Cysteamine supplementation during in vitro maturation (IVM) of rabbit oocyte improves the developmental capacity after intracytoplasmic sperm injection
Abstract
Purpose
Current approaches to in vitro maturation (IVM) may result in low efficiency and inadequate quality of the oocytes due to insufficient cytoplasmic maturation. Although positive effects of the cysteamine supplementation in IVM medium for oocyte nuclear maturation or male pronuclear formation have been confirmed, it is still controversial whether the cysteamine addition affects embryo development after IVM. We aimed here to confirm the effect of cysteamine addition into IVM medium for subsequent embryo development in vitro.
Methods
We administered the cysteamine to the IVM culture of rabbit immature oocytes at various concentrations and observed the developmental rate, speed to reach blastocyst stage and cell numbers at the blastocyst stage.
Results
Cysteamine supplementation improved developmental rate to blastocyst stage of the IVM oocytes. On the other hand, addition of glutathione (GSH) inhibitor buthionine sulfoximine inhibited GSH accumulation in the oocytes and subsequent embryo development to the blastocyst stage.
Conclusions
Controlling the GSH quantity of IVM oocytes may be an important factor for success of embryo development, and it is quite probable that a cysteamine supplementation can contribute to an increase of GSH content in oocyte.
Introduction
Important challenges in assisted reproductive technologies (ART) are reducing the amount of gonadotropins for oocyte collection in in vitro fertilization (IVF) to avoid the risk of ovarian hyperstimulation syndrome (OHSS), and enhancing the numbers and improving the qualities of the usable oocytes in poor responders to conventional gonadotropin therapies. For current practice in some IVF clinics, in vitro maturation (IVM) of oocytes, which can be collected from pre-matured antral follicles, is thought as possibly an effective procedure for the oocyte preparation in ART. IVM is a process by which immature oocytes are harvested from the ovaries and induced maturation and resumption of meiosis by a short-time culture is done in the laboratory. Recent approaches to the IVM have never achieved the same level as conventional methods, such as IVF/intracytoplasmic sperm injection (ICSI) with fully matured oocytes in vivo [1–3]. An important possible cause is that the oocytes obtained by IVM possess poor developmental ability because of insufficient cytoplasmic maturation.
It has been described that glutathione (l-γ-glutamyl-l-cysteinyl-glycine; GSH) content in the cytoplasm of the oocyte is an important factor for the oocyte qualities [4, 5]. GSH is known to have multiple functions such as amino acid transport, cellular proliferation, protein synthesis and protection of cells from oxidative stress [6–8]. Moreover, GSH participates in sperm decondensation and male pronuclear formation after fertilization [9, 10], and it also plays a critical role in cytoplasmic maturation of oocytes [11]. GSH synthesis occurs during the maturation in vivo [12, 13], and cytoplasmic concentration of it in oocytes peaks at the MII stage [14]. However, it was reported that quantities of synthesized GSH in IVM are low due to inadequate content of cysteine in the culture environment and, furthermore, it is easily auto-oxidized during culture for maturation [15–17].
Cysteamine supplementation to the IVM medium increases cytoplasmic GSH contents in various species by enhancing cysteine uptake and increases the supply of substrate to the GSH synthetase, which translates into an increased synthesis of GSH [6]. To date, especially the positive effects of the cysteamine for oocyte nuclear maturation or male pronuclear formation have been confirmed [13]. On the other hand, effects of the cysteamine supplementation to the IVM medium on cleavage and subsequent development are still controversial, and the mechanisms for the effect are also unclear. For example, Oyamada and Fukui [18] showed that the cysteamine supplementation increased the cleavage and developmental rates following IVM in bovine, and Anand et al. [19] and Singhal et al. [20] also demonstrated the increased rate of cleavage and developmental rate by cysteamine in buffalo IVM oocytes. The positive effects of cysteamine supplementation on the developmental rate after IVM and IVF were reported also in sheep and goats, whereas Kobayashi et al. [21], Deleuze et al. [22], and Chen et al. [23] reported that the cysteamine administration into IVM culture does not function to improve cleavage and the developmental rate in pig, horse or mouse IVM oocytes. The effects of the thiol compounds could be different among species, age of the host individuals or kinds of basement mediums for oocyte cultures [24, 25], therefore, it is essential to reproduce and confirm the effect with more various animals and situations.
