Outcomes After Major Surgical Procedures in Octogenarians: A Nationwide Cohort Study
Arthur K. E. Elfrink, Anna J. Alberga, Michel W. J. M. Wouters and Joost M. Klaase have equally contributed to this work.
Supplementary Information: The online version contains supplementary material available at https://doi.org/10.1007/s00268-022-06642-6.
Abstract
Introduction
Aging of the worldwide population has been observed, and postoperative outcomes could be worse in elderly patients. This nationwide study assessed trends in number of surgical resections in octogenarians regarding various major surgical procedures and associated postoperative outcomes.
Methods
All patients who underwent surgery between 2014 and 2018 were included from Dutch nationwide quality registries regarding esophageal, stomach, pancreas, colorectal liver metastases, colorectal cancer, lung cancer and abdominal aortic aneurysms (AAA). For each quality registry, the number of patients who were 80 years or older (octogenarians) was calculated per year. Postoperative outcomes were length of stay (LOS), 30 day major morbidity and 30 day mortality between octogenarians and younger patients.
Results
No increase in absolute number and proportion of octogenarians that underwent surgery was observed. Median LOS was higher in octogenarians who underwent surgery for colorectal cancer, colorectal liver metastases, lung cancer, pancreatic disease and esophageal cancer. 30 day major morbidity was higher in octogenarians who underwent surgery for colon cancer, esophageal cancer and elective AAA-repair. 30 day mortality was higher in octogenarians who underwent surgery for colorectal cancer, lung cancer, stomach cancer, pancreatic disease, esophageal cancer and elective AAA-repair. Median LOS decreased between 2014 and 2018 in octogenarians who underwent surgery for stomach cancer and colorectal cancer. 30 day major morbidity decreased between 2014 and 2018 in octogenarians who underwent surgery for colon cancer. No trends were observed in octogenarians regarding 30 day mortality between 2014 and 2018.
Conclusion
No increase over time in absolute number and proportion of octogenarians that underwent major surgery was observed in the Netherlands. Postoperative outcomes were worse in octogenarians.
Introduction
Aging of the population in Western countries has been described in the last 20 years which might be accompanied by a higher incidence of surgical procedures in the elderly [1]. In the Netherlands, the proportion of population who were 80 years or older (octogenarians) increased from 3.2% in 2000 to 4.6% in 2019 [2]. Major surgical procedures were described to be performed more often in octogenarians after the year 2000 [3].
As people get older, an increase of the number comorbidities of a patient scheduled for surgery has been described [3]. Having multiple comorbidities is common in elderly patients and is estimated to be 80% in octogenarians [4, 5]. Comorbidities are a risk factor for occurrence of postoperative morbidity and mortality due to a decrease of functional reserve [6-8]. Several studies from various surgical fields on major surgical procedures in elderly patients have shown higher morbidity and mortality rates [3, 9-12]. Clinical studies have also shown that higher age and additional comorbidities of a patient can be a reason to refrain from major surgical procedures as occurrence of perioperative complications can decrease quality of life and long-term outcomes [13].
To improve quality of care for octogenarians, insights into current daily practice and outcomes are needed. Improvement measures to improve quality of care and patient selection before proceeding to major surgical procedures can be based on current outcomes. To date, it is unclear whether aging of the population leads to an increase in octogenarians who undergo major surgical procedures in the Netherlands. Also, nationwide assessment of postoperative outcomes in octogenarians who undergo major surgical procedures in the Netherlands is lacking.
The aim of this nationwide study is to assess trends in the number and proportion of octogenarians undergoing major surgical procedures and their associated postoperative outcomes over time.
Methods
Study Population
Patients were included using the prospective quality registries for several indications for surgery from the Dutch Institute for Clinical Auditing (DICA): the Dutch Institute for Clinical Auditing (DICA): the Dutch Upper GI Cancer Audit (DUCA), the Dutch Pancreatic Cancer Audit (DPCA), the Dutch HepatoBiliary Audit (DHBA), the Dutch ColoRectal Audit (DCRA), the Dutch Lung Cancer Audit-Surgery (DLCA-S) and the Dutch Surgical Aneurysm Audit (DSAA) [14]. The number of patients diagnosed with oncological conditions was retrieved using data from the Dutch cancer registry (NKR). These data were available until 2018 for esophageal, stomach, colorectal and lung cancer [14, 15].
