Intraperitoneal use of local anesthetic in laparoscopic cholecystectomy: systematic review and metaanalysis of randomized controlled trials
Abstract
Background/purpose
With the advent of minimally invasive gallbladder surgery, and now with natural orifice techniques emerging, visceral nociception has been neglected as a cause of postoperative pain. A systematic review and metaanalysis was carried out to investigate the use of intraperitoneal local anesthetic (IPLA) in order to assess its role in laparoscopic cholecystectomy (LC). The aim of this systematic review was to appraise the clinical effects of this modality.
Methods
Comprehensive searches were conducted independently without language restriction. Studies were identified from the following databases from inception to September 2009: Cochrane Central Register of Controlled Trials (CENTRAL/CCTR), Cochrane Library, Medline, PubMed, Excerpta Medica Database (EMBASE), and Cumulative Index to Nursing and Allied Health Literature (CINHAL). Relevant meeting abstracts and reference lists were manually searched. Data analysis was performed using Review Manager Version 5.0 software.
Results
Thirty randomized controlled trials were identified for review. The clinical heterogeneity of IPLA use was high. However, there appeared to be reduced pain, opioid use, and need for rescue analgesia, and reduced postoperative cortisol and glucose responses.
Conclusion
There is evidence in favor of IPLA in LC. Further trials of this modality in LC are not needed as these are unlikely to reduce clinical heterogeneity. IPLA should be trialled as future minimally invasive surgical techniques approach.
Introduction
Pain after cholecystectomy is thought to be multidimensional in nature 1. With the advent of minimally invasive techniques including single-port laparoscopy and transluminal endoscopic surgery there is potential to bypass the abdominal wall altogether for visceral access and resection [2, 3]. Although greatly reducing the need for analgesia, these advances in technique still cause visceral nociception, through disruption of the peritoneum and dissection of viscera.
Visceral pain is a distinctly separate form of pain compared to somatic pain 4. Visceral signaling occurs through the enteric nervous system (ENS), which is complex and partly independent of the central nervous system (CNS), with a vast network of distinct, and functionally diverse, neuronal subtypes 5. Viscera such as the gallbladder and covering peritoneum convey unpleasant sensations and autonomic reactions to injury through afferents in the vagus nerve 6. These so-called “silent nociceptors” are activated by intraperitoneal inflammation and injury 4, giving rise to both painful and nonpainful sensations that influence feeding and illness behaviors 7.
The ease of use and safety of local anesthetics is well recognized, and collectively they serve as one of the most important classes of drugs in perioperative care. The main advantage of local anesthetic agents is that they do not have the adverse effects of systemically administered opioids, such as postoperative sedation, nausea, gastrointestinal paralysis, and respiratory suppression, and they act directly on the tissue that they are applied to. Local anesthetics are commonly administered in abdominal surgery by skin infiltration or epidural administration, blocking somatic afferents and providing significant benefits in reducing postoperative pain, and improving recovery 8. It is also possible, however, to instill local anesthetic solutions into the peritoneal cavity, thereby blocking visceral afferent signaling, and potentially modifying visceral nociception and downstream illness responses. Local anesthetics applied to the peritoneal cavity have been used as “field blocks” from as early as 1950 9-11. Tubal ligation has been performed effectively and safely under abdominal wall and intraperitoneal local anesthetic (IPLA) alone 12. Peripheral techniques of using local anesthetic also seem to be gaining popularity 8. However, the practice of IPLA administration is not routine in modern-day laparoscopic cholecystectomy (LC).
The aim of this review was to systematically evaluate the literature on the use of IPLA in LC, focusing on pain and metabolic outcomes as clinical measures of the effectiveness of the reduction of visceral nociception by this modality.
Methods
All aspects of the QUOROM (Quality of Reporting of Meta-analyses) statement were followed 13.
Search strategy
A comprehensive database search was carried out independently by the first two authors (A.K., T.S.). The following databases were searched from inception to September 2009: Medline, Cumulative Index to Nursing and Allied Health Literature (CINHAL), Pubmed, Cochrane Controlled Trials Register, and Excerpta Medica Database (EMBASE). Truncated ($) search terms were used. The search terms used were: “local anaesth$”, “local anesth$”, “nerve conduct$”, “ropivicaine”, “bupivacaine”, “lidocaine”, lignocaine”, “procaine”, “intra-abdominal$”, “intraperitoneal”, “visceral”, “laparosc$”, “cholecystectomy”, “peritoneum”, “coeliosco$”, “surgery”. Language limitation was not used.
Additional articles or abstracts were retrieved by hyperlinks and by manually scrutinizing the reference lists of relevant publications. Animal trials were excluded. Academic meeting proceedings were also searched by the second author. Publications in languages other than English were translated into English with the help of academics at the Faculty of Medical and Health Sciences at the University of Auckland. Authors were contacted for additional data when required.
Study selection
Publications were selected for the review if they investigated, by double-blinded randomization, the effects of IPLA (treatment) administered pre-, intra-, or postoperatively, versus control, on pain outcomes in adults undergoing LC. Exclusion criteria included trials using this method in open cholecystectomy, or evaluating the use of preperitoneal or abdominal wall (incisional) local anesthetic use, unless IPLA use was solely being investigated in a controlled comparison group. A.K. and T.S. independently examined all retrieved articles, and any disagreement over inclusion or exclusion was discussed with the senior author (A.G.H.) and a consensus reached. The methodological quality of randomized controlled trials (RCTs) was assessed using the previously published criteria of Jadad et al. 14.
