Genetic Counseling Dilemmas for a Patient with Sporadic Amyotrophic Lateral Sclerosis, Frontotemporal Degeneration & Parkinson's Disease
Abstract
Amyotrophic lateral sclerosis (ALS), frontotemporal degeneration and Parkinson's disease may be different expressions of the same neurodegenerative disease. However, association between ALS and parkinsonism-dementia complex (ALS-PDC) has only rarely been reported apart from the cluster detected in Guam. We report a patient presenting with ALS-PDC in whom pathological mutations/expansions were investigated. No other family members were reported to have any symptoms of a neurological condition. Our case demonstrates that ALS-PDC can occur as a sporadic disorder, even though the coexistence of the three clinical features in one patient suggests a single underlying genetic cause. It is known that genetic testing should be preferentially offered to patients with ALS who have affected first or second-degree relatives. However, this case illustrates the importance of genetic counseling for family members of patients with sporadic ALC-PDC in order to provide education on the low recurrence risk. Here, we dicuss the ethical, psychological and practical consequences for patients and their relatives.
Introduction
The discovery of new genes involved in ALS pathogenesis has enlarged the clinical spectrum of motor neuron diseases, resulting in a bridge between ALS and other neurodegenerative disorders such as frontotemporal dementia (FTD) and Parkinsonisms (Renton et al. 2011).
During World War II, a high-prevalence of ALS was reported among the indigenous Chamorros on the island of Guam. Soon afterwards, the related disorder parkinsonism-dementia complex (PDC) was characterized in the same population. At that time, about 10% of deaths in Chamorro adults were due to ALS, 100 times the rate in any other population. PDC represented another ~7% of deaths. The clinical spectrum of ALS-PDC in Guam inhabitants is variable. Some patients have clinical features of PDC along with clinical evidence of upper motor neuron involvement. Other patients with PDC also have clinical evidence of upper and lower motor neuron involvement. In several cases, at least one family member was affected by ALS, PDC or both (Hirano et al. 1961).
A high prevalence of ALS was also found in the Kii peninsula of Japan in the 1960s (Kimura et al. 1961); however, the prevalence of ALS-PDC was limited (Kuzuhara et al. 2001). Clinical and neuropathological studies suggest that ALS and PDC cases observed in the Kii Peninsula and in Guam were similar and may represent the same disorder (Itoh et al. 2003).
Sporadic cases of association between ALS and PDC have been rarely reported: there are published reports of one autopsy case in Japan (Shintaku et al. 2007) and four Czech patients (Farníková et al. 2010). None of these patients were investigated for genetic mutations.
A positive family history is present in about 10% of cases of ALS (familial ALS, FALS), whereas the remaining cases are labelled as sporadic ALS (SALS). A genetic aetiology has been identified in up to 60% of FALS, with 126 ALS-related genes/loci identified to date (http://alsod.iop.kcl.ac.uk/). The distinction between SALS and FALS has proven to be complicated. In fact, in 50–75% of FALS only two affected relatives are reported and in these instances the pattern of inheritance is not clear (Sabatelli et al. 2013). Variable expressivity for genetic variants has also been seen. For example, expansion of a hexanucleotide repeat sequence in an intron of C9orf72 is responsible of several cases of ALS and FTD (Renton et al. 2011; DeJesus-Hernandez et al. 2011). In the families with expansion in this gene, individuals with ALS, FTD or both may be present, and the expansion can also cause Parkinsonism (O'Dowd et al. 2012).
We describe a patient presenting with sporadic ALS-PDC, in whom the most common known genetic mutations causative of ALS and FTD have been excluded, and we discuss the issues that arose in family genetic counseling.
