Liver transplantation for hepatocellular carcinoma: the Kyoto experience
Abstract
Background/purpose
The results of living donor liver transplantation (LDLT) for hepatocellular carcinoma (HCC) at Kyoto University were analyzed.
Methods
Between February 1999 and December 2006, 136 patients with HCC underwent LDLT. Of these, 74 patients met the Milan criteria, while 62 patients did not. Treatment for HCC had been previously performed prior to LDLT for 101 patients (74%).
Results
According to the results of multivariate analysis of risk factors for recurrence among preoperative tumor variables, we have defined new Kyoto criteria as ≤10 tumors all ≤5 cm in diameter and protein induced by vitamin K absence or antagonist-II (PIVKA-II) ≤400 mAU/ml. The 5-year recurrence rate was significantly lower for the 85 patients who met the Kyoto criteria than for the 45 patients who exceeded them (3 vs. 54%,p < 0.0001). Similarly, patients who met the Kyoto criteria showed a significantly better 5-year survival rate (87%) than those who did not (36%,p < 0.0001). Survival rates did not differ between pretreated and primary groups, and recurrence rates were similarly low when limited to patients who met the Kyoto criteria.
Conclusions
The proposed Kyoto criteria are expected to serve efficiently as expanded selection criteria for LDLT in patients with HCC. History of previous treatments did not affect outcomes after LDLT.
Introduction
In Japan, because the number of deceased donor liver transplantations (DDLT) has been critically limited, living donor liver transplantation (LDLT) plays an important role in the management of patients with hepatocellular carcinoma (HCC) 1. In February 1999, an LDLT program for patients with HCC was started at Kyoto University, using the following selection criteria [2, 3]: (1) HCC not suitable for resection or local ablation therapies due to advanced tumor spread, repeated uncontrolled recurrence or poor liver function reserve; (2) exclusion of extrahepatic metastasis or macroscopic venous invasion on preoperative imaging. No restrictions were placed on the number or size of tumors, so patients with tumors exceeding the Milan criteria (MC) 4 were included.
Several factors support the extension of criteria beyond the MC [5-8]. First, the MC are too restrictive. Against the background of donor organ shortage in DDLT, the MC were originally aimed at predicting good outcomes with low rates of recurrence, rather than poor outcomes with high recurrence rates. A substantial subset exists with the potential for good outcome after LT among patients with HCC beyond the MC. Second, particularly in LDLT, the wait time between preoperative tumor staging and transplantation is usually shorter than in DDLT. Third, thanks to recent progress in imaging technology, such as multidetector computed tomography (MDCT), the accuracy of imaging studies has increased. Accordingly, the wide “safety margins” offered by the MC for tumor stage underestimation by preoperative imaging studies can be diminished 9.
Conversely, LDLT involves significant disadvantages, including risks to the live donor and higher perioperative morbidity and mortality compared with other treatment modalities for HCC. Given these risks, the argument has been made that early stage HCC should initially be managed using resection or conventional methods, with LDLT held in reserve as a second-line option [1-3]. Most recipients of LDLT thus have a history of non-transplant treatments and receive LDLT as a rescue treatment for uncontrolled recurrent HCC.
The present article introduces our experiences of LDLT for HCC at our institute. By analyzing the results, optimal criteria expanded beyond the MC and effects of pre-transplant treatments on transplant outcomes are discussed.
Patients
Between February 1999 and December 2006, a total of 136 patients (96 men, 40 women) who were diagnosed with HCC by preoperative imaging studies underwent LDLT at Kyoto University. Another 17 patients with incidental tumors were excluded from this study. Median age was 55 years (range 22–69 years). A total of 76 patients were HCV-Ab positive and 47 were HBs-Ag positive, including 4 patients with co-infection. Child-Pugh classification was C for 61 patients, B for 53 patients, and A for 22 patients. Median model for end-stage liver disease (MELD) score was 14 (range 4–36). Of the 136 patients, 101 patients displayed a history of previous treatment for HCC using various non-transplant methods including hepatic resection, trans-arterial chemoembolization (TACE), percutaneous ethanol injection (PEI) or radiofrequency ablation (RFA). The remaining 35 patients had no history of HCC treatment before LDLT, in most cases due to advanced liver dysfunction. As of the end of December 2008, the median follow-up period was 43 months.
