Liver transplantation for hepatocellular carcinoma: the Hong Kong experience
Abstract
Orthotopic liver transplantation (OLT) is the best treatment option for selected patients with hepatocellular carcinoma (HCC) with the background of cirrhosis since this treatment modality can cure both diseases at once. Over the years, the applicability of OLT for HCC has evolved. In Asia, including Hong Kong, a shortage of deceased donor liver grafts is a universal problem having to be faced in all transplant centers. Living-donor liver transplant (LDLT) has therefore been developed to counteract organ shortage and the high prevalence of HCC. The application of LDLT for HCC is a complex process involving donor voluntarism, selection criteria for the recipient and justification with respect to long-term survival in comparison to the result of deceased donor liver transplant. This article reviews the authors’ experience with OLT for HCC patients in Hong Kong, with emphasis on the applicability and outcome of LDLT for HCC. Donor voluntarism has a significant impact on the application of LDLT. “Fast-track” LDLT in the setting of recurrence following curative treatment carries a high risk of recurrence even though the tumor stage fulfills the standard criteria. Although the survival outcome may be worse following LDLT than DDLT for HCC, LDLT is still the main treatment option for patients with transplantable HCC in Hong Kong, and a reasonable survival outcome can be achieved in selected patients with extended indications. It is particularly true that LDLT provides the only hope for patients with advanced HCC under the constricting problem of organ shortage.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common malignant tumor and the third leading cause of cancer-related death in the world [1, 2]. Orthotopic liver transplantation (OLT) is the best treatment option for selected patients with HCC with the background of cirrhosis, since this treatment modality can cure both diseases at once. In fact, the applicability of OLT for HCC has evolved over the past decades. The early results of OLT for HCC in the late 1980s were disappointing, with high tumor recurrence and poor 5-year survival (less than 40%) [3-5]. Such failure was mainly due to the poor patient selection with advanced tumor status. These unsatisfactory initial results of OLT for clinically advanced HCC had suggested that both microscopic and macroscopic vascular invasion by HCC was the most important poor prognostic factor. To achieve acceptable patient outcome after OLT for HCC, stringent criteria were necessary for patient selection, using tumor size and the number of tumors as the surrogate parameters for the likelihood of vascular invasion. In 1996, Mazzaferro et al. 6 introduced the Milan criteria (solitary tumor not exceeding 5 cm or no more than three tumors, each measuring less than 3 cm) based on a retrospective study of 48 patients who had undergone OLT for HCC. In that study, the 4-year overall and recurrence-free survival rates were 75 and 83%, respectively, if the tumor staging met the Milan criteria.
The clinical situation regarding the allocation of a deceased organ for patients with HCC is different between the Eastern and Western transplantation centers. Unlike the Western countries, the organ donation rate from deceased donors remains low in Asian countries, including Hong Kong (4.2 per million of population in 2005) 7. The critical shortage of deceased organ donation has posed a great obstacle in the applicability of deceased donor liver transplantation (DDLT) for HCC in Hong Kong. The mismatch between organ donation and high incidence of HCC necessitates the establishment of a strict organ allocation system. Patient priority on the waiting list is primarily determined by the severity of liver disease based on the model for end-stage liver disease (MELD) score 8. Unlike the organ allocation system in the US, which assigns an additional score to HCC patients, there is no prioritization of HCC patients for organ allocation in Hong Kong to avoid the fact that the limited number of deceased donor grafts would inevitably be given to the large number of HCC patients 7. Hence, tumor progression and eventually dropout of the listed HCC patients are inevitable with the prolonged waiting period for a deceased organ graft in our locality. With the advances of living-donor liver transplantation (LDLT) in Asia [9, 10], the role of liver transplantation for HCC patients has changed dramatically. LDLT can theoretically provide an unlimited source of liver grafts for patients with HCC that is within the selection criteria. The problem of prolonged waiting time on the list and the risk of drop-out can virtually be eliminated by LDLT. The unaffected donor pool of organs for patients with non-malignant liver disease would be another crucial advantage of LDLT since the living-donor graft is a dedicated gift directed exclusively to the recipient. In a survey up to the year 2005, 96% of liver transplants for HCC performed among Asian transplantation centers were from live donors 7. This article reviews the experience of OLT for HCC patients in Hong Kong, with emphasis on the applicability and outcome of LDLT for HCC.