Here we administered the cysteamine to the culture of rabbit immature oocytes and examined its effect on embryo development in vitro.
Materials and methods
Collection of oocytes
Fertile female New Zealand White rabbits were super-ovulated with 80 IU pregnant mare's serum gonadotropin (PMSG; Novartis Animal Health K.K., Tokyo, Japan) by intramuscular administration and 60 IU human chorionic gonadotropin (hCG; Novartis) by intravenous dosage after 72 h of PMSG injection. Ovulated oocytes were collected after 14–16 h of hCG injection by flushing the removed oviducts with M2 medium for ICSI.
Fertile female New Zealand White rabbits were stimulated with 80 IU PMSG intramuscularly before 72 h of follicle recovery. Pre-ovulated immature follicles at approximately 1 mm in diameter were collected, and cumulus-oocyte complexes (COCs) were aspirated from the follicles using 21 G needles and applied for IVM.
In vitro maturation
The IVM medium consists of TCM199 (NISSUI PHARMACEUTICAL CO., Tokyo, Japan) supplemented with 10 ng/ml Epidermal Growth Factor (Sigma Aldrich, St. Louis, MO, USA), 100 μM β-Estradiol Water Soluble (SIGMA), and 0.1 mg/ml Polyvinyl alcohol (PVA; SIGMA). For the study, the IVM medium was further supplemented with 0, 100, 200, and 500 μM cysteamine (SIGMA). For IVM culture, collected COCs were gently washed twice, transferred into 50 μl drops of IVM medium and cultured for 14–16 h at 37 °C in a humidified atmosphere of 5 % CO2 in air.
Intracytoplasmic sperm injection
Following the IVM culture, cumulus cells were removed by exposing them to M2 medium containing 0.1 % hyaluronidase (Nacalai tesque Co., Kyoto, Japan). Denuded IVM-oocytes were washed and maintained for ICSI in culture medium described in the following section for 1 h at 37 °C in a humidified atmosphere of 5 % CO2 in air. Ejaculated sperms were obtained from fertile male New Zealand White rabbits. Sperms were washed once in 5 ml of M2 medium followed by centrifugation at 700–800 rpm for 1 min. The sperm pellet was collected and placed to the bottom of 2 ml of M2 medium to perform swim-up preparation method for 15 min at room temperature. Treated sperms were transferred to 10 % Polyvinylpyrrolidone (PVP; SIGMA) and only the head of a sperm was injected into the oocytes with a piezo-driven micromanipulation system (PRIME TECH LTD., Ibaraki, Japan).
In vitro culture
Sperm-injected oocytes were washed twice in 50 μl drops of CMRL1066 (Invitrogen, Carlsbad, CA, USA) supplemented with 30 % fetal bovine serum (FBS; Thermo Fisher Scientific K.K, Waltham, MA, USA), 0.55 mg/ml sodium pyruvate (SIGMA), 0.146 mg/ml l-glutamine, non-animal source (SIGMA), 1.861 μl/ml lactate (SIGMA), 0.063 mg/ml penicillin G potassium salt (Nacalai tesque), and 5.0 μl/ml gentamicin solution (SIGMA). Then, the oocytes were transferred into 50 μl drops of the above culture medium under mineral oil and cultured for 5 days at 38 °C in a humidified atmosphere of 5 % CO2, 5 % O2, and 90 % N2 in air. Embryo observations were performed at 6–8 h (fertilization), 24 h (cleavage), 72 h (morula to blastocyst stage), 96 and 120 h (blastocyst stage) after ICSI (Fig. 1).