Evaluation of the extent of tumor involvement using contrast-enhanced CT was usually performed within 1 month before LDLT. Tumor staging was determined by counting only viable and enhancing nodules on CT. For patients with previous treatment, resected tumors or nodules that were judged as nonviable were not counted. A total of 74 patients (54%) met the MC according to pre-transplant imaging, while 62 patients did not.
Results
Patient survival
As of the end of December 2008, a total of 90 patients remained alive. The overall patient survival rate at 5 years was 69%. Univariate analysis of preoperative tumor characteristics (Table 1) revealed that maximal tumor size >5 cm in diameter, tumor number ≥11, alfa-fetoprotein (AFP) >400 ng/ml, and protein induced by vitamin K absence or antagonist-II (PIVKA-II) >400 mAU/ml were significantly associated with 5-year patient survival. Conversely, survival rates were similar for patients who met the MC and those who did not (75 vs. 60% at 5 years, respectively; p = 0.3110).
| Variables | n | 5-year survival rate (%) | P | 5-year recurrence rate (%) | P |
|---|---|---|---|---|---|
| Tumor size | |||||
| ≤3 cm | 90 | 75 | 0.0001 | 14 | <0.0001 |
| 3–5 cm | 31 | 71 | 9 | ||
| >5 cm | 15 | 23 | 78 | ||
| Tumor number | |||||
| ≤3 | 88 | 72 | <0.0001 | 14 | <0.0001 |
| 4–10 | 30 | 86 | 11 | ||
| ≥11 | 18 | 21 | 62 | ||
| Milan criteria | |||||
| Meet | 74 | 75 | 0.3110 | 7 | 0.0002 |
| Exceed | 62 | 60 | 33 | ||
| AFP (ng/ml) | |||||
| <400 | 107 | 74 | 0.0358 | 12 | 0.0001 |
| ≥400 | 29 | 49 | 45 | ||
| PIVKA-IIa (mAU/ml) | |||||
| <400 | 99 | 81 | <0.0001 | 11 | <0.0001 |
| ≥400 | 29 | 34 | 51 |
- Univariate analysis was performed using the log-rank test
- a PIVKA-II, protein induced by vitamin K absence or antagonist-II. This value was not available for 8 patients
Recurrence of HCC
Postoperative recurrence of HCC occurred in 22 patients, occurring ≤2 years after LDLT in 19 (86%) of these patients. Sites of first recurrence comprised bone (n = 7), lung (n = 6), graft liver (n = 3), abdominal lymph nodes (n = 2), right subphrenic space (n = 2), adrenal gland (n = 1), and brain (n = 1). After excluding death without recurrence, the overall cumulative recurrence rate was 19% at 5 years.
Univariate analysis of preoperative risk factors for recurrence showed tumor diameter >5 cm, tumor number ≥11, exceeding the MC, AFP >400 ng/ml, and PIVKA-II >400 mAU/ml all represented significant predictors of higher recurrence rates (Table 1).
Extended criteria beyond the MC: the Kyoto criteria
The recurrence rate was significantly higher for the over-MC group than for the within-MC group. However, when the recurrence rate was analyzed based on tumor number and size separately, the recurrence rate was significantly lower for patients with ≤10 tumors than for those with ≥11 tumors (p < 0.0001). In addition, patients with tumors ≤5 cm in maximum diameter showed a significantly lower recurrence rate than those with tumors >5 cm. Accordingly, tumor number and maximal size for each patient in the over-MC group were plotted in relation to outcomes (Fig. 1). In total, 18 patients developed recurrence, and 7 patients died of HCC-unrelated causes. However, in patients beyond the MC, but with ≤10 tumors all ≤5 cm in diameter (n = 34), only 2 patients developed HCC recurrence, at 3 and 26 months after LDLT, while another 2 patients died of HCC-unrelated causes at 1 and 5 months after LDLT. The remaining 30 patients were alive without recurrence at a median follow-up of 61 months (range, 24–113 months).