Data extraction
Data abstraction was performed by A.K. and T.S. independently. The primary outcomes used for metaanalysis were pain scores measured on the visual analogue scale (VAS, 0–100 mm or 0–10 cm) at rest, opioid analgesia requirement, rescue analgesia requirement, and postoperative cortisol and glucose as markers of neuroendocrine response to surgery. If additional modalities were tested in the treatment arms, such as additional agents added to the IPLA, intraabdominal gas drains, or incisional local anesthetic without control, then these groups were excluded from the metaanalysis.
Quantitative data synthesis was performed and entered into tables based on the following postoperative time points for pain: 1, 2, 4, 6, 8, and 24 h. The median score was used as an estimate of the mean where the latter was not reported. Standard deviations were obtained from the corresponding author if not published, or they were calculated based on the methods described in the Cochrane hand book 15. If data were insufficient after the above efforts, the study was excluded based on missing information and the reason provided. Total opioid analgesia requirement within 24 h was extracted from the reports where possible and converted into morphine equivalence [16, 17]. Nonsteroidal analgesia was not converted into morphine equivalence and was not included in the metaanalysis. The percentage of patients needing additional or ‘rescue’ opioid analgesia was also used as an outcome variable for metaanalysis if this was reported. Neuroendocrine measures were similarly obtained and categorized based on time interval postoperatively and converted into SI units before being used for metaanalysis.
Statistical analysis
Metaanalysis was performed using Review Manager Version 5.0 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008). A random-effects model was favored to allow for variation of normal distribution between studies 18. Results of the metaanalysis were assessed by graphical presentations of mean or standardized mean difference according to the outcome and 95% confidence intervals represented on forest plots with P < 0.050 considered statistically significant.
Heterogeneity between studies was assessed by three methods. First, publication bias was tested using the funnel plot graphical exploration method 15. Secondly a χ2 (chisquare) test for statistical heterogeneity was performed and P < 0.100 was considered statistically significant for this. I2 statistics were used to assess clinical heterogeneity 19. If statistical heterogeneity existed, sensitivity analysis was performed to detect small study effect by comparison of the fixed and random effects estimates of the intervention, as detailed in the Cochrane handbook for systematic review of interventions 15. Finally, in the case of moderate or high clinical heterogeneity (defined as I2 ≥ 50%) methodological subgroup analysis was performed 19 with Jadad 4 and 5 rated trials grouped together as “high quality” and all other trials grouped as “low quality” to further investigate the cause of heterogeneity. Study weight was assessed by sample size.
Results
Study characteristics
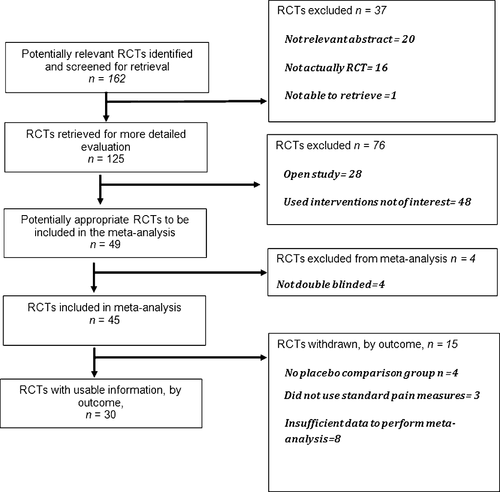
The QUOROM 13 trial flow diagram for systematic review is presented in Fig. 1. The characteristics of all 30 trials included, and 19 trials excluded, in this review are presented in Tables 1 and 2, respectively. Reasons for exclusion were inadequate information regarding blinding 20-23, inability to obtain adequate data to perform metaanalysis 24-31, use of additional nonanesthetic intraperitoneal agents 32-35, and inadequate outcome measures 36-38. The local anesthetic agents in the included studies were bupivacaine in 21 trials 39-59, ropivacaine in five trials [57, 60–63], lignocaine in four trials [41, 58, 64, 65], and levobupivacaine in three trials 66-68.
| First author, year, reference | Trial method, procedure, N (intervention/control groups) | Timing of LA use with respect to dissection, method of delivery | Agent used, comparison group | Pain outcome, analgesia use | Bowel outcome, PONV | Authors' conclusion, other relevant findings | Jadad score Used for meta-analysis? |
|---|---|---|---|---|---|---|---|
| Chundrigar 1993 39 | RCT, LC 28/30 | Post, into the region of the GB bed | 20 ml of 0.25% bupiv, NS | Reduced pain, no difference in analgesia use | NA | Safe, easy to administer and may become routine practice | 3 Used for pain |
| Pasqualucci 1994 40 | RCT, LC 14/14/14 | Post, both, surface of liver, right subdiaphragmatic space, deperitonalized area | 20 ml 0.5% bupiv with adren, NS | Decreased pain, decreased ketorolac use | Reduced PONV | Safe, easy to perform. Reduced pain and NSAID use, reduced endocrine response (cortisol). Reduced resp rate. Could become method of choice | 4 Used for pain & cortsiol response |
| Rademaker 1994 41 | RCT, LC 15/15/15 | Post, right subdiaphragmatic area | 20 ml 0.5% lignocaine, 20 ml 0.25% bupiv, NS | No difference in pain, no difference in nicomorphine use | NA | No difference in pain or metabolic measures (glucose, cortisol) indicating inadequate pain relief. Serum levels measured, peaked 15 min, No difference in resp outcomes | 2 Used for pain analgesia, glucose, cortiso |