Donor voluntarism in LDLT for HCC
Hypothetical decision analytic models have demonstrated the potential survival benefits of LDLT over DDLT for patients with HCC within the Milan criteria when the waiting time for a deceased liver graft exceeds 6 months [11, 12]. Nevertheless, the assumptions that a live donor for a HCC patient is readily available and that LDLT can be carried out within a short period of time are not always true. In fact, donor voluntarism is a major contributing factor to be considered when the survival outcome following LDLT for HCC is evaluated. This has been demonstrated by an intention-to-treat analysis of a cohort of 51 HCC patients undergoing LDLT and DDLT in the authors' center 13. More than half of the HCC patients did not have a volunteer live donor available (26 patients without vs. 25 patients with voluntary live donors). LDLT was successfully performed in 84% of patients who had voluntary live donors available. On the other hand, only 20% of patients who lacked a voluntary live donor received DDLT. The median waiting time for DDLT was 344 days, and the drop-out rate was up to 70% among the listed HCC patients without voluntary live donors. In contrast, the median waiting time for the live donor graft was only 24 days. The intention-to-treat survival of HCC patients with voluntary live donors was significantly higher than that of those without voluntary live donors (4-year survival: 66 vs. 31%). Hence, in the authors' experience, the option of LDLT has increased the overall applicability of liver transplantation for HCC from 12 to 53%, taking into account donor voluntarism and a vigilant donor evaluation process.
LDLT versus DDLT for HCC
Another important issue to justify the option of LDLT for HCC is the comparison between its survival outcome and that of DDLT for HCC. Roayaie et al. 14 found that there was a tendency for early tumor recurrence after LDLT (mean time: 8.7 months) compared with DDLT (mean time: 19.6 months) in a cohort of 311 patients with histologically confirmed HCC after OLT. Another multicenter LDLT cohort study (A2ALL) of 106 HCC patients from the US has reported a significantly higher 3-year tumor recurrence rate after LDLT (29%) compared with that after DDLT (0%) 15. In authors' center, a retrospective study was conducted comparing the outcome after LDLT with that after DDLT in 60 patients with HCC 16. Given the standard radiological selection criteria based on tumor size and number [University of California at San Francisco (UCSF) criteria] 17, there was an obvious selection bias for some important clinical characteristics in the LDLT group in that study. Patients undergoing LDLT for HCC had fewer incidental tumors, a lower rate of preoperative transarterial chemoembolization, a lower rate of salvage transplantation, shorter waiting time on the list and lower graft weight to standard liver weight ratio when compared to those receiving DDLT. These confounding risk factors led to the inferior oncological outcome in the LDLT group because of the possible aggressive biological tumor behavior and small-for-size graft injury and regeneration 18. Although the overall survival rates were comparable between the LDLT and DDLT groups, the cumulative 5-year recurrence rate was significantly higher in LDLT group than in the DDLT group (29 vs. 0%). Thus, selection of patients with early HCC based on standard tumor size-number criteria for LDLT and DDLT may eventually result in different clinical outcomes. Considering the unique nature of live donor graft as a dedicated gift to the recipient, additional clinical characteristics need to be included in the selection criteria for LDLT in order to justify the potential donor risk and the expected recipient outcome.
Extended criteria in LDLT for HCC
Rationale
Since the decision for LDLT for HCC is exclusively based on the balance between donor risks and recipient benefits, expansion beyond the standard criteria (Milan criteria 6 or UCSF criteria 17) to more advanced tumor staging in patient selection has been proposed in many transplantation centers. The Kyoto group has adopted the extended criteria that include any size or number of tumors provided there is no gross vascular involvement or distant metastasis. The reported 4-year overall patient survival was 64% in all HCC patients and 59% in patients whose tumors were beyond the Milan criteria 19. In a multicenter study from 49 transplantation centers in Japan on 316 HCC patients receiving LDLT, the Milan criteria were adopted in one-third of the transplantation programs. The overall survival and recurrence-free survival at 3 years were significantly worse when the Milan criteria were not met (60.4 vs. 78.7% and 52.6 vs. 79.1%). The preoperative alpha fetoprotein level, tumor size, vascular invasion and bilobar distribution were identified as independent risk factors for recurrence after LDLT 20. Similar results were also reported by Hwang et al. 21 based on four transplantation programs in Korea. The results of these studies confirm the predictive power of the Milan criteria on patient outcome following LDLT for HCC.