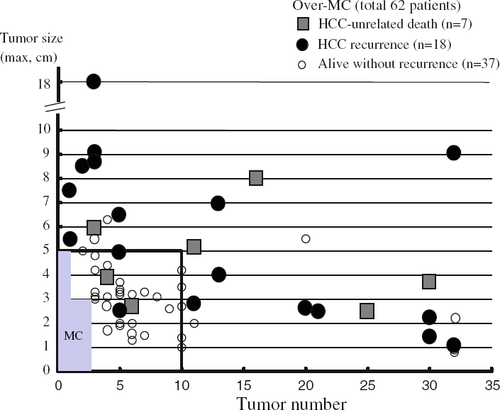
Among preoperative tumor variables including ≥11 tumors, maximal tumor diameter >5 cm, MC, AFP level >400 ng/ml, and PIVKA-II level >400 mAU/ml, multivariate analysis using Cox's proportional hazard model revealed that ≥11 tumors, tumor diameter >5 cm, and PIVKA-II >400 mAU/ml represented independent risk factors for postoperative recurrence (Table 2). We therefore defined new and extended criteria to minimize the risk of tumor recurrence as HCC with ≤10 tumors all ≤5 cm in diameter and PIVKA-II ≤400 mAU/ml (Kyoto criteria). The 5-year recurrence rate was significantly lower for the 85 patients who met the new Kyoto criteria (3%) than for the 45 patients who exceeded these criteria (54%, p < 0.0001). Similarly, patients who met the Kyoto criteria showed a significantly better 5-year survival rate (87%) than those who did not (36%, p < 0.0001).
| Variables | Risk ratio | 95% Confidence interval | P |
|---|---|---|---|
| Tumor number ≥11 nodules | 3.048 | 1.129–8.196 | 0.0277 |
| Tumor diameter >5 cm | 8.333 | 2.109–32.258 | 0.0024 |
| Beyond MC | 1.423 | 0.183–2.695 | 0.6073 |
| AFP >400 ng/ml | 1.429 | 0.192–2.545 | 0.5880 |
| PIVKA-II >400 mAU/ml | 5.618 | 2.123–14.925 | 0.0005 |
- Multivariate analysis was performed using Cox's proportional hazard model
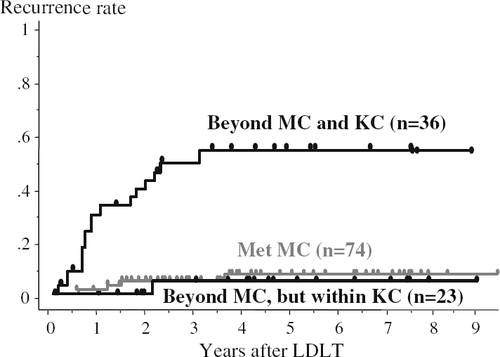
From the perspective of extended criteria beyond the MC, patients were divided into 3 groups: patients who met the MC (n = 74); patients beyond the MC, but within the Kyoto criteria (n = 23); and patients beyond both the MC and Kyoto criteria (n = 36). Nine patients who met the MC but displayed PIVKA-II >400 mAU/ml were included in the within-MC group. A comparison of recurrence rates among these 3 groups (Fig. 2) showed that 5-year recurrence rates were similar for the first and second groups (7 and 5%, respectively), while the rate for the third group was significantly higher than those for the other groups (55%, p < 0.0001). Notably, the PIVKA-II level was >400 mAU/ml for 3 of the 4 patients developing recurrence in the within-MC group.