| Joris 1995 42 | RCT, LC 20/20 | Post, right subdiaphragmatic area | 80 ml 0.125% bupiv with adren, NS | No significant difference, no difference in metamizol, paracetamol and piritramide | NA | IP Bupiv is not effective at reducing visceral pain | 2 Used for pain and number needing rescue analgesia |
| Raetzell 1995 43 | RCT, LC 22/10/22 | Post, subphrenic and GB bed | 50 ml 0.125% bupiv, 50 ml 0.25% bupiv, NS | No difference, no difference in piritramid use | No difference in PONV | Not recommended as reduced vital capacity occurred in 0.25% LA group. Serum levels reached peak within 5–30 min. One patient exceeded toxic limit but no clinical effects | 2 Used for pain and analgesia |
| Fuhrer 1996 44 | RCT, LC 12/10 | Post, subdiaphragmatic and GB wound | 0.6 ml/kg 0.375% bupiv, NS | No significant difference, no difference in morphine | NA | Not effective and may be harmful as toxic level was reached in one patient. Serum levels peaked at 10–30 min | 3 Used for pain and analgesia |
| Pasqualucci 1996 45 | RCT, LC 30/30/30/30 | Pre, post, both, upper surface of liver, subdiaphragmatic space, deperitonalized zone | 20 ml 0.5% bupiv with adren, NS | Reduced pain in LA groups but more so in preemptive group, reduced ketorolac use | Reduced PONV | Reduced physiological, glucose and cortisol response, reduced pain, reduced respiratory rate. Timing important | 3 Used for pain, analgesia use, and cortisol |
| Szem 1996 46 | RCT, LC 26/29 | Pre, under right hemidiaphragm, over Glisson's capsule, subhepatic space, serosa of GB | 100 ml 0.1% bupiv, NS | Reduced early pain, no difference in morphine equivalence use | No difference PONV | Detectable difference albeit small in pain reduction | 3 Used for overall pain and analgesia |
| Mraovic 1997 47 | RCT, LC 40/40 | Both, hepato-diaphragmatic space, near and above the hepato-duodenal ligament, above GB, hepatodiaphragmatic space and GB bed | 30 ml 0.5% bupiv, NS | Reduced pain, reduced metamizol consumption | NA | Recommended as a safe method to reduce pain from surgical manipulation and second dose for visceral pain | 4 Used for pain |
| Tsimoyiannis 1998 48 | RCT, LC 50/50/50/50/50/50 | Post, infused under right hemidiaphragm at the end of case | 1.5 mg/kg bupiv ± NS wash ±drain, controls | Reduced abdo and shoulder tip pain, reduced analgesia use (Paracetamol/Codeine suppositories and parenteral pethidine) | Reduced PONV in treatment arms with suction drains and IP LA | Recommend using saline wash and using IP bupiv when a drain is not necessary to reduce postop pain | 3 Used for pain and number needing rescue analgesia |
| Elfberg 2000 49 | RCT, LC 32/32 | Post, in GB bed | 2 mg/kg bupiv solution, NS | No difference in pain scores or analgesia use | NA | No change in pain or resp function. Safe serum levels measured at 1 and 4 h | 3, Used for pain |
| Elhakim 2000 64 | RCT, LC 25/25 | Post, under right diaphragmatic area | 200 ml 0.1% lignocaine, NS | Reduced abdo pain, reduced pain on deep inspiration, reduced nalbuphine use | No difference in PONV | Improved pain. No difference in respiratory function and no clinical adverse events. Safe | 2 Used for pain and analgesia use |
| Gupta 2002 60 | RCT, LC 20/20 | Post, IP catheter placed in GB bed | 20 ml 0.5% ropiv then intermittent 10 ml injections via IP catheter q1 h as needed for 20 h, NS | Reduced early deep pain and cough pain. No difference in ketobemidone use | No difference in PONV | Reduced early postop deep and cough pain. Serum levels decreased from 30 min onwards and no infections related to IP catheter | 5 Used for pain and number needed rescue analgesia |
| Jiranantarat 2002 50 | RCT, LC 39/41 | Post, right subdiaphragmatic area, GB bed, HD ligament | 20 ml 0.5% bupiv, NS | No difference in pain, analgesia use | NA | No difference in groups | 2 Used for pain and analgesia use |
| Labaille 2002 61 | RCT, LC 14/11/13 | Both, hepatodiaphragmatic space, gallbladder and area between liver and kidney | 40 ml 0.25% ropiv, 40 ml 0.75% ropiv, NS | Reduced abdo pain, reduced morphine use | No difference in PONV | 100 mg IP ropiv recommended as routine technique for pain relief. Safe. Max serum levels at 20–40 min | 3 Used for pain and analgesia |
| Maestroni 2002 62 | RCT, LC 30/30 | Pre pneumo via Veress needle then wait 10 min | 5 mg/kg ropiv in 200 ml NS, NS | Reduced pain up to 8 h, no difference in analgesia consumption | NA | Reduced plasma cortisol levels, safe. Preemptive analgesia, works best in order to prevent central sensitization to pain. No difference in resp rate | 3 Used for pain, number needing rescue and cortisol |
| Hernandez-Palazon 2003 51 | RCT, LC 30/30/30 | Post, sprayed on diaphragem, GB bed and right subhepatic space | 30 ml 0.25% bupiv ±2 mg morphine, NS | Reduced early abdo and incision pain, reduced metamizol use | No difference in PONV | Reduced pain and reduced metamizol requirement. IP morphine had no benefits | 3 Used for pain |
| Lepner 2003 65 | RCT, LC 20/20/20/20 | Post, right subdiaphragm and incisional | 200 ml 1.5% ligno + incisional bupiv, incisional NS, control | No significant pain benefit by adding IP ligno, no difference in pethidine use | No difference in PONV | Incisional infiltration best for pain relief, use of IP LA deserves further study | 5 Used for pain and number needing rescue |
