemia. Diuretics, which are invariably needed for the treatment of volume retention, increase uric acid levels. The treatment of gout in patients with heart failure is complicated by their fragile volume state and chronic renal failure, both of which prohibit the use of non-steroidal anti-inflammatory drugs (NSAIDs) and corticosteroids. Moreover, drug interactions exist between drugs used for the treatment of hyperuricemia and gout and pharmacological agents used for the therapy of heart failure. This review therefore focuses on the treatment of hyperuricemia and gout in patients with heart failure.
2. Uric acid
At physiologic pH, urates, i.e. the ionized forms of uric acid (mostly monosodium urate), predominate in blood. The normal concentration range is below 420 μmol/l (7.0 mg/dl). Urate concentrations vary with sex, age, weight, blood pressure, renal function, alcohol intake, and purine content of the diet. Levels begin to rise during puberty in males but remain low in females until menopause due to higher excretion attributable to hormonal influences, i.e. uricosuric effects of estrogens.
Urate is the catabolic end-product of adenine and guanine, the purine bases essential for nucleic acids and purine nucleotides. Although purine nucleotide breakdown occurs in all cells, urate is produced only in tissues that contain xanthine oxidase, i.e. primarily in liver and small intestine. Normally, three-quarters of urate produced is excreted by the kidneys, the remainder is eliminated through the intestines. After glomerular filtration of the urate, almost all of it is reabsorbed in the proximal tubule. It is then secreted in the proximal tubule and again reabsorbed (Fig. 1). As pH is low in the urinary tract, urate is excreted in the urine as uric acid.
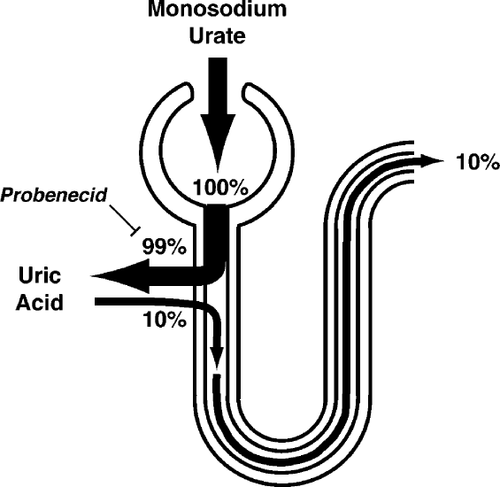
3. Hyperuricemia
Hyperuricemia, the cardinal biochemical feature and prerequisite for gout, is classified as primary (idiopathic/genetic) or secondary (acquired). Both types of hyperuricemia can result from increased production or decreased excretion of urate (or both). For example, alcohol consumption and obesity are not only associated with increased urate formation but also with reduced renal excretion 1–4. In acute or chronic renal failure, less urate is filtered in the glomeruli. A greatly enhanced purine turnover rate, such as in the tumor lysis syndrome (caused by lymphomas, hematologic malignancies, solid tumors and cytolytic therapy for these disorders), hemolysis, and psoriasis can dramatically increase urate levels 5. Further secondary causes of hyperuricemia include intake of certain drugs (Table 1), starvation, heat stroke, lactic acidosis, lead poisoning, trisomy 21, and glycogen storage disease 6.
| Increased urate levels | Decreased urate levels |
|---|---|
| Diuretics | Ascorbic acid |
| Salicylates (low-dose) | Calcitonin |
| Nicotinic acid | Calcium channel blockers |
| Cyclosporine | Dicumarol |
| Lead | Estrogens |
| Levodopa | Fenofibrate |
| Ethanol | Glucocorticoids |
| Ethambutol | Salicylates (>2 g/day) |
| Pyrazinamid | Uricosurics |
| Radiographic contrast agents | Verapamil |
| (in patients with left | |
| ventricular dysfunction) | |
| Vitamin B12 | ACE inhibitors |
| (pernicious anemia) | Losartan |
The annual incidence of gouty arthritis among patients with urate levels exceeding 540 μmol/l (9.0 mg/dl) is lower than 5% 7. Asymptomatic hyperuricemia does not have any adverse effects before the development of gout 8–10. Consequently, routine screening for hyperuricemia is not indicated. There is no evidence that treatment of asymptomatic hyperuricemia is beneficial or cost-effective. The only exception are patients with tumor lysis syndrome at risk of developing acute uric acid nephropathy 11,12.