In recent years, new extended selection criteria for LDLT for HCC have been reported with encouraging results. The HangZhou group from mainland China proposed an extended criteria using tumor size, serum alpha fetoprotein (AFP) and tumor histopathologic grade (total tumor diameter ≤8 cm, or histopathologic grade I or II with preoperative AFP ≤400 ηg/ml if total tumor diameter >8 cm). There was no significant difference in survival outcome between patients fulfilling the Milan criteria and those exceeding them but fulfilling the HangZhou criteria 22. In another study by Lee et al. 23 from Korea, extended criteria of LDLT for HCC using more advanced tumor staging was analyzed (largest tumor ≤5 cm and number of tumor ≤6, and no gross vascular invasion). The overall 5-year patient survival rates were 76.3 and 18.9%, respectively, within and beyond this criteria, which had a higher discriminatory power than the Milan and UCSF criteria. With these satisfactory results following the extended criteria, the applicability of LDLT for advanced HCC is expected to be expanded. More clinical evidence is therefore necessary to justify the donor risks and recipient benefits in such clinical circumstances.
Hong Kong experience
We have retrospectively studied the outcome of patients with HCC undergoing LDLT at Queen Mary Hospital in Hong Kong. Patients with HCC were selected based on the preoperative radiological tumor staging. The tumor evaluation process consisted of computed tomography of the abdomen and thorax, and a radionucleotide bone scan. Positron emitting tomography using C 11 acetate was occasionally performed in selected patienta to exclude extrahepatic metastasis. Before 2002, the radiological Milan criteria were used. From 2002 till 2005, the selection criteria were expanded to match the radiological UCSF criteria. From 2006 onwards, selected patients with more advanced HCC were evaluated for LDLT according to the following exclusion criteria: (1) no evidence of gross vascular tumor invasion, (2) no evidence of distant metastases and (3) no evidence of diffuse HCC. The evaluation processes for both donor and recipient regarding the suitability for LDLT has been described previously [13, 16, 24]. The donor voluntarism was ensured before proceeding to the assessment of medical and surgical suitability. The psychological status of the voluntary donor was assessed by a dedicated clinical psychologist, and a separate interview was conducted in the absence of the recipient and their family. A suitable donor ought to satisfy the following criteria: (1) compatible ABO blood group, (2) negative serology for hepatitis B and C virus, (3) no evidence of any acute and chronic illness and (4) adequate CT volumetry of the liver graft for the recipient (40% of recipient standard liver weight) 25 and liver remnant for the donor (30% of total liver volume) 26.
From January 1997 to June 2007, 83 consecutive patients with HCC underwent LDLT. The diagnosis of HCC was confirmed by histological examination of the explanted liver. Preoperative radiological examination revealed 70 patients within and 13 patients beyond the Milan criteria, and 75 patients within and 8 patients beyond the UCSF criteria. Based on the pathological examination of the explanted liver, 50 patients and 33 patients had tumor staging fulfilling and beyond the Milan criteria, respectively. Clinical details and outcome of patients within and beyond the Milan criteria were compared. Table 1 shows the patients' clinical characteristics. There were no significant differences between the two groups in age, sex, proportion of patients with hepatitis B or C viral infection, blood group distribution and serum AFP level. The severity of liver disease was similar between the two groups in terms of Child-Pugh score and MELD score. A similar proportion of patients (14% in the within-Milan group and 15.2% in the beyond-Milan group) received LDLT as the salvage procedure for recurrence after curative hepatic resection. There were significantly more patients in the beyond-Milan group who underwent transarterial chemoembolization or local ablation therapy before LDLT than those in the within-Milan group (60.6 vs. 32%). The waiting time while on the list was similar between the two groups.