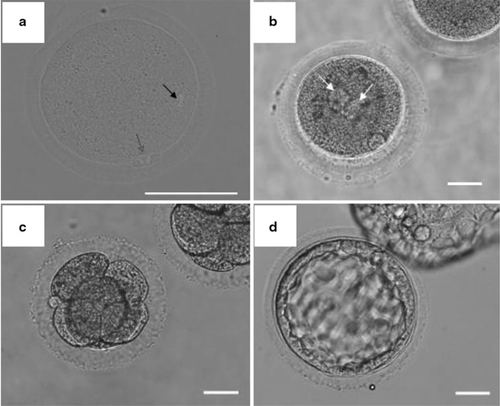
Oocytes after maturation and subsequent development. a DAPI stained IVM-oocyte. The black arrow shows oocyte nucleus, and red arrow shows the nucleus of first polar body. b Pronuclear formation. The white arrows show the male and female pronuclei. c 4–8 stage embryos, and d blastocyst stage embryo. Scale bars are 50 μm
To assess the effect of GSH during in vitro culture, the collected COCs were gently washed twice and cultured in 50 μl drops of IVM medium with or without 5 mM buthionine sulfoximine (BSO, SIGMA), which is an inhibitor of GSH synthesis, during IVM cultures and the developmental rate was observed to the blastocyst stage.
Calculation of the GSH containing level in the oocyte
ThiolTracker Violet dye (Invitrogen) was diluted in PBS at 20 μM. Oocytes were incubated in the ThiolTracker containing PBS for 30 min at room temperature, and used for fluorescent observation. To compare the fluorescent intensity, captured fluorescent images were analyzed using NIH Image J software (W. Rasband, NIH, USA).
Cell count at blastocyst stage
To evade the bias or dispersion of the cell number by the condition of the blastocyst embryo, we excluded blastocysts at an early stage or hatched, shrunk or over-expanded embryos. Cell numbers were compared between the experimental groups of in vivo matured control (normal oocyte with ICSI and in vitro culture), non-treated IVM control (IVM oocyte with ICSI and in vitro culture) and 200 μM cysteamine added group (IVM oocyte with ICSI and in vitro culture). Blastocysts were washed twice in M2 medium and fixed by 4 % Paraformaldehyde Phosphate Buffer Solution (Wako Pure Chemical Industries, Ltd., Oosaka, Japan) for 15 min using 96-well micro plate (BD Falcon™, Franklin Lakes, NJ USA). Then, the blastocysts were permeabilized in 0.1 % Triton-X (SIGMA) diluted in PBS for 15 min and washed three times in 0.1 % PVA diluted in PBS (0.1 % PBS-PVA). Samples were penetrated in DAPI (Vector Laboratories, Ltd., Peterborough, England) for counterstaining for 10 min and washed three times in 0.1 % PBS-PVA again. Thereafter, these samples were covered with VECTASHIELD mounting medium (Vector Laboratories) to avoid signal reduction onto the glass slide. The DAPI stained nuclei was counted under UV light with a fluorescent microscope.
Statistical analysis
To assess the effects of cysteamine on the maturation, fertilization and development, differences among the groups were analyzed by Fisher's exact test. The effect of cysteamine on the developmental speed to the blastocyst was analyzed by ANOVA followed by Fisher's PLSD post-test. Blastocyst cell number differences among each experimental group were detected using the Tukey–Kramer HSD test. P < 0.05 was considered a significant difference.
Results
Cysteamine addition improved the developmental rate to blastocyst stage
Here, we examined the effect of the cysteamine supplementation to the IVM culture of rabbit pre-matured oocytes. In this study, all oocytes used for IVM culture reached MII stage in all groups, thus, we could not observe any effects of the cysteamine addition on the meiosis. However, the cysteamine addition clearly affected the development after fertilization. When 200 μM cysteamine was added to culture, the developmental rate to the blastocyst stage was significantly increased, and the efficiency was improved approximately two times higher than that of untreated control (Table 1). In order to examine whether the cysteamine effect is related to the GSH quantity in the oocyte cytoplasm, GSH synthesis inhibitor BSO was added to the IVM medium. At first we confirmed whether BSO administration could decrease GSH in oocyte using ThiolTracker violet dye. Measuring fluorescent intensity by Image J revealed that (1) IVM oocyte contains a lower level of GSH than control (ovulated) oocyte, (2) cysteamine supplementation recovered the GSH quantity of the IVM oocytes to a comparable level with the control, and (3) BSO administration decreased the GSH quantity (Fig. 2). And as we expected, BSO administration dramatically inhibited the developmental rate to blastocyst; it decreased to 8.0 % (4/50) compared to control group (34.0 %, P < 0.05).