Genetic Analysis
Written informed consent was obtained from the patient. Genomic DNA was extracted from peripheral blood according to standard protocols. Superoxide dismutase 1 (SOD1;NM_000454), angiogenin (ANG; NM_001145), TAR DNA-binding protein 43 (TARDBP; NM_007375), fused in sarcoma/traslocated in liposarcoma (FUS/TLS; NM_004960), progranulin (PGRN; NM_002087) and valosin-containing protein (VCP; NM_007126) genes were amplified by polymerase chain reaction (PCR) with primer sets described elsewhere (Leverenz et al. 2007; Millecamps et al. 2010). Direct DNA sequencing included coding and splice site regions. For TARDBP, FUS and VCP, analysis was limited to exon 6, exons 5–6–13-14-15 and exons 2–3–5-6-10-14, respectively. ANG gene analysis included the 600-bp promoter region, and PGRN gene analysis included 219 bp in the promoter region and 304 bp in the 3'UTR (Corrado et al. 2007). For C9orf72, a repeat-primed PCR assay was used to screen the presence of the GGGGCC hexanucleotide expansion in the first intron, as described (Renton et al. 2011). Sequencing was performed with an ABI 3730 automated sequencer (Applied Biosystems, UK). The ATXN-2 CAG repeat in exon 1 (NM_002973.3) was amplified using a fluorescent primer and sized by capillary electrophoresis on an ABI 3130 genetic analyzer (Applied Biosystem, Foster City, CA).
Patient Report
A 75-year-old man (II:1; Fig. 1), originating from a small town in the north of Italy, came to our attention with a two-year history of amnesia, ideomotor apraxia and impairment of speech. Caregivers reported dysphagia, cramps and nocturnal agitation. He had a medical history of hypertension, benign prostatic hyperplasia and glaucoma. There was no consanguinity. The family history was negative for neurological diseases. He was treated with venlafaxine and donepezil for one year.
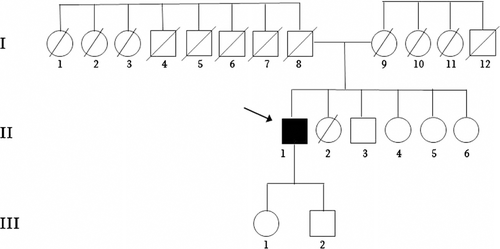
Pedigree of the family. Arrow indicates index patient; diagonal line indicates individual deceased. Individual I:8 died at 40 years during the War; Individual I:9 died at 101 years and I:1–1:7 and I:10-I:12 died in old age not for neurological disorders; II:2 died at 1 years for an infectious disease
The neurological examination showed hypomimia, slurred speech, bradykinesia and slow gait with camptocormic posture and reduced arm swing bilaterally. There was extrapyramidal stiffness of both upper and lower limbs with bilateral wrist cogwheeling prevalent on the left side. Fasciculations and atrophy were diffusely present. Deep tendon reflexes were brisk at all four limbs. There were bilateral Babinski and Hoffman signs. Snout, palmomental and glabellar tap reflexes were present and masseter reflex was increased. There were no signs of cerebellar or vestibular impairment, nor tremor. Superficial and proprioceptive sensations were normal, and upward gaze palsy was present.
Routine hematological and biochemical tests were normal. Cerebral spinal fluid examination showed a slight increase of tau and decrease of beta-amyloid protein 42. Magnetic resonance imaging (MRI) of the brain showed diffuse cortical and subcortical atrophy and few small focal T2-hyperintense lesions of the white matter due to chronic ischemic damage (Fig. 2a). Spinal cord MRI was normal. Motor evoked potentials showed normal central motor conduction. Needle electromyography (EMG) showed spontaneous activity (fibrillation and positive sharp waves) and chronic neurogenic changes (polyphasic motor unit potentials with increased amplitude and duration) at proximal and distal lower limb muscles. Single-photon emission computed tomography (SPECT) analysis with 123I–ioflupane showed reduced dopaminergic innervation in the right striatum (Fig. 2b). Neuropsychological tests showed marked frontal syndrome conditioning deficits of language, semantic memory and praxis with numerous perseverations and intrusions; impairments in tests for selective attention and verbal phonological; and a frontal assessment battery (FAB) score of 5. The Mini Mental State Examination (MMSE) score was 17/30.