| Within-Milan group (n = 50) | Beyond-Milan group (n = 33) | P-value | |
|---|---|---|---|
| Age, years | 54 (3–64) | 54 (11–67) | 0.508 |
| Sex ratio, M:F | 41:9 | 31:2 | 0.186 |
| Hepatitis B surface antigen positive | 42 (84) | 28 (84.8) | 0.917 |
| Hepatitis C antibody positive | 7 (14) | 3 (9.1) | 0.733 |
| Blood group A:B:O:AB | 20:13:13:4 | 14:14:4:1 | 0.225 |
| Child-Pugh score | 8 (5–14) | 7 (5–15) | 0.271 |
| MELD score | 13 (7–59) | 13 (6–34) | 0.481 |
| Serum alpha fetoprotein, ηg/ml | 41 (3–6557) | 41 (3–117850) | 0.434 |
| Previous hepatic resection | 7 (14) | 5 (15.2) | 0.884 |
| Previous TACE or local ablation | 16 (32) | 20 (60.6) | 0.01* |
| Waiting time on the list (days) | 29 (1–406) | 24 (1–473) | 0.696 |
- Continuous variables are expressed as median and range
- Figures represent number of patients (percentage)
- MELD Model of the end-stage liver disease, TACE transarterial chemoembolization
- * Statistically significant
Operation and perioperative details
The donor and recipient operations were described previously [10, 27]. The transplant operation details and perioperative outcome are described in Table 2. The cell-saver device was routinely not used because of the malignant condition of the recipient. Intraoperative blood transfusion requirement was similar between the two groups. The majority of recipients in both groups received a right liver graft. For adult-to-adult LDLT in the authors' center, a right liver graft with inclusion of the middle hepatic vein was routinely procured from the live donor in order to facilitate the hepatic venous drainage of the right anterior section. In selected patients, the left liver with inclusion of the middle hepatic vein was used if the donor's left liver was of adequate size. There was no significant difference between the two groups in the actual graft weight as well as the graft weight to recipient standard liver weight ratio. There was no hospital mortality in either group. No patients required early retransplantation. The morbidity rate was similar between the two groups. Early complication occurred in 27 patients and 14 patients in the within-Milan group and the beyond-Milan group, respectively. Nineteen patients in the within-Milan group and 13 patients in the beyond-Milan group developed late complications.
| Within-Milan group (n = 50) | Beyond-Milan group (n = 33) | P-value | |
|---|---|---|---|
| Intraoperative blood transfusion, units of red cells | 4 (0–43) | 2 (0–30) | 0.309 |
| Right versus left lobe graft | 48:2 | 31:2 | 0.455 |
| Graft weight (gm) | 577 (240–930) | 585 (330–1120) | 0.981 |
| Graft weight/ESLM (%) | 46.5 (37.2–79.2) | 46.9 (27.3–76.1) | 0.675 |
| Hospital mortality | 0 | 0 | 1.000 |
| Complication | |||
| Early complications | 27 (54) | 14 (42.4) | 0.302 |
| Symptomatic pleural effusion | 16 | 5 | |
| Chest infection | 4 | ||
| Hepatic artery thrombosis | 1 | ||
| Bile leakage | 2 | 2 | |
| Others | 4 | 6 | |
| Late complications | 19 (38) | 13 (39.4) | 0.898 |
| Bile duct stricture | 9 | 4 | |
| Portal vein stenosis | 8 | 3 | |
| Hepatic artery thrombosis | 1 | ||
| Opportunistic infection | 3 | 2 | |
| Others | 3 | 3 |
- Continuous variables are expressed as median and range
- Figures represent the number of patients (percentage)
- ESLM Estimated standard liver mass
Histological examination of the explanted liver regarding tumor characteristics is detailed in Table 3. As expected, the tumor staging was more advanced in the beyond-Milan group than in the within-Milan group in terms of larger tumor size, more tumor number, and more advanced TNM stages. The proportion of patients with pathological tumor characteristics beyond the UCSF criteria was higher in the beyond-Milan group than in the within-Milan group (22 patients vs. 0 patients). Microvascular tumor invasion was more common in the beyond-Milan group than in the within-Milan group.