| Ng 2004 66 | RCT, LC 23/24 | Post, injected into GB bed and peritoneal cavity | 30 ml 0.25% levobupivacaine with adren, NS with adren | Reduced inspiration pain, no difference in analgesia usage | No difference in PONV | Reduced pain on inspiration. This has continued to be routine practice by study surgeons | 5 Used for number needing rescue analgesia |
| Jabbour Khoury 2005 52 | RCT, LC 20/20/20/20 | Post, sprayed to subdiaphragmatic and GB area | 40 ml 0.25% bupiv, 40 ml 0.25% bupiv + 200 mg IV or IP ketoprofen, control | Reduced abdo pain and STP in all treatment groups, reduced ketoprofen only in IP bupiv + IV ketoprofen group | POV reduced only in IP bupiv + IV ketoprofen group | Reduced pain in IP LA group but this is improved with IV NSAID at end of procedure due to synergism. Safe and cheap | 3 Used for pain and number needing rescue |
| Louizos 2005 67 | RCT, LC 26/28/25 | Post, onto GB bed | 20 ml 0.25% levobupivacaine ± trocar site infiltration, NS | Reduced shoulder pain, educed dextropropoxyphene use | NA | Safe and effective in reducing pain, but most effective when combined with trocar site infiltration | 3 Used for pain and number needing rescue |
| Barczynski 2006 53 | RCT, LC 30/30/30/30 | Pre and post pneumoperitoneum (pre-dissection), sprayed in the direction of the liver and GB | 2 mg/kg bupiv in 200 ml NS, NS | Reduced pain in LA group but effect bigger in prepneumo group with elimination of shoulder tip pain. Reduced ketoprofen use | NA | Administering IP LA prior to creation of the pneumoperitoneum signifies greater benefit compared to administering after pneumoperitoneum | 4 Used for pain mean and number needing rescue |
| Karaaslan 2006 54 | RCT, LC 16/18/16/15 | Pre-pneumo, pre and post dissection, injected by needle in right subcostal region, or subhepatic area by vision | 20 ml 0.5% bupiv after intubation, after pneumoperitoneum or at end, control | Reduced pain and diclofenac consumption in group receiving LA after pneumoperitoneum, pre dissection | NA | Safe, instillation before dissection has most benefit | 3 Used for pain |
| Alkhamesi 2007 55 | RCT, LC 20/20/20/20 | Post, aerosolized to cover entire peritoneal cavity or injected to cover GB bed | 10 ml 0.5% aerosolized bupiv, 10 ml 0.5% bupiv wash, NS, control | Reduced pain and morphine consumption in the aerosolized group | Reduced vomiting in nebulized group | Quicker mobility, greatly reduced morphine use and pain. Aerosolized LA should be advocated | 2 Used for pain and analgesia use |
| Garcia 2007 56 | RCT, LC 19/13 | Post, into GB bed and suprahepatic region | 80 ml 0.125% S75-R25 bupivacaine, NS | Reduced pain at 12 h, no significant difference in tramadol use | No difference in PONV | Reduced pain but larger studies needed to show statistical significance in other measures using this new mix of LA | 4 Used for pain |
| Kucuk 2007 57 | RCT, LC 20/20/20/20 | Post, subdiaphragmatic and GB bed | 21 ml 0.5% bupiv with adren, 21 ml 0.5% ropiv with adren, 21 ml 0.75% ropiv with adren, NS | Reduced pain in all 3 LA groups, reduced morphine use, mostly in 0.75% ropiv group | No difference in PONV | 150 mg ropiv more effective than smaller doses of LA, no difference in respiration rate | 2 Used for pain and analgesia |
| Ahmed 2008 58 | RCT, LC 50/50/50/50 | Post, diaphragmatic space and GB fossa | 20 ml 0.5% bupiv, 20 ml 2% lignocaine, 20 ml, NS, control | Reduced abdominal pain, shoulder pain, reduced meperidine and diclofenac suppository use | No difference in PONV | Safe, reduces pain and analgesia use. Studies needed to identify optimal timing of application. Reduced respiratory rate in LA groups | 4 Used for pain and number needing rescue |
| Pappas-Gogos 2008 63 | RCT, LC 20/20/20/20/20/20 | Pre, post, right subdiaphragmatic | 40 ml 0.2% ropiv combined with or without 30 ml/kg NS, and a drain in various timings, control | Reduced pain and Paracetamol/Codeine suppositories and ketoprofen use in groups receiving LA at beginning | No difference in PONV | IP LA at the beginning and NS wash at the end of the procedure had most impressive pain relief and reduction of analgesic use | 4 Used for pain |
| Papadima 2009 68 | RCT, 36/35 | Post, silastic drain tube placed in the left subdiaphragm and removed 8 h post op | 10 ml 0.5% levobupivacaine and repeated at 8 h, NS | Reduced pain, reduced fentanyl and meperidine use and reduced number needing rescue analgesia on the ward | NA | Safe method to reduce pain and need for opioid use | 5, Used for pain, analgesia and number needing rescue |
| Golubovic 2009 59 | RCT 30/30 | Post, hepatodiaphragmatic ligament, above hepatoduodenal ligament and above the GB bed | 50 ml 0.25% bupiv, NS | Reduced pain, reduced pethidine use | NA | Reduced pain and need for opioid use | 3, Used for pain and number needing rescue |