3.1. Hyperuricemia and heart failure
Coronary atherosclerosis, and as a consequence myocardial ischemia and infarction, is the chief etiology of heart failure accounting for approximately 50% of cases 13. The four major coronary risk factors (hyperlipidemia, hypertension, diabetes mellitus, and smoking) leading to endothelial dysfunction and atherosclerosis are therefore frequently present in patients with heart failure. Despite the association of hyperuricemia with insulin resistance, diabetes mellitus, hypertension, hypertriglyceridemia, and obesity (metabolic syndrome), hyperuricemia is not an independent cardiovascular risk factor 14–19.
However, patients with chronic heart failure very often present with hyperuricemia 20,21. Remarkably, urate concentrations are correlated with maximal oxygen uptake and NYHA functional class in patients with cardiac failure 22. Urate levels are also related to circulating markers of inflammation in patients with chronic heart failure 23. These associations may be partly explained by the fact that tissue hypoxia—a hallmark of heart failure—is a stimulus for the production of urate 24. Indeed, there is an inverse relationship between serum urate concentrations and peripheral blood flow in patients with chronic heart failure 25. Moreover, serum uric acid levels predict mortality in patients with chronic heart failure, as well as after acute myocardial infarction 26,27.
In patients with congenital heart disease, serum urate levels are also elevated and correspond directly to the degree of polycythemia in cyanotic patients 28,29. Further, serum urate levels correlate with the severity and the mortality of primary pulmonary hypertension 30,31.
3.2. Drug-induced hyperuricemia
3.2.1. Diuretics
Diuretics are invariably needed for the treatment of volume retention in patients with chronic heart failure. When given acutely, they enhance renal uric acid excretion, whereas chronic diuretic therapy is associated with reduced excretion of uric acid. Mechanisms are increased uric acid reabsorption in the proximal tubule secondary to volume depletion, and competition between the diuretic and uric acid for the organic acid secretory mechanism in the proximal tubule.
In the Hypertension Detection and Follow-up Program, therapy with thiazide-type diuretics tended to increase levels of urate and creatinine 32. The increase in both was less in those patients with higher baseline urate levels than in those with lower levels. There were only 15 episodes of gout in 5 years among 3693 participants. The risk of gout in thiazide-treated patients is therefore very small. However, the initiation of diuretic therapy may rarely lead to rapid development of gout tophi in susceptible individuals 33.
Urate retention occurs with all diuretic agents 34–42. However, there may be slight differences between classes. Acute gout may relate more strongly to the use of loop diuretics than thiazides 43, and spironolactone may cause less pronounced urate retention than thiazide diuretics 44. In patients taking a thiazide and no other diuretic, an increased incidence of gout attacks was associated with obesity and high alcohol intake. These risk factors should thus be controlled in patients with heart failure at risk of diuretic-induced hyperuricemia and gout. There are reports about correction of diuretic-induced hyperuricemia by concomitant administration of potassium 45.
Low-dose diuretic therapy in hypertensive patients does not seem to alter serum urate levels significantly 46. Indeed, the requirement for anti-gout therapy in hypertensive patients is doubled for thiazide doses of ≥25 mg per day (in hydrochlorothiazide equivalents); no significant increase in risk is seen for lower doses 47. Similarly, low-dose therapy with a loop diuretic (e.g. 5 mg of torasemide) is not associated with hyperuricemia 48. However, low-dose diuretic therapy may be effective in hypertension but insufficient in patients with chronic heart failure who often additionally suffer from chronic renal failure.
Diuretic agents with uricosuric activity, e.g. ticrynafen and tienilic acid, are potentially useful for the management of patients with chronic heart failure and hyperuricemia 49–53. However, reports about unexpected adverse effects (hepatotoxicity and nephrotoxicity) limit their use 54,55.
3.2.2. ACE-inhibitors
ACE-inhibitors, such as captopril and enalapril, enhance the renal urate excretion (Table 1). In fact, urate levels can be significantly reduced by monotherapy with captopril in hypertensive patients suffering from hyperuricemia. Moreover, diuretic-induced hyperuricemia can be prevented or counteracted by the administration of an ACE-inhibitor such as captopril and enalapril 56.
3.2.3. Angiotensin receptor antagonists
Angiotensin receptor antagonists also enhance urinary uric acid excretion and therefore decrease urate levels. Losartan, an angiotensin receptor antagonist, decreases urate levels in hypertensive patients with thiazide-induced hyperuricemia 57, as well as in heart transplant recipients 58. In combination with fenofibrate, which also decreases urate levels as a single regimen, losartan further decreases urate levels in hypertensives 59.