All patients who were 18 year or older and underwent primary surgery from the several quality registries between the 1st of January 2014 and 31st of December 2018 were included. From the DUCA, DPCA, DHBA, DCRA and DLCA-S, patients were included who underwent resection of esophageal or stomach cancer, resection for benign or malignant pancreatic disease, colorectal liver metastases (CRLM), colorectal cancer, or lung cancer, respectively. From the DSAA, patients who underwent elective repair of an abdominal aneurysm (AAA) were included.
Patients were divided for analyses in two groups: patients who were younger than 80 years (80-) or those who were 80 years or older (octogenarians).
For this study, no ethical approval was needed under Dutch Law. However, clinical audit boards of each quality registry approved this study. This study was performed according to the Strobe guidelines for cohort studies [16] (Appendix A).
Outcomes
Primary outcomes were postoperative outcomes which consisted of length of hospital stay (LOS), 30 day major morbidity defined as complications graded Clavien–Dindo 3a or higher, and 30 day mortality [17]. If complications were not scored according to the Clavien–Dindo classification in a registry, a postoperative complicated course was used. This composite outcome is defined as a complication accompanied by either one of the three: prolonged LOS (> 14 days), reintervention or death within 30 days of surgery [18-20]. This outcome was used for the DLCA, DCRA and DSAA. All outcomes were compared between patients who were younger than 80 years and octogenarians.
Secondary outcomes were trends in number of patients over the years who were younger than 80 per quality registry compared to octogenarians over the years, and postoperative outcomes.
Statistical Analyses
Categorical variables were displayed as appropriate using numbers accompanied by percentages. Continuous variables were displayed as mean accompanied with standard deviation (SD) in case of normal distribution. If non-normal distribution of data was observed, the median was shown accompanied by interquartile ranged (IQR).
Postoperative outcomes were compared between the groups using the Chi-squared test in case of dichotomous outcomes or Mann–Whitney U test in case of continuous outcomes. Analysis regarding the number of octogenarians within quality registries compared to total number of patients over the years and compared to the total number of diagnosed patients with the same oncological condition was performed using linear regression analysis.
For trends in postoperative outcomes, trend analysis over time were performed using linear or logistic regression as appropriate for continuous or dichotomous outcomes. The beta coefficient (ß) or odds ratio (OR), including 95% confidence intervals, was displayed as influence per year if a trend was observed.
In all analyses, a two-sided p-value < 0.05 was seen as significant.
Results
The total included number of patients per quality registry ranged from 2509 to 38,229 patients (Table 1). The percentage of octogenarians ranged between quality registries between 3.5% (esophageal cancer) and 21.4% (AAA). The percentage of octogenarians in oncological quality registries compared to the total number of octogenarians diagnosed with cancer ranged between 4.6% (lung cancer) and 73.7% (colon cancer).