- RCT randomized controlled trial, IP intraperitoneal, LC laparoscopic cholecystectomy, NS normal saline, PONV postoperative nausea and vomiting, LA local anesthetic, IM intramuscular, GB gallbladder, IV intravenous, NA not applicable, S75-R25 50% enantiomeric excess bupivacaine, NSAID nonsteroidal anti-inflammatory drug, HD hepatoduodenal ligament, ligno lignocaine, bupiv bupivacaine, ropiv ropivacaine, adren adrenaline, abdo abdominal, resp respiratory, postop postoperative, pre-pneumo pre-pneumoperitoneum, STP shoulder tip pain
| First author, year, reference | Trial method, procedure, N (intervention/control groups) | Timing of LA use with respect to dissection, method of delivery | Agent used, comparison group | Pain outcome, analgesia use | Bowel outcome, PONV | Authors' conclusion, other relevant findings | Jadad score, reason for exclusion |
|---|---|---|---|---|---|---|---|
| Goegler 1993 21 | RCT, LC 20/20 | Post, subphrenic suprahepatic catheter | 10 ml 0.25% bupiv and 10 ml 1% prilocaine, controls | Reduced pain within 6 h. Analgesia use not reported | NA | Easy to perform method. Faster recovery and vigilance. No changes in pulmonary function | 1 No mention of double blinding |
| Thiry 1994 30 | RCT, LC 20/20 | Post, instilled under the right diaphragm | 80 ml 0.125%bupiv + adren, NS | No difference in pain over 24 h, no difference in dipyrone/paracetamol use | NA | Visceral pain dominates and bupiv did not attenuate this | 2 Data not sufficient |
| Hafez 1995 22 | RCT, LC 15/15/15 | Pre (insufflation), Intraperitoneal | 40 ml 1% lignocaine, IV fentanyl, control | Reduced pain, NA | NA | Reduced plasma cortisol levels, reduced glucose up to 120 min. Serum levels measured and peaked at 15 min | 1 No mention of double blinding |
| Scheinin 1995 37 | RCT, LC 20/20/20 | Post, right subphrenic space | 100 ml 0.15% bupiv with and without adrenaline, NS | No difference, in pain or ketoprofen use | NA | Serum levels lower in adren group, no difference in pain or NSAID use | 2 Pain scores not reported using VAS |
| Schulte-Steinberg 1995 35 | RCT, LC 15/18/17 | Post, onto GB bed and subphrenic surface of liver | 20 ml 0.25% bupiv, 1 mg IP morphine, NS | No significant reduction in pain or analgesic use | NA | Lack of analgesia likely due to small dose and rapid dilution | 3 Comparison arm was IP morphine |
| Fornari 1996 24 | RCT, LC 25/25 | Postop, Right subdiaphragmatic space | 60 ml 0.167% bupiv, NS | No difference in pain or analgesic use | NA | No difference seen but larger number of patients needed in future trials | 2 Unable to obtain report |
| Kilic 1996 36 | RCT, LC 20/20 | Post, subdiaphragmatic area | 80 ml 0.125% bupiv, NS | Reduced scapular pain, no difference in analgesia | No difference in time to flatus or rate of PONV | Reduced postop pain, especially scapular pain | 2 Pain scores not reported using VAS |
| Wulf 1998 38 | RCT, LC 18/20/21 | Post, directly on the surface of the liver | 50 ml 0.125% bupiv, 50 ml 0.25% bupiv, NS | NA | NA | No difference in hepatic enzymes postop. IP bupiv is not associated with cholestasis | 3, Did not measure pain outcomes |
| Bisgaard 1999 32 | RCT, LC 25/25 | Both, hepatoduodenal lig infiltrated, GB bed, upper liver and subdiaphragm, right subhepatic space | 38 ml 0.2% ropiv, NS | Reduced, reduced morphine use | Reduced nausea | Reduced pain, nausea, and opioid use after somatovisceral regimen used | 4 Experimental group also received incisional LA with no control for IP LA alone |
| Elhakim 2000 33 | RCT, LC 30/30/30 | Post, under right diaphragm and GB bed | 200 ml 0.1% lignocaine + IP tenoxicam, 200 ml. 1% lignocaine +IV tenoxicam, NS | Reduced pain in both IP groups, reduced nalbuphine use in tenox IP group | No difference in PONV, faster return of bowel function in IP tenox group | Adding tenoxicam to IP lignocane reduces pain further when compared to IP ligno and IV tenoxicam. No side effects | 4 Experimental group also given IP tenoxicam |
| Gharaibeh 2000 20 | RCT, LC 37/38 | Post, GB bed and Calot's triangle area | 10 ml 0.25% bupiv, control | Reduced shoulder pain, no report on analgesia use | NA | Safe. Reduced shoulder pain effectively | 1 No mention of double blinding |
| Moreira 2000 28 | RCT, LC 20/20 | Both, liver and GB surface | 20 ml 0.5% bupiv + adren, NS | No difference in pain or analgesia use | NA | This study has not shown benefits from the technique used | 2 Data not sufficient |
| Zmora 2000 31 | RCT, LC 26/25 | Post, right subphrenic space and GB bed | 50 ml 0.2% bupiv, NS | No difference in pain or meperidine use | NA | No benefits seen in this study, more studies needed to establish the role of IP bupivacaine | 4 Data not sufficient |
| Lee 2001 27 | RCT, LC 20/21/25 | Pre, post, upper surface of liver, and subdiaphragmatic | 40 ml 0.25% bupiv with adren, control | No difference, no difference | No difference in PONV | Somatic pain contributes more than visceral pain | 3 Data not sufficient |
| Karadeniz 2003 26 | RCT, LC 15/15/15/15 | Pre, post, as a bolus over GB region in 2 arms, postop infusion via a catheter in one arm for 4h | 20 ml 0.5% bupiv, postop infusion for 4 h in one arm, NS | Reduced pain and meperidine use in infusion group and preemptive group | No difference in PONV | IPLA recommended for postop pain | 3 Data not sufficient |