3.2.4. Other drugs
Further cardiovascular drugs, e.g. beta-blockers such as timolol, which influence urate levels are listed in Table 1 60–62. It is of interest that radiocontrast agents used for coronary angiography increase urate levels in patients with left ventricular dysfunction 63.
4. Pathophysiology of gout
Gout is characterized by hyperuricemia, acute arthritis, deposits of urate crystals called tophi, and uric acid kidney stones. Established criteria for diagnosis further include the presence of urate crystals in joint fluid 64,65. The leukocyte count in the synovial fluid is usually increased. The prevalence of gout is 0.7 percent in men and 0.1 percent in women. The vast majority of primary gout is due to undersecretion of uric acid with the underlying renal defect not yet identified.
4.1. Gouty arthritis
Gouty arthritis occurs as a result of an inflammatory reaction to crystals of sodium urate that are deposited in the joint tissue. Locally infiltrating granulocytes phagocytize the urate crystals and subsequently secrete leukotrienes, various cytokines, and chemotactines, which can elicit an intense inflammatory reaction. Lysosomal and other enzymes released can lead to joint destruction. Increased lactate production caused by the inflammatory process decreases pH, which favors further deposition of urate crystals. Clinical gout usually develops after 20–30 years of sustained hyperuricemia. The first attack is usually a monoarticular arthritis of the first metatarsophalangeal joint called podagra. Precipitating factors such as alcohol ingestion, systemic infection, trauma, or surgery can often be identified. Initial episodes are usually self-limiting but recurrent arthritis often occurs within 1 year of the first attack. Later attacks are more prolonged and more commonly involve multiple joints.
4.2. Tophi
Tophi are deposits of urate crystals that elicit a foreign body reaction of mononuclear cells with granuloma formation. They may involve joints and cause a destructive arthropathy. Tophi can develop in every organ except the central nervous system. In the heart, tophi can cause serious conduction defects such as total heart block and rarely valve regurgitation due to valvular tophi 66,67.
4.3. Gout and renal disease
At a low pH (<5.5), uric acid rather than monosodium urate is present in urine. The prevalence of nephrolithiasis correlates with serum urate concentrations, reaching approximately 50% with serum urate levels of 770 μmol/l (13 mg/dl). The prevalence of uric acid stones in patients with gout averages 10–20%.
Urate nephropathy is a late and nowadays rare manifestation of gout with urate crystals causing a giant cell inflammatory reaction in the medullary interstitium and pyramids causing chronic renal failure. Prior to aggressive treatment of gout, urate nephropathy was the cause of death in approximately one-quarter of patients with gout.
Uric acid nephropathy is a reversible cause of acute renal failure resulting from precipitation of uric acid in renal tubules and collecting ducts. Predisposing factors include dehydration and acidosis. Uric acid nephropathy often occurs during the blastic crisis of leukemia or lymphoma or due to cytolytic therapy. In acute renal failure due to uric acid nephropathy urinary uric acid levels are elevated, whereas most forms of acute renal failure with oliguria, urinary uric acid excretion is normal or reduced.
5. Treatment of gout
5.1. Acute gouty arthritis
Usually, acute attacks of gouty arthritis can be effectively treated with non-steroidal anti-inflammatory drugs (NSAIDs) or colchicine 68,69. A non-salicylic NSAID like indomethacin is given in the highest approved dose (150–300 mg per day in divided doses) until 5–7 days after all signs of inflammation have resolved 70. Other NSAIDs also work but are no better than indomethacin 71–74.
5.1.1. NSAIDs in chronic heart failure
Constitutive cyclooxygenase (COX-1) is present in cells under physiological conditions, whereas COX-2 is induced in inflammation. NSAIDs unselectively inhibit both isoforms of the enzyme.
Whereas NSAIDs have little effect on renal function in healthy human subjects, the use of NSAIDs may cause sodium and volume retention, hyperkalemia, and renal failure in the elderly, in hypertensives, and in patients with chronic heart failure 75–81, especially if there is concomitant treatment with an angiotensin-converting enzyme (ACE)-inhibitor and/or spironolactone, a potassium-sparing diuretic 82. In patients with heart failure, renal perfusion is particularly dependent upon vasodilator prostaglandins that oppose the vasoconstrictor influences of angiotensin II and norepinephrine that result from activation of neurohumoral systems. Indeed, as much as one-fifth of hospital admissions with chronic heart failure may be due to the use of NSAIDs 82,83. High-doses of NSAIDs and long plasma drug half-life hold a particularly high risk. Furthermore, drug interactions with furosemide and ACE-inhibitors limit their use in heart failure 84,85. Alternative therapeutic strategies for patients with gout and heart failure are thus needed.