| Within-Milan group (n = 50) | Beyond-Milan group (n = 33) | P-value | |
|---|---|---|---|
| Number of tumors in explant | 1 (1–3) | 3 (1 – multiple) | 0.000* |
| Largest tumor size in explant | 2.4 (1–5) | 4 (2–19.5) | 0.000* |
| Explant UCSF criteria | 0.000* | ||
| Within | 50 | 11 | |
| Beyond | 0 | 22 | |
| Microvascular invasion | 14 (28) | 19 (57.6) | 0.003* |
| TNM staging | 0.003* | ||
| Stage I | 9 (18) | 0 | |
| Stage II | 22 (44) | 1 (3) | |
| Stage III | 19 (38) | 32 (96) |
- Continuous variables are expressed as median and range
- Figures represent number of patients (percentage)
- UCSF University of California at San Francisco, TNM tumor-node metastasis
- * Statistically significant
Following transplantation, immunosuppression using a double regimen of steroids with cyclosporine or tacrolimus was adopted. The steroid was tailed off at the end of 6 months after transplantation. Since 2002, all recipients received a quadruple regimen consisting of induction with interleukin 2 receptor antibody, two perioperative doses of steroid, mycophenolate mefetil (MMF) and tacrolimus. MMF was discontinued at the end of 3 months, whereas tacrolimus monotherapy with a target trough level of 3–5 ηg/ml was continued. Lamivudine with add-on adefovir dipivoxil for viral breakthrough or entecavir monoprophylaxis was prescribed to the recipient with chronic hepatitis B. The patients were monitored regularly every 3 months by measurement of serum AFP concentration and CT scan of the abdomen and thorax. Suspected recurrences on clinical grounds were confirmed by histological examination as far as possible.
Clinical long-term outcome
With a median follow-up period of 36 months (range 4–120 months), 12 patients developed intrahepatic tumor recurrence (6 patients in the within-Milan group and 6 patients in the beyond-Milan group). Among them, 2 patients in the within-Milan group and 4 patients in the beyond-Milan group had extrahepatic metastases. Another 4 patients (2 patients in each group) developed extrahepatic metastases in the absence of intrahepatic recurrence. The overall 1-year, 3-year and 5-year survival rates were 96, 82.8 and 71.7% in the within-Milan group, and 100, 73 and 65.3% in the beyond-Milan group, respectively. There was no statistical difference between the two groups (P = 0.780). The recurrence-free survival rates were also similar between the two groups (Fig. 1). The recurrence-free 1-year, 3-year and 5-year survival rates were 90, 79.1 and 73.4% in the within-Milan group, and 87.9, 68.9 and 62.6% in the beyond-Milan group, respectively. Figure 2 illustrates the recurrence-free survival when patients were categorized into three subgroups (within-Milan, beyond-Milan but within UCSF, and beyond UCSF). Patients with tumor beyond UCSF had worse recurrence-free survival than those with tumors within the Milan criteria and those with tumors beyond the Milan criteria but within UCSF, although the difference was not statistically significant.