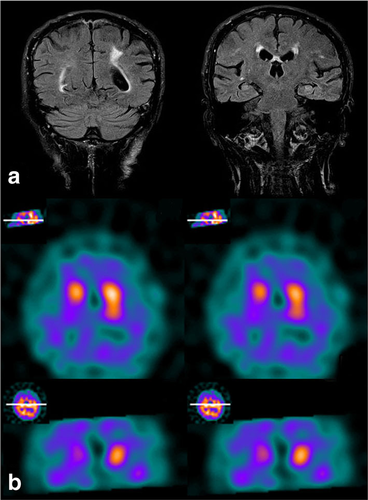
a Coronal plane FLAIR-weighted MRI scan showing a mild cortical atrophy and bilateral hyperintense lesions due to chronic ischaemic damage; b Axial and coronal SPECT images with 123I–ioflupane showing reduced dopaminergic innervation in the right striatum
The patient was diagnosed with parkinsonism, FTD and probable motor neuron disease. Therapy with levodopa was started. At 3-month follow-up, muscle atrophy and weakness had worsened, while EMG showed diffuse spontaneous activity and chronic neurogenic changes in all four limb muscles, leading to the diagnosis of definite ALS according to El Escorial criteria (Brooks et al. 2000). Treatment with riluzole 100 mg daily was started. Two years after diagnosis, the patient died due to respiratory failure. No exposure to known environmental risk factors for neurodegenerative disorders were reported by the proband and his relatives.
The analysis for mutations in the SOD1, TARDBP, FUS, ANG, VCP, PGRN, ATXN2 and C9ORF72 genes was negative.
Genetic testing was offered to the patient within a genetic counseling session with a multidisciplinary team represented by geneticists and neurologists. The patient was accompanied at this session by his children. The proband's offspring stated their willingness to undergo genetic testing in case of a positive test result in their father. They were aware of the possibility of the need for a psychological evaluation before starting any genetic tests. Moreover, they stated their desire to be part of international research programs. The post-test genetic counseling session occurred three months later and again included the patient's offspring. The patient was informed of the negative genetic results before he died. The multidisciplinary team underlined the limits of the genetic test performed and explained that the clinical complexity of the patient's condition could be due to either involvement of hitherto unidentified genetic factors or environmental factors could be not genetically determined. The team, according to the signed informed consent, will continue genetic evaluation of other causative gene/s that might be discovered. This information was shared with the offspring, who expresed their desire to be informed about future possible results. At the end of the counseling session, the offspring had realized most of the difficulties inherent to diagnosis of genetically-determined neurodegenerative disease in cases such as that of the proband, and also realized that only a non-conclusive judgement could be issued about the low probability of their future risk of developing the same disease as their relative. The above approach follows an ongoing experimental model used at a specialized center dedicated to ALS patients. Likewise, counseling for Huntington's disease (HD) also involves a multidisciplinary team. In contrast, our approach requires more sessions to ensure that patients have sufficient awareness of the limitations of genetic testing, given the clinical differences of ALS-PDC versus HD (ie incomplete versus complete penetrance).
Discussion
We describe a case of sporadic ALS-PDC with negative genetic analysis. Neither consanguineous marriages nor cases of ALS, Parkinson disease, parkinsonisms or dementia were reported in the large kindred (Fig. 1). However, the patient's father (I:8) died at the age of 40 years during the Second World War.
The association between ALS, FTD and parkisonism is very rare. ALS-PDC has been described as an endemic syndrome in the indigenous Chamorros of Guam (Hirano et al. 1961) and in the inhabitants of the Kii peninsula of Japan (Kimura et al. 1961). Genetic and environmental factors (cycad seed, mineral deficiency, infectious agents, heavy metal toxicity) were considered but a shared pathogenetic explanation has not been found (Steel and McGeer 2008; Spencer et al. 2009). The analysis of C9orf72 hexanucleotide repeat expansion in 89 Chamorro individuals affected by ALS and FTD did not find any correlation (Dombroski et al. 2013). Conversely, C9orf72 expansion and SOD1 mutations have been reported in patients with ALS-PDC from the Kii peninsula (Dombroski et al. 2013; Kikugawa et al. 1997) and in further kindreds presenting with ALS, FTD, PD, ALS-FTD or ALS-PDC (Renton et al. 2011; DeJesus-Hernandez et al. 2011; O'Dowd et al. 2012). Mutations in TDP43 (Borghero et al. 2011; Mosca et al. 2012) and DJ-1 (Park-7) genes (Annesi et al. 2005) have been also identified in multigenerational ALS-PDC families.