5.1.2. Selective COX-2 inhibitors
Recently, selective inhibitors of COX-2 have been introduced 86–91. Selective inhibitors of COX-2 are of special interest, since some of the detrimental effects of NSAIDs on renal function may be mediated by blockade of COX-1. Indeed, a preliminary study of a selective COX-2 inhibitor, celecoxib, in elderly patients with mild renal impairment, reported no significant change in glomerular filtration rate in contrast to naproxen, a NSAID 92. However, contradictory results were reported with the use of rofecoxib, another selective COX-2 inhibitor, which decreased renal function in elderly patients similar to indomethacin, a NSAID 93. Experimental data and case reports indicate that care is needed with their use in patients with impaired renal function 94–96.
5.1.3. Colchicine
Colchicine was introduced for the specific treatment of acute gout almost 250 years ago. Colchicine is an antimitotic agent decreasing the functional activity of granulocytes migrating into the inflamed area and inhibiting the release of pro-inflammatory substances 97. Urate blood concentrations are not altered. Given promptly within the first hours of an attack, few patients fail to obtain pain relief 69.
The drug is usually administered orally in a dose of 1 mg initially, followed by 0.5 mg every 2 h until abdominal discomfort or diarrhea develops or a total dose of 8 mg has been administered. Colchicine has been replaced by NSAIDs as a first-line agent because it invariably causes diarrhea when given orally. It may be given intravenously but the risk of toxic effects (bone marrow suppression, renal and hepatic injury, central nervous effects) is much greater. An initial dose of 2 mg is given intravenously, two additional doses of 1 mg each may be given at 6-h intervals; the total dose should never exceed 4 mg.
The doses should be reduced by 50% in patients with renal or hepatic insufficency and in the elderly. Drug interactions with cimetidine and erythromycin enhance its toxic effects. Apart from its inhibiting effects on spindle formation and cell division, colchicine constricts blood vessels, enhances the response to sympathomimetic agents, and has stimulating effects on central vasomotor centers. Care should thus be exercised with its use in patients with chronic heart failure.
5.1.4. Further therapeutic measures in patients with heart failure
Intra-articular injections of glucocorticoids are very useful when the use of NSAIDs and colchicine is problematic, as is the case in patients with chronic heart failure, or in refractory cases. The dose of corticosteroids is related to the size of the joint. An intra-articular dose of methylprednisolone acetate ranges from 5 to 10 mg for a small joint to 20–60 mg for a large joint such as the knee. Diagnostic aspiration of joints alone can sometimes greatly reduce the pain of gout. Oral glucocorticoids (30–50 mg/day, tapered during a period of 7–10 days) can be problematic in patients with chronic heart failure because they cause fluid retention due to their mineralocorticoid effects.
Acetaminophen has good analgesic properties but only weak anti-inflammatory effects. It may thus only be used as an adjunctive therapy for gout. Hepatotoxicity, the most serious side effect, may be more frequent in the presence of impaired liver function caused by right ventricular failure.
Additionally, opioid analgesics may be used to relieve moderate to severe pain. All opioid analgesics are metabolized by the liver; however, in the presence of renal disease, accumulation of active metabolites may occur. Morphine and related drugs cause vasodilation and may thus induce hypotension in patients with reduced blood volume.
5.2. Chronic gout
The management of symptomatic hyperuricemia includes diet and specific urate-lowering agents. It should address the control of hyperlipidemia, diabetes mellitus, and hypertension, as well as advice about alcohol intake and obesity. The purine content of the diet does not usually contribute more than 60 μmol/l (1 mg/dl) to urate levels in the blood. However, reducing the intake of meat, yeast, peas, beans, lentils, spinach, mushrooms, and beer may help to achieve blood urate levels below 360 μmol/l, but in most cases pharmacological therapy is necessary.
A complication of these drugs is the precipitation of acute attacks of gout. The risk can be minimized by increased fluid intake (which is not feasible in patients with heart failure), concomitant administration of prophylactic drugs (e.g. colchicine, 0.5–1 mg per day), delaying urate-lowering therapy for several (usually 2) weeks, and initiating the drug in less than full dose and increasing it over 3–4 weeks 98.