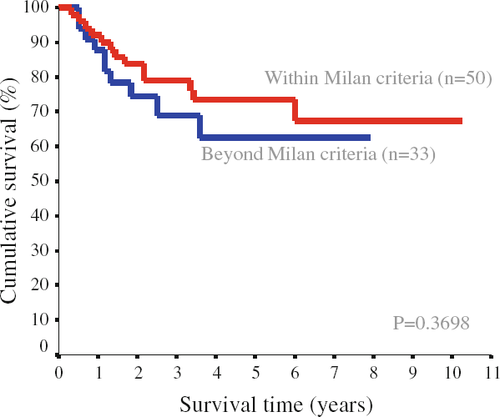
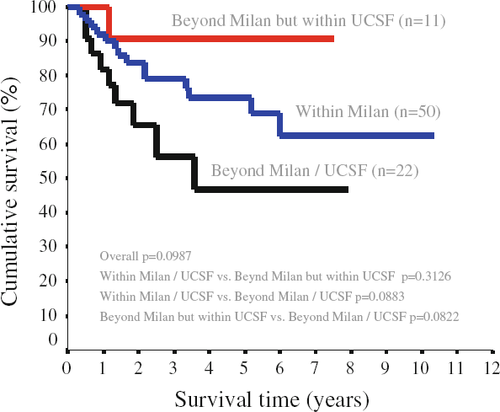
Univariate analysis was performed to identify possible preoperative and operative factors that might carry a significant prognostic effect on recurrence-free survival. The UCSF criteria based on explant pathology were included in the analysis. Four variables were found to have significant influence in predicting recurrence (Table 4). These included sex, UCSF criteria based on explant, salvage transplant (LDLT after curative hepatic resection or local ablation therapy) and TNM staging. Meanwhile, other variables were not significant in univariate analysis, including Child-Pugh score, MELD score, tumor size, tumor numbers, serum AFP level, Milan criteria based on explant, graft weight, intraoperative blood transfusion and waiting time on the list. Multivariate analysis using a Cox proportional hazard model for recurrence-free survival revealed only salvage transplant to be the independent prognostic factor affecting recurrence-free survival after LDLT for HCC (risk ratio 3.73; 95% confidence interval 1.66–8.38; P = 0.001).
| 3-year (%) | 5-year (%) | P-value | |
|---|---|---|---|
| Sex | 0.046* | ||
| Male (n = 62) | 72.1 | 65.5 | |
| Female (n = 11) | 100 | 100 | |
| Explant UCSF criteria | 0.033* | ||
| Within (n = 61) | 84.1 | 76.4 | |
| Beyond (n = 22) | 55.9 | 46.6 | |
| Salvage transplantation | 0.0006* | ||
| No (n = 64) | 81.6 | 74.8 | |
| Yes (n = 12) | 54.5 | 36.3 | |
| TNM staging | 0.05* | ||
| Stage I | 82.2 | 77.8 | |
| Stage II | 77.6 | 72.8 | |
| Stage III | 48 | 36 |
- UCSF University of California at San Francisco
- * Statistically significant
The debate on the choice of primary transplantation versus primary hepatectomy followed by salvage transplantation is still going on. On one hand, the approach of salvage transplant can reduce the number of HCC patients to be recruited into the waiting list since those HCC patients are rendered tumor-free after hepatectomy or local ablation, and there is a time lag between primary hepatectomy and tumor recurrence or liver decompensation. On the other hand, LDLT as a salvage procedure for poor-risk patients with failed primary treatment would deprive the natural selection process whereby patients with aggressive tumor biology may drop-out due to tumor progression. Conflicting results were reported from two French groups on the comparison of primary and salvage transplantation. Belghiti et al. 28 compared 18 patients who underwent salvage transplantation following primary hepatectomy with 70 patients undergoing primary transplantation for HCC. The outcomes of the two strategies were comparable. However, Adam et al. 29 reported that salvage transplantation was associated with a higher operative mortality, an increased risk of recurrence and a poorer outcome compared with primary transplantation for HCC. Recently, Hwang et al. 30 from the Asan group have shown that the overall survival rate after salvage transplantation was similar to that after primary transplantation, particularly when the extent of the recurrent tumor was within the Milan criteria. In the authors' opinion, “fast-track” LDLT in the setting for recurrence following curative treatment carries a high risk of recurrence even the tumor stage fulfills the standard criteria. It is therefore necessary to further explore other biological markers that might have a prognostic influence to facilitate the clinical decision of whether to proceed with this salvage approach.
Conclusion
The application of LDLT for HCC is a complex process involving donor voluntarism, selection criteria for the recipient and justification with respect to long-term survival in comparison to the result of DDLT. Donor voluntarism has a significant impact on the application of LDLT. Although survival outcome may be worse following LDLT than DDLT for HCC, with the critical shortage of deceased donor grafts, practicing LDLT will give the only hope for patients with advanced HCC who cannot be treated by other treatment modality.