Few sporadic cases of ALS-PDC have been previously described. Two patients with ALS plus parkinsonism and four patients with ALS-PDC have been reported from Canada (Uitti et al. 1995), in addition to four patients with sporadic ALS-PDC from the Czech republic (Farníková et al. 2010). However, all these cases were published before the discovery of the C9orf72 expansion, and no other genetic analyses were performed.
It is necessary to consider the genetic tests and the genetic counseling that are offered for family members of patients with sporadic ALS-PDC. We think that in cases of a negative familial history, the analysis of rare pathogenetic genes should not be necessarily performed. In a paper on genetic counseling in ALS, Chiò et al. (2014) suggest that genetic testing should be offered only to patients with ALS who have a first-degree or second-degree relative with ALS and/or FTD, and should be discussed with, but not offered to, all other patients with ALS, with special emphasis on the uncertainties and limitations of genetic testing. Performing or offering genetic tests in patients with late-onset ALS and no family history is questionable, since the majority of ALS are sporadic. Nonetheless, patients wth ALS are increasingly aware of the importance of genetics, and probands and relatives frequently request genetic testing and seek to participate in international research programs (Mandich et al. 2015). Moreover, clinical trials often require genetic tests for participating patients. These considerations can lead to discussion of genetic testing even in the absence of family history, as for our patient.
It is also essential to consider the genetic counseling provided in these cases. For example, in cases in which a relative of a patient with a known genetic mutation asks to undergo genetic testing, we apply the ALS international guidelines (Chiò et al. 2014): we provide accurate information, including a discussion of the uncertainties of ALS genetics including the limitations in predicting future development of the disease, age of onset, symptoms severity or progression rate. At that point, only if the patient is sure, we perform the test. In addition, we apply our experimental multidisciplinary counseling approach to family members.
Finally, we highlight the disclosure of genetic data to relatives after a patient's death. The ethical obligation to respect a patient's confidentiality extends beyond death. However, in special cases, if the test results are considered essential for patient's family health, the Italian Data Protection Authority can authorize test result disclosure to the family's General Practitioner. We think that it should be essential to address this possibility with patients during the pre-test counseling session, discuss the research/diagnostic protocols, and establish who in the family can be notified of these results in the event of patient's death. In case of a positive genetic test, even if the results became available after the proband's death, we organize a multidisciplinary team that includes a psychologist to communicate the genetic testing results and provide genetic counseling to the family members. Several meetings are generally requested with the goals of communicating the genetic results; ensuring that offspring are aware of the meaning of genetic testing results and of the consequences of a possible positive test to other family members; and providing offspring the time they need to make a decision about learning genetic results when these are available.
As recently highlighted by Arthur et al. (2016), the use of genetic testing in ALS is very important because of the future possibility of gene therapy. Moreover, those authors point out the difficulties in establishing permanent genetic counseling guidelines for ALS patients because of the complexity of this devastating disease (Arthur et al. 2016).
Conclusions
Our case demonstrates that ALS-PDC can occur as a sporadic disorder, even though the coexistence of the three clinical features in one patient could suggest a single underlying genetic cause despite a late age at onset. Whether a genetic cause can be ascertained may depend on the number of genes assayed and the approach used. As research in the field of ALS and related disorders accelerates, the process of genetic testing should consider the issue of complex phenotypes. Challenging decisions also occur when deciding how many genes are to be tested to search for the mechanism in sporadic cases. In our opinion, a multidisciplinary approach (neurologists, geneticist and eventually psychologists) to genetic counseling is essential to reassure the proband and his relatives about the low probability for recurrence of degenerative disease in other family members in the absence of familial history. Genetic counseling is an effective way to support patients and their families and to make them aware of the present limits of our knowledge, increasing reciprocal confidence between patients and the medical team.
Compliance with Ethical Standards
Conflicts of Interest
Vittorio Mantero, Claudia Tarlarini, Angelo Aliprandi, Giuseppe Lauria, Andrea Rigamonti, Lucia Abate, Paola Origone, Paola Mandich, Silvana Penco and Andrea Salmaggi declare that they have no conflict of interest.
Human Studies and Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2000.
Animal Studies
No animal studies were carried out by the authors for this article.