Type of surgery |
2014 |
2015 |
2016 |
2017 |
2018 |
Total |
p-value trend |
|---|---|---|---|---|---|---|---|
Abdominal aortic aneurysm |
|||||||
N—total |
2542 |
2464 |
2564 |
2488 |
2528 |
12,586 |
|
N—80 + total |
553 |
509 |
540 |
536 |
552 |
2690 |
0.720 |
% 80 + |
21.8 |
20.7 |
21.1 |
21.5 |
21.8 |
21.4 |
0.586 |
Mean age (SD) |
73.3 (7.6) |
73.2 (7.6) |
73.2 (7.5) |
73.2 (7.5) |
73.4 (7.7) |
73.2 (7.7) |
0.626 |
Colon cancer |
|||||||
N—total |
7783 |
8470 |
8244 |
7094 |
6608 |
38,199 |
|
N—80 + total |
1562 |
1496 |
1515 |
1421 |
1324 |
7318 |
0.019 |
% 80 + |
20.1 |
17.7 |
18.4 |
20.0 |
20.0 |
19.2 |
0.600 |
Mean age (SD) |
70.9 (10.4) |
69.8 (10.1) |
70.0 (10.2) |
70.3 (10.6) |
70.3 (10.9) |
70.2 (10.4) |
0.662 |
Colorectal liver metastases |
|||||||
N—total |
771 |
884 |
1008 |
1009 |
838 |
4510 |
|
N—80 + total |
56 |
62 |
64 |
82 |
50 |
314 |
0.867 |
% 80 + |
7.3 |
7.0 |
6.3 |
8.1 |
6.0 |
7.0 |
0.649 |
Mean age (SD) |
65.5 (10.1) |
66.1 (10.0) |
65.8 (10.4) |
66.3 (10.5) |
65.2 (10.6) |
65.7 (10.7) |
0.838 |
Lung cancer |
|||||||
N—total |
1507 |
1631 |
1907 |
2070 |
2241 |
9356 |
|
N—80 + total |
92 |
93 |
91 |
100 |
126 |
502 |
0.101 |
% 80 + |
6.1 |
5.7 |
4.8 |
4.8 |
5.6 |
5.4 |
0.384 |
Mean age (SD) |
66.0 (9.4) |
66.1 (9.0) |
65.9 (9.5) |
66.4 (9.0) |
66.8 (9.1) |
66.3 (9.9) |
0.077 |
Stomach cancer |
|||||||
N—total |
578 |
485 |
552 |
441 |
453 |
2509 |
|
N—80 + total |
86 |
82 |
109 |
89 |
80 |
446 |
0.913 |
% 80 + |
14.9 |
16.9 |
19.7 |
20.2 |
17.7 |
17.8 |
0.240 |
Mean age (SD) |
69.0 (10.9) |
70.0 (42.4) |
69.3 (11.5) |
69.6 (11.5) |
69.7 (11.7) |
69.9 (11.5) |
0.140 |
Pancreas resection |
|||||||
N—total |
871 |
870 |
880 |
945 |
904 |
4470 |
|
N—80 + total |
53 |
48 |
58 |
69 |
66 |
294 |
0.069 |
% 80 + |
6.1 |
5.5 |
6.6 |
7.3 |
7.3 |
6.6 |
0.063 |
Mean age (SD) |
64.8 (11.8) |
65.0 (11.7) |
65.6 (11.0) |
65.2 (11.8) |
65.9 (11.6) |
65.3 (11.6) |
0.074 |
Rectal cancer |
|||||||
N—total |
2957 |
3280 |
3046 |
3063 |
2644 |
15,005 |
|
N—80 + total |
365 |
368 |
345 |
401 |
329 |
1808 |
0.713 |
% 80 + |
12.3 |
11.2 |
11.3 |
13.1 |
12.4 |
12.0 |
0.491 |
Mean age (SD) |
67.8 (10.6) |
67.4 (10.0) |
67.0 (10.2) |
67.3 (10.6) |
67.1 (10.8) |
67.3 (10.4) |
0.201 |
Esophageal cancer |
|||||||
N—total |
770 |
837 |
793 |
820 |
799 |
4019 |
|
N—80 + total |
28 |
38 |
24 |
26 |
26 |
142 |
0.440 |
% 80 + |
3.6 |
4.5 |
3.0 |
3.2 |
3.3 |
3.5 |
0.334 |
Mean age (SD) |
65.2 (9.0) |
65.3 (8.8) |
65.3 (8.4) |
65.8 (8.5) |
66.3 (8.7) |
65.8 (8.9) |
0.022 |
- Bold values are statistically significant (p < 0.05)
- N number of patients, SD standard deviation
Trends in Number of Octogenarians
The mean age ranged between 65.3 and 73.2 years for the different indications for surgery. An increasing number of older patients that underwent resection of esophageal cancer were observed with a mean age of 65.2 years in 2014 and 66.3 years in 2018 (p = 0.022). In other quality registries, no significant increase in mean age was observed.
No increase in the proportion of octogenarians was observed for esophageal cancer, CRLM, colon cancer, rectal cancer, lung cancer and abdominal aortic aneurysms (Table 1, Fig. 1). The proportion of octogenarians who underwent pancreas resection slightly increased from 6.1% in 2014 to 7.3% in 2018 (p = 0.063).