Effects of pre-transplant treatments
Of the 136 patients, 101 patients had a history of previous treatments for HCC before LDLT (pretreated group), while 35 patients received LDLT without previous treatment (primary group). In the pre-treated group, previous non-transplant treatments included hepatic resection (n = 15), TACE (n = 31), PEI or RFA (n = 12), or TACE plus PEI or RFA (n = 43). These treatments were performed not as a bridge to transplant or for down-staging, but with intent for curative ablation in other hospitals before referral to our institute. The median period between the first diagnosis of HCC and LDLT was 22 months in this group (range 2–171 months). Forty patients received 1–2 treatments and 61 patients received ≥3 treatments.
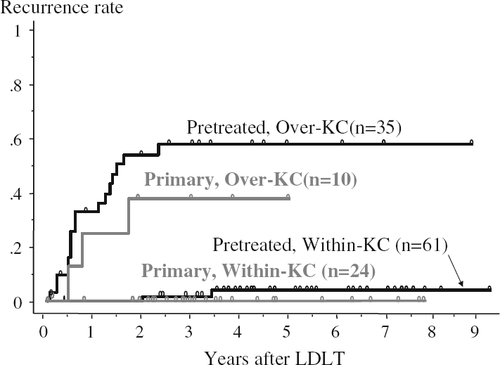
Comparing transplant outcomes between pretreated and primary groups, patient survival rates were similar (68 vs. 70% at 5 years, respectively; p = 0.6807). The recurrence rate was slightly, but not significantly, higher for the pretreated group than for the primary group (22 vs. 10% at 5 years, respectively; p = 0.1789). However, if recurrence rates were examined by stratifying patients on the basis of the Kyoto criteria (Fig. 3), the 61 patients meeting the Kyoto criteria in the pretreated group showed a similarly low recurrence rate to that for the 24 within-Kyoto patients in the primary group (4 vs. 0% at 5 years).
Since pre-transplant treatments, especially hepatic resection, may complicate the operative transplant procedure and increase the risk of postoperative complications 10, the effects of the type of pre-transplant treatments on perioperative results were evaluated (Table 3). Patients were divided into 3 groups: patients who had received hepatic resection before LDLT (group 1, n = 15), patients treated with TACE and/or PEI/RFA (group 2, n = 86), and patients without previous treatment (group 3, n = 35; identical to the “primary group” defined above). In group 1, hemihepatectomy was performed for 5 patients, sectionectomy 11 for 3, and partial resection for 7. All 15 patients in group 1 developed recurrence after hepatic resection, which was treated by TACE and/or PEI/RFA. Before LDLT, the mean (±SEM) preoperative MELD score was significantly higher in group 3 (20 ± 1) than in group 2 (15 ± 1; p < 0.01) or group 1 (11 ± 2; p < 0.001). The proportion of patients who exceeded the MC tended to be higher in group 1 (60%) than in group 3 (34%). Indeed, operation time was significantly longer in group 1 (903 ± 44 min) than in group 2 (788 ± 18 min; p < 0.001) or group 3 (755 ± 19 min; p < 0.001), and blood loss also tended to be greater in group 1, although no significant difference was identified. However, both short-term (90-day) survival rates, which may be compromised by surgical complications, and 5-year survival rates were similar among the 3 groups.