| Paulson 2003 29 | RCT, LC 18/15/19/14 | Pre, post, both, over GB fossa and over liver in the subdiaphragmatic area, perihepatic area before closing | 15 ml 0.5% bupiv, NS | Reduced pain in LA group, NA | NA | Increased same day discharge, safe, cheap, and feasible. Data combined in pre/post group so unable to comment on difference | 3 Did not use VAS for pain and data not sufficient |
| Hazinedaroglu 2006 25 | RCT, LC 25/25 | Post, surface of liver, GB bed, subdiaphragmatic, HD ligament | 30 ml 0.25% bupiv, NS | No difference in pain, analgesia use | No difference in PONV | No difference in pain seen, perhaps due to low IP tissue penetrance of LA | 3 Data not sufficient |
| Sozbilen 2007 23 | RCT, LC 14/17/14 | Pre, post, onto GB and subdiaphragmatic and GB bed | 40 ml 0.375% ropiv + incisional ropiv pre or post dissection, no control group | No significant advantage in pain score based on timing of LA, no difference in IM pethidine use | No difference in PONV | No control or placebo group to compare with. Timing was not significant. Preemptive group had higher levels of adrenaline | 1 No mention of blinding |
| Maharajan 2009 34 | RCT, LC 20/20 | Post, intraperitoneal and incisional sites | 40 ml 0.25% bupivacaine + 20 ml 2.25% bupivacaine incisional site | Fewer patients had severe pain and reduced need for rescue analgesia | NA | Reduced severity of postoperative pain and reduced analgesic requirement | 2, treatment group also received incisional LA |
- RCT randomized controlled trial, IP intraperitoneal, LC laparoscopic cholecystectomy, NS normal saline, PONV postoperative nausea and vomiting, LA local anesthetic, IM intramuscular, GB gallbladder, TV intravenous, NA not applicable, VAS visual analogue scale, tenox tenoxicam
Results of the overall metaanalysis are outlined in Table 3. A number of agent and dose combinations were studied at various timings in relation to dissection. Many methods for the intraperitoneal use of local anesthetic were described, most commonly direct application of the solution to the gallbladder bed, liver, and subdiaphragmatic spaces. Use of intraperitoneal catheters [60, 68] was reported in 2 trials and these were used to infiltrate the intraperitoneal cavity postoperatively for attempted prolonged analgesia.
| Outcome | No. of patients (IPLA/Control) | No. of studies | SMD (95% CI) | P value | χ2 | P value | I2 (%) |
|---|---|---|---|---|---|---|---|
| Overall results | |||||||
| VAS pain (post dissection instillation) | 495/487 | 20 | –0.77 [−0.98, −0.57] | <0.00001 | 855.30 | <0.00001 | 90 |
| VAS pain (pre-dissection instillation) | 124/124 | 5 | –1.36 [−1.94, −0.77) | <0.00001 | 168.08 | <0.00001 | 92 |
| VAS pain (pre and post dissection instillation) | 85/83 | 4 | –1.16 (−1.85, −0.46) | 0.001 | 139.78 | <0.00001 | 91 |
| Opioid use (up to 24 h post operation) | 340/335 | 14 | –1.13 (−1.83, −0.44) | 0.001 | 233.45 | <0.00001 | 94 |
| Number needing rescue analgesia | 396/392 | 13 | 0.28 (0.13, 0.60)a | 0.001 | 42.31 | <0.0001 | 72 |
| Post operation cortisol | 100/98 | 5 | –0.64 (−0.98, −0.30) | 0.0002 | 5.38 | 0.25 | 26 |
| Post operation glucose | 58/57 | 3 | –0.69 (−1.34, −0.05) | 0.03 | 5.45 | 0.07 | 63 |
| Jadad quality score ≥4 | |||||||
| VAS pain (post dissection instillation) | 142/137 | 6 | –0.43 (−0.56, −0.30) | <0.00001 | 193.43 | <0.00001 | 88 |
| VAS pain (pre-dissection instillation) | 50/50 | 2 | –1.08 (−1.51, −0.65) | <0.00001 | 20.45 | 0.002 | 71 |
| VAS pain (pre and post dissection instillation) | 54/51 | 2 | –1.24 (−1.85, −0.63) | <0.00001 | 27.31 | <0.0001 | 82 |
| Opioid use (up to 24 h post operation) | 84/77 | 3 | –2.38 (−4.09, −0.66) | 0.007 | 47.23 | <0.0001 | 94 |
| Number needing rescue analgesia | 176/177 | 6 | 0.39 (0.12, 1.26)a | 0.11 | 30.53 | <0.0001 | 84 |
| Post operation cortisol | NA | NA | NA | NA | NA | NA | NA |
| Post operation glucose | NA | NA | NA | NA | NA | NA | NA |
- SMD standard mean difference (less than 0 favors the local anesthetic group), IPLA intraperitoneal local anesthetic, VAS visual analogue scale
- a Odds ratio: less than 1 favors local anesthetic group
Safety
There were no reports of any adverse events following instillation of local anesthetic to the intraperitoneal cavity; however, serum bupivacaine reached potentially toxic concentrations (as reported by the original study authors) in two patients [43, 44] after instillation of 125 mg and 2.25 mg/kg into the peritoneum, respectively. These serum concentrations were lower than the range at which central nervous and cardiovascular system toxicity have been observed 69.
Pain results
Twenty-one trials investigated post-dissection instillation [40, 42-44, 48-52, 54-60, 63-65, 67, 68] in 1053 patients. There was a reduction in pain scores with the use of IPLA at each time point, except at 8 h postoperatively, according to metaanalysis [overall standard mean difference; SMD −0.77 (−0.98, −0.57); P < 0.00001] (Fig. 2). The funnel plot was symmetrical (results not shown); however, heterogeneity was high (χ2 = 855.30, I2 = 90%). The results of meta-analysis were similar, except that the 8 h time point became significant when a fixed-effects, rather than a random-effects, model was used and methodology subgroup analysis did not alleviate high heterogeneity (Table 3).