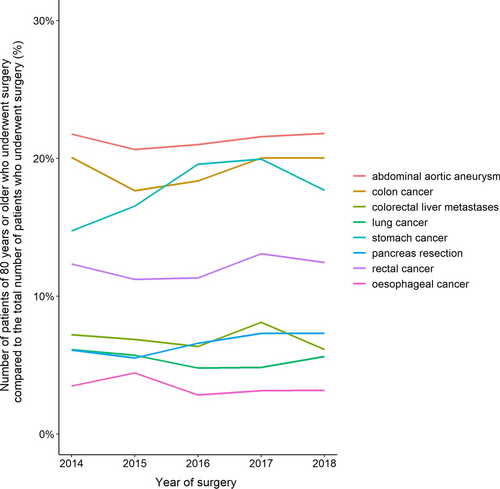
Trend in number of patients of 80 years or older who underwent surgery per quality registry compared to the total number of patients who underwent surgery per quality registry per year
The proportion of octogenarians in oncological quality registries did not increase compared to the Dutch cancer registry (supplementary Fig. 1, supplementary table 1).
Postoperative Outcomes
Median LOS was longer in octogenarians who underwent surgery for colon cancer compared to non-octogenarians (7 days (IQR 5–12) vs. 5 days (IQR 4–8), p < 0.001), CRLM (7 days (IQR 5–10) vs. 6 days (IQR 4–9), p < 0.001), lung cancer (7 days (IQR 5–10) vs. 6 days (IQR 4–9)), p < 0.001), pancreatic disease 13 days (IQR 9–22) vs. 11 days (IQR 8–16), p < 0.001), rectal cancer (7 days (IQR 5–12) vs. 6 days (IQR 4–10), p < 0.001), esophageal cancer (15 days (IQR 10–22) vs. 11 days (IQR 8–18), p < 0.001) as compared to younger patients. Median LOS in octogenarians who underwent AAA-repair was shorter compared to non-octogenarians (3 (IQR 2–5) vs. 3 (IQR 2–6), p = 0.046). This difference was due to more endovascular AAA-repair in octogenarians. For stomach cancer, no difference in median LOS was observed (Table 2 and Fig. 2).
Type of surgery |
< 80 years |
≥ 80 years |
RR |
p-value |
|---|---|---|---|---|
Median (IQR)/N (%) |
Median (IQR)/N (%) |
|||
Abdominal aortic aneurysm |
9896 (78.6) |
2690 (21.4) |
||
Length of stay |
3 (2–6) |
3 (2–5) |
0.046 |
|
30 day major morbidity |
1207 (12.2) |
368 (13.7) |
1.12 |
0.045 |
30 day mortality |
159 (1.6) |
59 (2.2) |
1.37 |
0.043 |
Colon cancer |
30881 (80.8) |
7318 (19.2) |
||
Length of stay |
5 (4–8) |
7 (5–12) |
< 0.001 |
|
30 day major morbidity |
4211 (13.6) |
1568 (21.4) |
1.57 |
< 0.001 |
30 day mortality |
449 (1.5) |
425 (5.8) |
3.99 |
< 0.001 |
Colorectal liver metastases |
4196 (93.0) |
314 (7.0) |
||
Length of stay |
6 (4–9) |
7 (5–10) |
< 0.001 |
|
30 day major morbidity |
360 (8.6) |
37 (11.8) |
1.37 |
0.067 |
30 day mortality |
59 (1.4) |
6 (1.9) |
1.36 |
0.632 |
Lung cancer |
8836 (94.6) |
502 (5.4) |
||
Length of stay |
6 (4–9) |
7 (5–10) |
< 0.001 |
|
30 day major morbidity |
791 (9.0) |
52 (10.4) |
1.16 |
0.322 |
30 day mortality |
162 (1.8) |
28 (5.6) |
3.05 |
< 0.001 |
Stomach cancer |
2063 (82.2) |
446 (17.8) |
||
Length of stay |
8 (6–12) |
8 (6–12) |
0.527 |
|
30 day major morbidity |
360 (17.5) |
76 (17.0) |
0.90 |
0.890 |
30 day mortality |
96 (4.7) |
34 (7.6) |