| Cysteamine (μM) | No. of oocyte | Survived after ICSI | Fertilized (%) | Cleaved (% from fertilized oocytes) | Blastocysts (% from fertilized oocytes) |
|---|---|---|---|---|---|
| 0 | 56 | 51 | 44 (78.6) | 38 (86.4) | 15 (34.0)a |
| 100 | 35 | 34 | 31 (91.2) | 29 (93.5) | 12 (38.7)a |
| 200 | 36 | 33 | 30 (90.9) | 28 (93.3) | 19 (63.3)b |
| 500 | 35 | 34 | 29 (85.3) | 28 (96.6) | 18 (62.1)b |
| Ovulated | 42 | 42 | 35 (83.3) | 35 (100) | 33 (94.3)c |
| BSO | 65 | 57 | 50 (87.7) | 38 (76.0) | 4 (8.0)d |
- Different characters in each column show that statistically significant differences are detected (P < 0.05)
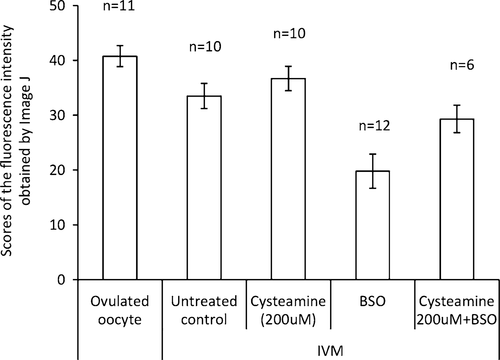
Intracellular GSH levels were determined by oocytes obtained from each experimental condition with ThiolTracker dye and analyzed by NIH Image J. Statistically significant difference exists among all experimental groups with each other (P < 0.05). Bars depict standard deviations (SD) in each group
Cysteamine supplementation accelerated the developmental speed to the blastocyst stage
In order to estimate the developmental speed, we observed the embryo stages after ICSI. When observed at 72 h after sperm injection, embryos did not reach the blastocyst stage except for 7 of 33 ovulated embryos. At 96 h after sperm injection, approximately 90 % of embryos reached the blastocyst stage in the ovulated embryo-group, but only 6 of 10 IVM-derived embryos (60.0 %) reached the blastocyst stage. Interestingly, when cysteamine was added to the IVM culture, most embryos (84.2 %) reached the blastocyst stage at 96 h after sperm injection although no blastocyst was observed at the 72 h time-point (Table 2). From these, it was suggested that the cysteamine supplementation accelerated the developmental speed of the IVM-derived embryos.
| Cysteamine (μM) | No. of embryos reached to blastocyst at each time | Total blastocysts | ||
|---|---|---|---|---|
| 72 h (%) | 96 h (%) | 120 h (%) | ||
| 0 | 0 (0) | 6 (60.0) | 4 (40.0)a | 10 |
| 200 | 0 (0) | 16 (84.2) | 3 (15.8)a,b | 19 |
| Ovulated | 7 (21.2) | 23 (69.7) | 3 (9.1)b | 33 |
- Different characters in each column show that statistically significant differences are detected (P < 0.05)
Cysteamine improved the cell numbers of the IVM-derived embryos at blastocyst stage
To further examine the effect of the cysteamine supplementation, we compared the cell numbers between the embryos derived from ovulated oocytes, IVM-oocytes cultured with and without 200 μM cysteamine. In the control group, 360.6 (mean) ± 101.4 cells were contained in embryos. On the other hand, 267.2 (mean) ± 89.6 cells were contained at the blastocyst stage in the IVM group, and this reduction of cell numbers was statistically significant (P < 0.05). The cysteamine administered group, however, contained 379.1 (mean) ± 132.9 cells at blastocyst stage (Fig. 3). These results evidenced that the cysteamine supplementation improved IVM culture systems.