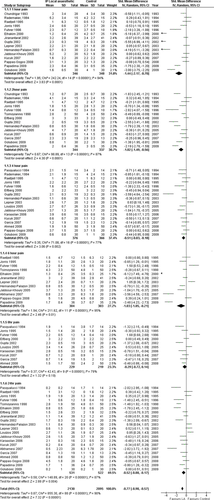
Five trials investigated predissection instillation [26, 46, 53, 62, 63] in 248 patients. There was a reduction in pain scores with the use of IPLA at all time points except 6 and 24 h postoperatively (overall SMD, −1.36 [−1.94, −0.77]; P < 0.00001) (Fig. 3). The funnel plot of these five trials was not symmetrical, indicating the probable existence of publication bias. Heterogeneity was high (χ2 = 168.08, I2 = 92%) and the overall result of metaanalysis was similar, the 24 h time point became significant when a fixed-effects, rather than a random- effects, model was used. Methodology subgroup analysis did not alleviate high heterogeneity (Table 3).
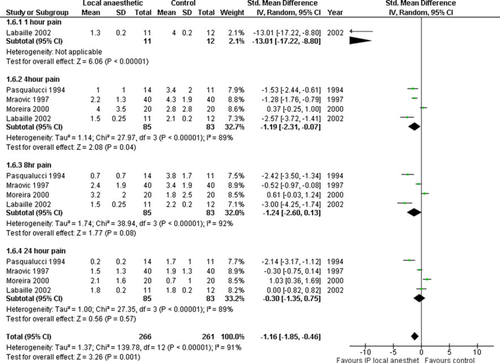
Four trials investigated pre- and post-dissection IPLA instillation [28, 40, 47, 61] in 168 patients. There was reduction of pain scores with IPLA at 1 and 4 h time points (overall SMD, −1.16 [−1.85, −0.46]; P < 0.00001) (Fig. 4). Heterogeneity was high (χ2 = 139.78, I2 = 91%) and the result of overall metaanalysis was similar. However, the 8 h time point became significant when a fixed-effects, rather than a random-effects, model was used and methodology subgroup analysis did not alleviate this (Table 3). The funnel plot was symmetrical (results not shown).
Postoperative opioid use
Fourteen trials reported on postoperative opioid use in 675 patients [36, 40, 41, 43-46, 50, 55, 57, 61, 63, 64, 68]. There was a reduction of 24 h total opioid use with IPLA postoperatively according to metaanalysis (overall SMD −1.13 [−1.83, −0.44]; P = 0.001) (Fig. 5). The funnel plot was symmetrical (results not shown), but heterogeneity was high (χ2 = 233.45, I2 = 94%). The results of metaanalysis were similar when a fixed-effects, rather than a random-effects, model was used and methodology subgroup analysis did not alleviate high heterogeneity (Table 3).
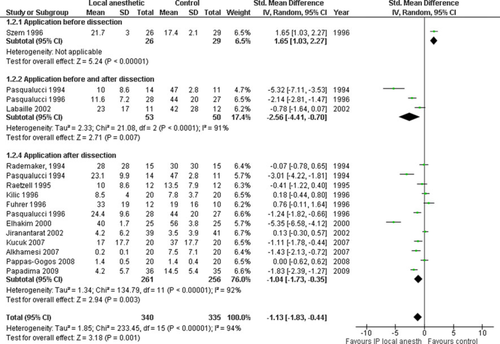
Need for rescue analgesia
Twelve trials reported need for rescue analgesia in 788 patients [48, 52-54, 58-60, 62, 65-68]. Metaanalysis showed a reduced need for rescue analgesia with IPLA (overall odds ratio [OR], 0.28 [0.13, 0.60]; P = 0.001) (Fig. 6). Heterogeneity was moderate (χ2 = 233.45, I2 = 72%). The results of metaanalysis were similar when a fixed-effects, rather than a random-effects, model was used and methodology subgroup analysis showed no benefits from IPLA; however, heterogeneity was actually higher when this was undertaken (Table 3). The funnel plot was symmetrical (results not shown).
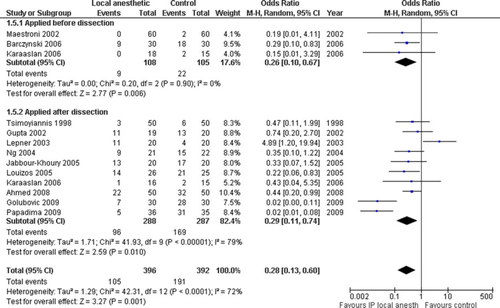
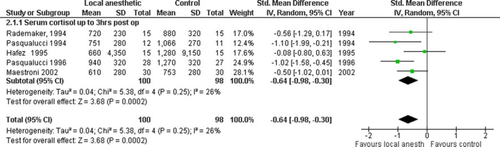
Metabolic outcomes
Five trials investigated postoperative cortisol response, up to 3 h, in 198 patients [22, 40, 41, 45, 62]. Metaanalysis revealed a blunted response to surgery in the IPLA arm (−0.64 [−0.98, −0.30]; P = 0.0002) (Fig. 7). Heterogeneity was low (χ2 = 5.38, I2 = 26%) (Table 3). The funnel plot was symmetrical (results not shown).
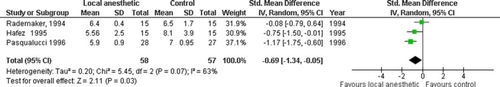
Three trials investigated postoperative glucose response, up to 3 h, in 115 patients [22, 41, 45]. Metaanalysis revealed a blunted response to surgery in the IPLA arm (−0.69 [−1.34, −0.05]; P = 0.03) (Fig. 8). Heterogeneity was moderate (χ2 = 5.45, I2 = 63%) (Table 3) and methodology subgroup analysis could not be performed.