1.64 |
0.037 |
Pancreas resection |
4176 (93.4) |
294 (6.6) |
||
Length of stay |
11 (8–16) |
13 (9–22) |
< 0.001 |
|
30 day major morbidity |
1151 (27.6) |
86 (29.3) |
1.06 |
0.434 |
30 day mortality |
127 (3.0) |
17 (5.8) |
1.90 |
0.016 |
Rectal cancer |
13182 (88.0) |
1808 (12.0) |
||
Length of stay |
6 (4–10) |
7 (5–12) |
< 0.001 |
|
30 day major morbidity |
2594 (19.7) |
370 (20.5) |
1.04 |
0.450 |
30 day mortality |
101 (0.8) |
70 (3.9) |
5.06 |
< 0.001 |
Esophageal cancer |
3877 (96.5) |
142 (3.5) |
||
Length of stay |
11 (8–18) |
15 (10–22) |
< 0.001 |
|
30 day major morbidity |
1118 (28.8) |
56 (39.4) |
1.37 |
0.008 |
30 day mortality |
115 (3.0) |
12 (8.5) |
2.85 |
0.001 |
- Bold values are statistically significant (p < 0.05)
- RR relative risk
- p-value is for statistical significance of percentage difference between < 80 and 80 years or older
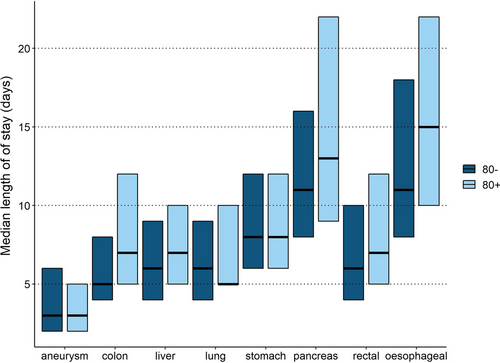
Median length of stay with interquartile range per type of surgery stratified for patients who were younger than 80 years versus octogenarians
30 day major morbidity was higher in octogenarians who underwent surgery for AAA (13.7 vs. 12.2%, p = 0.045), colon cancer (21.4% vs. 13.6%, p < 0.001) and esophageal cancer (39.4% vs 28.8%, p = 0.008) (Table 2).
Thirty-day mortality was higher in octogenarians who underwent surgery for AAA (2.2% vs. 1.6%, p = 0.043), colon cancer (5.8% vs 1.5%, p < 0.001), lung cancer (5.6% vs 1.8%, p < 0.001), stomach cancer (7.6% vs 4.7%, p = 0.037), pancreatic disease (5.8% vs 3.0%, p = 0.016), rectal cancer (3.9% vs. 0.8%) and esophageal cancer (8.5% vs 3.0%, p = 0.001) (Table 2).
Trends in Postoperative Outcomes in Octogenarians
A decrease in LOS was observed in octogenarians who underwent surgery for colon cancer from median 9 days (IQR 6–14) in 2014 to 6 days in 2018 (IQR 4–11), ß − 0.54, 95% CI – 0.71 to − 0.36, p < 0.001) and rectal cancer (median 8 days (IQR 6–13.25) in 2014 to 6 days (IQR 5–10) in 2018, ß – 0.67, 95% CI – 1.09 to − 0.25, p < 0.001). In other quality registries, no difference in LOS was observed between 2014 and 2018.
A decrease in 30 day major morbidity between 2014 and 2018 was observed in octogenarians who underwent surgery for colon cancer (25.4% vs. 19.9%, OR 0.92, 95% CI 0.89–0.96, p < 0.001). No trends were observed in octogenarians regarding 30 day major morbidity in other quality registries.
No trends were observed in octogenarians regarding 30 day mortality between 2014 and 2018.