5.3. Allopurinol
Two classes of drugs are available for the treatment of hyperuricemia 99: uricosuric drugs and xanthine oxidase inhibitors. Allopurinol, the only xanthine oxidase inhibitor in clinical use, inhibits biosynthesis of uric acid. It is the first-line agent in the treatment of chronic gout. The daily single dose of allopurinol ranges from 100 to 600 mg, with a mean of 300 mg. The risk of precipitating acute gout is reduced if therapy is begun with a dose of 50–100 mg per day.
Allopurinol toxicity is increased in patients taking thiazide diuretics. Further, there are clinically relevant interactions with oral anticoagulants, theophylline, and azathioprine. Main side effects are a rash with an incidence of 2% and hypersensitivity manifesting as renal failure, hepatitis, exfoliative dermatitis and Stevens–Johnson syndrome with a frequency of 0.4% 100. Concomitant ampicillin administration greatly increases the incidence of hypersensitivity to up to 20%. Renal failure patients and patients taking diuretics are at particularly high risk if dose reduction is not made 101,102. The hypersensitivity syndrome carries a high mortality despite the therapeutic use of corticosteroids. Table 2 shows the appropriate doses of allopurinol for patients with reduced renal function.
| Creatinine clearance (ml/min) | Dose |
|---|---|
| 0 | 100 mg every third day |
| 10 | 100 mg every other day |
| 20 | 100 mg/day |
| 40 | 150 mg/day |
| 60 | 200 mg/day |
| 80 | 250 mg/day |
| ≥100 | 300 mg/day |
- a Modified after Cameron and Simmonds 106.
5.4. Uricosurics
Uricosuric agents increase the urinary excretion of urate by inhibiting renal reabsorption in the proximal tubule (Fig. 2). They may be useful in diuretic-induced hyperuricemia. Probenecid, developed in 1951 to depress renal excretion of penicillin which then was in short supply, is started at 250 mg twice daily and increased to 500 mg twice daily after 1–2 weeks 103,104. Sulfinpyrazone is begun at 50 mg twice daily and increased to 300–400 mg twice daily 105. Benzbromarone is given in a single dose of 40–80 mg per day.
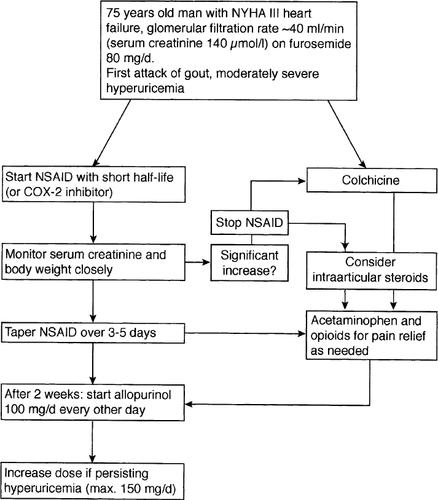
Uricosuric agents are inefficient or hazardous when urinary uric acid concentration is high, as it is in urate overproduction, in the presence of reduced renal function (creatinine clearance <50 ml/min), and if the patient has a history of nephrolithiasis because they increase the risk for the latter. Sulfinpyrazone causes gastrointestinal irritation in as much as 10–15% of patients. It reduces platelet aggregation, may induce reversible leuko- and thrombopenia, and lowers the efficacy of beta-blocking agents. With probenecid, caution is advised in patients with a history of peptic ulcer and in those taking NSAIDs because their plasma concentrations are increased by probenecid. Similarly, the plasma levels of captopril, an ACE-inhibitor, are increased by probenecid. The effects of uricosuric drugs are diminished by coadministration of low-dose aspirin and thiazide diuretics.
6. Conclusions
The use of NSAIDs as first line agents for the treatment of acute gouty arthritis is problematic in patients with heart failure because of potentially detrimental effects on renal function. Therefore, alternative therapeutic approaches are needed. Intra-articular glucocorticoid injections can be very helpful in these patients. Whether selective COX-2 inhibitors have a lower rate of adverse effects on the kidney and cardiovascular system and therefore may be used as a substitute for NSAIDs in patients with heart failure remains to be established. For the chronic management of hyperuricemia, proper dose adjustment of allopurinol is mandatory according to the severity of renal dysfunction.
7 Acknowledgements
Original research of the authors was supported by grants of the Swiss National Research Foundation (grants nos. 32–51069.97/1 and 32–52690.97), the Swiss Heart Foundation, and the Stanley Thomas Johnson Foundation.