| Group 1 (n = 15) | Group 2 (n = 86) | Group 3 (n = 35) | P | |
|---|---|---|---|---|
| Pre-operation | ||||
| MELD score | 11 ± 2 | 15 ± 1 | 20 ± 1 | 0.0001a |
| Rate of over-MC patients | 60% | 48% | 34% | 0.2010 |
| Operation | ||||
| Operation time (min) | 903 ± 44 | 788 ± 18 | 755 ± 19 | 0.0088b |
| Blood loss (g) | 9962 ± 3265 | 6912 ± 974 | 7624 ± 1117 | 0.4713 |
| Post-operation | ||||
| 90-day survival rate | 93% | 92% | 89% | 0.8077 |
| 5-year survival rate | 72% | 67% | 70% | 0.8959 |
- Group 1, pretreated with hepatic resection; group 2, pretreated with TACE and/or PEI/RFA; group 3, no pretreatment (primary group) Continuous variables are expressed as means ± SEM
- Comparisons of continuous variables between groups were performed using analysis of variance and Fisher's PLSD test. Comparisons of proportions between groups were performed using χ2 tests
- a Group 1 versus group 2, p < 0.05; group 1 versus group 3, p < 0.0001; group 2 versus group 3, p < 0.01
- b Group 1 versus group 2, p < 0.001; group 1 versus group 3, p < 0.001
Discussion
Attempting to offer the chance of cure to patients outside the MC, many centers have performed liver transplantation using extended criteria beyond the MC [5-8, 12, 13]. However, expansion of criteria naturally contains a risk of increased post-transplant recurrence. Mazzaferro et al. recently introduced the concept of the metro ticket paradigm 14. This means the higher the number or size of nodules away from the conventional limits is, the lower the expected patient survival. The issue of concern is thus how to preoperatively identify categories beyond the MC that still offer predictably good outcomes. Particularly in LDLT, in weighing the risks to the living donor, a reasonable survival rate should be ensured. Although several studies have proposed extended criteria based on tumor number and size as an estimate of tumor burden, these are considered insufficient to stratify patients in such categories, and additional parameters for tumor biological features related to risk of recurrence are necessary 15. Moreover, patient selection criteria before LDLT should only incorporate tumor variables available in preoperative evaluation.
In the present study, since HCC recurrence occurred within 2 years after LDLT in most cases, patients who underwent LDLT by December 2006 and had been followed for >2 years were enrolled. Recurrence rates were significantly higher for patients who exceeded the MC than for those within the MC based on preoperative imaging studies. However, when over-MC patients were divided into 2 subgroups, patients who exceeded the MC but presented with ≤10 tumors all ≤5 cm displayed recurrence rates comparable to those who met the MC. In addition, multivariate analysis revealed not preoperative AFP, but PIVKA-II level as an independent risk factor for recurrence (Table 2). This indicates a significant association between PIVKA-II and recurrence irrespective of tumor size or number. In the previous study 8, PIVKA-II was shown to be closely related to pathological tumor features such as microvascular invasion or histological tumor grade. All these results suggest that the preoperative PIVKA-II level offers additional information on tumor biological behavior related to histological findings usually obtained by explant pathology and represents a useful predictor of recurrence in conjunction with tumor size and number. We have therefore proposed new Kyoto criteria: tumor number ≤10; all tumors ≤5 cm; PIVKA-II ≤400 mAU/ml. Of the 136 patients in the present series, 23 patients (17%) who were beyond the MC but within the Kyoto criteria showed a similarly low recurrence rate comparable to that for within-MC patients. As the Kyoto criteria appear effective for predicting a better subgroup outside the MC, we have adopted these as expanded selection criteria for HCC patients since January 2007 and have started a prospective study to validate their feasibility.
Another issue is the effect of pre-transplant treatment on transplant outcomes. In the present study, survival rates were similar for the pretreated and primary groups. Although recurrence rates were slightly higher for the pretreated group, rates for patients who met the Kyoto criteria were similar between pretreated and primary groups. Recurrence rates after LDLT appeared to be associated with tumor stage immediately before LDLT, irrespective of history of pre-transplant treatment.
Recently, several trials of down-staging of HCC before liver transplantation have been reported [16, 17]. One of the merits of down-staging is considered to be the selection of a subset of tumors with more favorable biology that are more likely to respond to treatment and do well after transplantation 16. In our experience, pretreatments before LDLT were not performed for down-staging, but with intent for curative ablation. However, patients may have been naturally selected during these treatments, and most patients with aggressively recurrent HCC may have been excluded as candidates for LDLT before referral to our institute. Thus, the possibility of selection bias resulting in reduced recurrence rates in the pretreated group remains.
Although blood loss and operation time during LDLT were greater for patients with a history of hepatic resection, the implication is that these prior treatments neither increased the incidence of surgical complications nor affected immediate postoperative survival. These results may support our policy of holding LDLT as an optional treatment with a chance of cure for patients displaying otherwise uncontrolled HCC.