Discussion
The current nationwide study on major surgical procedures in octogenarians did not show an increase in the proportion and absolute number of octogenarians that undergo surgery for esophageal cancer, stomach cancer, lung cancer, CRLM, pancreatic disease, colorectal cancer or AAA. For several major surgical procedures, postoperative outcomes such as LOS, 30 day major morbidity and 30 day mortality were worse in octogenarians. Improvement regarding LOS has been made after colorectal surgery in octogenarians. Postoperative morbidity and mortality did not improve during the study period in these patients.
Data from the Dutch Institute for Epidemiology show that aging of the population reached a plateau. An increase in octogenarians from 3.1% in 2000 to 4.3% in 2015 was observed while during the current study period an increase from 4.3% to 4.5% was observed [2]. In absolute numbers, this meant an increase from 717089 octogenarians in 2014 to 798820 octogenarians in 2018. The current study showed that no increase in the number of surgical procedures in octogenarians was observed in the past years. Several explanations are proposed such as earlier detection of malignancies due to early age surveillance and possibly stricter patient selection in multidisciplinary team meetings. Other reasons for no increase in octogenarians undergoing major surgical procedures might be new and less invasive multimodal alternatives for elderly patients such as definitive chemotherapy or (stereotactic) radiotherapy [21-24]. It was also described that a decrease in major surgical resection in elderly patients had taken place before the inclusion period of the current study [25]. Only for pancreatic cancer resection, a positive trend in the number of treated elderly patients was described [12]. It could be that we are now witnessing a plateau phase after a historical decrease in major surgical procedures in octogenarians. It is intriguing that this plateau phase has been witnessed in the current study as with current innovations such as less invasive surgery one would have expected to observe an increase in surgical procedures in octogenarians. However, with upcoming initiative such as prehabilitation, a future increase in the number of surgical procedures in octogenarians can possibly be expected.
Assessment of postoperative outcomes could aid clinicians in considering whether performing major surgical procedures is safe in octogenarians. Several postoperative outcomes were worse in octogenarians after esophageal cancer, stomach cancer, lung cancer, CRLM, pancreatic disease, colorectal cancer or AAA [12, 26-31]. This compares equally to the results in the current study. However, higher LOS might also reflect structural problems after surgery in octogenarians, such as problems regarding rehabilitation or transfer to a nursing home [32, 33]. The lower LOS of octogenarians who underwent AAA-repair can be attributed to the more frequent use of endovascular surgery in octogenarians [34]. As several factors influence LOS, other outcomes such as 30 day major morbidity and 30 day mortality better reflect outcomes in octogenarians. Unfortunately, only LOS improved after stomach and colorectal cancer surgery in the current study, while other postoperative outcomes did not improve for the various surgical fields. These observed decreases in LOS might be a result of the implementation of Enhanced Recovery After Surgery in these fields, but could also be attributed to the different patient selection per type of major surgical procedures. Measures to improve postoperative outcomes and therewith quality of care should be searched for by clinicians treating octogenarians. This study can be used as a benchmark to measure improvement of outcomes in elderly patients.
Results of the current study show that there is room for improvement regarding major surgical procedures in octogenarians. Preoperative selection should be (evidence) based on proper risk stratification and by making use of the optimalization of octogenarians by prehabilitation [35]. Using prehabilitation, modifiable risk factors can be optimized before surgery resulting in better postoperative outcomes [36]. As a result, it might be possible to perform major surgical procedures in more octogenarians due to more favorable outcomes resulting from implementation of multimodal prehabilitation [24]. Future studies should focus on this subject to improve quality of care for octogenarians undergoing major surgical procedures.
The current study has several limitations. First, the retrospective design of the study using registry data results in the lack of perioperative information and the differences between registries in the used outcomes can result in overestimation and underestimation of outcomes. Also, only surgically treated patients are registered in the several quality registries. No data are available on patients who were not surgically treated. Patient selection can therefore not be fully assessed in this study. Second, due to the inclusion of several large quality registries, no multivariable correction was performed when assessing postoperative outcomes. Assessment of the worse outcomes in octogenarians should therefore be assessed in depth by future studies on outcomes in separate quality registries. Third, a very recent inclusion period means that no information was available concerning the years before 2014. Before 2014, aging of the population probably increased significantly and it would have been interesting to have studied if a trend in surgical treatment of octogenarians and outcomes was observed. However, this study can be a benchmark for the coming years regarding trends and outcomes in octogenarians in various surgical fields.