Discussion
In this systematic review we identified 30 randomized double-blinded trials which reported the use of IPLA in LC. Metaanalysis of these trials revealed an overall reduction of pain, opioid analgesia use, need for rescue analgesia, postoperative cortisol, and glucose response. There was no report of adverse effects in any patients, although potentially toxic serum levels were reached in two patients, with no subsequent consequences. This review confirms that pain arising from the parietal and visceral peritoneum can be controlled and IPLA may be used as part of multimodal, balanced analgesia, after LC. This approach should be particularly useful in the increasing ambulatory setting that this operation is performed in.
Cervero and Laird argue that although visceral nociception is not easily excitable in health, there is sensitization in inflammation; for example, after visceral surgery 4. Afferent signaling may therefore be greater in magnitude and prolonged in duration after surgical insult 4. A proinflammatory cytokine cascade in the peritoneal cavity, with direct action on the visceral afferents and the vagus as a major vehicle, is a feasible contributor to postoperative visceral pain perception and the “sickness response” 70. By using IPLA it may be possible to modulate peritoneal and visceral signaling to the brain, thereby attenuating the metabolic impact of visceral surgery.
The mechanism of action of IPLA is not fully understood, although it is likely that there is a blockade of free afferent nerve endings in the peritoneum. Systemic absorption of local anesthetic from the peritoneal cavity may also play a part in reduced nociception. It is known that systemic levels of local anesthetic are detectable in the serum circulation as soon as 2 min after bolus instillation into the peritoneum 44, and a systematic review has recently confirmed that low-dose intravenous local anesthetic infusion is advantageous in patients having abdominal operations, with decreased pain and reduced opioid consumption noted 71. It is also known that local anesthetics have anti-inflammatory actions [72, 73]. Topical intraperitoneal lignocaine and bupivacaine have been shown to inhibit chemical peritonitis in an animal model 74. Intraperitoneal infusion of procaine has also been shown to reduce adhesion formation and density significantly in rabbits after abdominal surgery 75. Therefore, it is logical that IPLA should be used in addition to other perioperative pharmacological agents in visceral surgery. The underlying mechanism of these anti-inflammatory effects may be prostaglandin antagonism, inhibition of leukocyte migration, and lysosomal enzyme release, all effects seen in in vitro and animal studies 76-80.
In this systematic review we only included randomized double-blinded trials. The nature of clinical trials extending over 18 years that utilized IPLA meant that there was a variety of different local anesthetic agents and methods of administration utilized. Therefore, there is fundamental heterogeneity in such clinical studies that must be considered. Publication bias did not stand out. However, subgroup analysis of higher-quality studies did not reduce heterogeneity greatly (Table 3). This indicates that the heterogeneity between studies is most likely due to the various methods of IPLA administration, types of local anesthetic, duration of administration, and dosages which varied between studies. Intraoperative anesthetic and postoperative pain protocols also varied.
To reduce this level of heterogeneity we included double-blinded RCTs that reported VAS abdominal pain at rest when comparing only IPLA to controls. Hence, we excluded other modalities used, such as additional intraperitoneal drugs or gas suction drains. Studies were grouped based on the timing of IPLA application to the dissection. Standardization of postoperative analgesia by conversion of opioids into morphine equivalence was attempted. Numbers needing rescue analgesia were also analyzed separately and trials reporting the use of nonsteroidal anti-inflammatory drugs were not included in the metaanalysis.
Three reviews have previously been performed on the use of IPLA in LC 81-83. Boddy et al. 81 identified several factors that may influence the benefits of IPLA; namely, dose, concentration, timing, site, spillage of bile and blood, instillation in the head-down versus supine position, and volume of residual gas left in the abdomen. Gupta 82 concluded that pr-emptive intraperitoneal instillation combined with trocar site LA should be used.
Given the number of trials identified in the present study, there is probably no need for further study of IPLA in LC. Given the potential benefits demonstrated in this review there is evidence for the use of IPLA in LC. Furthermore, novel methods in the delivery of agents to the peritoneal cavity are emerging with the advent of a peritoneal nebulizer, and the use of elastomeric pumps to maintain a continuous infusion system for drug delivery 84-88. These modalities should perhaps be trialed in patients undergoing natural orifice surgery, as this field grows further, in order to study visceral nociception as a clinically relevant issue.
Conclusion
Various methods of IPLA in LC have been described. Based on a metaanalysis of 30 RCTs, there is benefit to be gained in terms of pain reduction, analgesia use, and blunting of endocrine response, after LC. Clinical heterogeneity was high due to the variation of IPLA protocols. We conclude that the use of IPLA is beneficial to reduce pain in LC, and further trials in this area are not needed. An effective and safe dose seems to be 20 ml of 0.5% bupivacaine applied to the dissection bed prior to pneumoperitoneum release.
Acknowledgments
This research was conducted during tenure of the Ruth Spencer fellowship from the Auckland Medical Research Foundation, held by Arman Kahokehr, and the Surgeon Scientist Scholarship from the Royal Australasian College of Surgeons, held by Tarik Sammour. The authors would like to thank Drs Emmanuelle Cognard, Elizaveta Sopina, and Irene Vorontsova for their valuable time and help with translation of articles. The authors would also like to thank the library team at Middlememore Hospital: Jill Unsworth, Joanna Martin, Patricia Hayward, Heather Steedman, and Vicki Chetham, for their efforts in retrieving articles.
Conflict of interest statement
None.