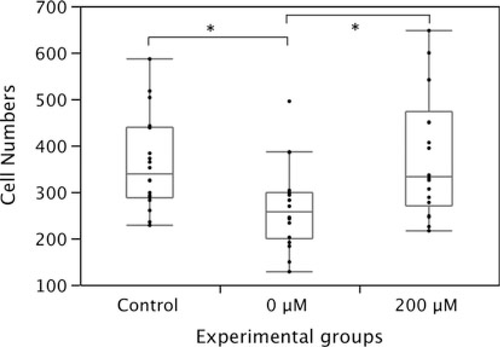
The cell numbers at blastocyst stage in the control (obtained from ovulated oocytes), non-treated IVM group and cysteamine added IVM group. Asterisks mean a statistically significant difference (P < 0.05). Bars depict ± SD of 18 specimens
Discussion
Although pregnancies and live births have been obtained from IVM oocytes collected from unstimulated cycles in the human, the successful rate is still low. To increase the developmental potential of the IVM-derived embryo, improving the culture systems for IVM is essential [26].
In this study, we examined the effect of cysteamine addition during IVM focusing on cleavage and subsequent preimplantation development. Cysteamine supplementation improved the developmental rate to the blastocyst stage. On the contrary, addition of a GSH inhibitor BSO drastically inhibited embryo development. From these results, it was assumed that controlling the GSH quantity of IVM oocytes may be an important factor for the success of embryo development, and it is quite probable that a cysteamine supplementation can contribute to an increase of GSH content in the oocyte. Results of our study may support the opinion suggested in the previous researches such as Oyamada and Fukui in bovine or Anand et al., and Singhal et al., in buffalo IVM oocytes.
An accumulation of reactive oxygen species (ROS) causes the peroxidation of nucleic acids, lipids, amino acids, and carbohydrates. Excessive amounts of ROS affect various cellular functions via directly damaging cellular components such as RNA and DNA [27], and it also results in avocation of some intra-cellular molecular signaling. In these responses to ROS stimulation, the mechanisms mediated by cellular stresses relating to MAPK activity are well documented [28]. ROS increases the activity of Jun N-terminal kinase (JNK) and p38, which are members of MAPK and related to some inflammatory processes. In oocytes, increasing the p38 activity affects embryo development negatively. On the other hand, the relation of JNK activity with oocyte fragmentation is demonstrated in a recent study [29]. As the other major mechanism of ROS induced cellular damage is a process mediating the Nuclear factor kappa B (NF-kB) [30]. It is well known that the NF-kB also plays a crucial role in immune responses, inflammation and apoptosis. Chaube et al. [31] treated rat oocyte with H2O2, which is a major inducer of ROS, and determined that degeneration and initiation of apoptosis in both immature and matured oocytes. Furthermore, ROS significantly affected mitochondrial functions and distributions. Malfunction and abnormal distribution of mitochondria could directly lead to a decline of the developmental ability of early embryos. Until now, it has been reported that the ROS level is gradually increased during in vitro culture of the embryo [32], or oocyte deriving IVM containing a lower level of GSH than in vivo matured oocytes [33]. Based on this evidence, containing a suitable quantity of the GSH is necessary for dealing with the above impediments and, thus, the idea that the cysteamine administration, which could directly result in the GSH accumulation in the oocyte, enhancing embryo development is very reasonable. Furthermore, in the present study, it was observed that the developmental speed of the cysteamine-added group caught up with that of in vivo matured control. Some evidence suggests that the developmental speeds could be proposed as a good criterion for expectation of the developmental ability [34–37], thus, cysteamine may normalize the developmental property of the IVM oocyte.
Possibly, toxicity of cysteamine is a considerable major problem. Now, cysteamine is used as a scavenger of ROS in various cells. Contrarily, Shin et al., reported that an over-dose of cysteamine could induce cell death in human corneal epithelial cells in vitro, although the GSH accumulation and anti-oxidative effects are increased depending on the dose. Detailed elucidation for the function and toxicity of cysteamine in mammalian development will directly lead to the effective procedure for ART.
Acknowledgments
We gratefully acknowledge Ms. Naomi Backes Kamimura, Department of Biology-Oriented Science and Technology, Kinki University, for English editing.
Conflict of interest
We have no conflict of interest.