In this study, it was shown that despite aging of the population, no increase in major surgical procedures in octogenarians was observed for esophageal cancer, stomach cancer, lung cancer, CRLM, pancreatic disease, colorectal cancer or AAA. Postoperative outcomes such as LOS and 30 day mortality, however, were worse in octogenarians for several major surgical procedures, and improvement is therefore warranted.
Acknowledgements
The authors would like to thank all surgeons, administrative nurses and other administrative collaborators for data registration in the DICA quality registries database, as well as all Clinical Audit Boards for their scientific input. Collaborators: Daan M. Voeten, J. Annelie Suurmeijer, Anne-Loes Warps, Lisa van der Woude, Robin Detering, Nienke Wolfhagen.
Funding
None to be reported.
Declarations
Conflict of interest
None to be reported.
Appendix 1
See Table 3
Item No |
Recommendation |
|
|---|---|---|
Title and abstract |
1 |
(a) Indicate the study’s design with a commonly used term in the title or the abstract |
Done, page 1 |
||
(b) Provide in the abstract an informative and balanced summary of what was done and what was found Done, page 1 |
||
Introduction |
||
Background/rationale |
2 |
Explain the scientific background and rationale for the investigation being reported Done, page 2 |
Objectives |
3 |
State specific objectives, including any prespecified hypotheses Done, page 2 |
Methods |
||
Study design |
4 |
Present key elements of study design early in the paper Done, page ¾ |
Setting |
5 |
Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow-up, and data collection Done, page 3 |
Participants |
6 |
(a) Give the eligibility criteria, and the sources and methods of selection of participants. Describe methods of follow-up Done, page 3 |
(b) For matched studies, give matching criteria and number of exposed and unexposed Not a matched study |
||
Variables |
7 |
Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers. Give diagnostic criteria, if applicable Done as far as outcomes, exposures is concerned, page 4 Rest not applicable for this study |
Data sources/measurement |
8* |
For each variable of interest, give sources of data and details of methods of assessment (measurement). Describe comparability of assessment methods if there is more than one group Done, page 4 |
Bias |
9 |
Describe any efforts to address potential sources of bias Done, in limitations (page 9) |
Study size |
10 |
Explain how the study size was arrived at Done, page 4 |
Quantitative variables |
11 |
Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why Done, page 5 |
Statistical methods |
12 |
(a) Describe all statistical methods, including those used to control for confounding Done as far as applicable, page 5 |
(b) Describe any methods used to examine subgroups and interactions Done, page 5 |
||
(c) Explain how missing data were addressed Done, page 5 |
||
(d) If applicable, explain how loss to follow-up was addressed Not applicable |
||
(e) Describe any sensitivity analyses Done, page 5 |
||
Results |
||
Participants |
13* |
(a) Report numbers of individuals at each stage of study—e.g., numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow-up, and analyzed Not applicable |
(b) Give reasons for non-participation at each stage Done (page 4 methods, and page 6 in total numbers) |
||
(c) Consider use of a flow diagram Was considered, but was not included as a result of conclusions by all authors |
||
Descriptive data |
14* |
(a) Give characteristics of study participants (e.g., demographic, clinical, social) and information on exposures and potential confounders Done, Table 1 page 13/14 |
(b) Indicate number of participants with missing data for each variable of interest Done, Table 1 page 13/14 (missing data patients were excluded) |
||
(c) Summarize follow-up time (e.g., average and total amount) Not applicable |
||
Outcome data |
15* |
Report numbers of outcome events or summary measures over time |
Main results |
16 |
Give unadjusted estimates and, if applicable, confounder-adjusted estimates and their precision (e.g., 95% confidence interval). Make clear which confounders were adjusted for and why they were included Done, confounder adjusted estimates not applicable. Tables 1 and 2 page 13/14 |
(b) Report category boundaries when continuous variables were categorized Not applicable |
||
(c) If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period Not applicable |
||
Other analyses |
17 |
Report other analyses done—e.g., analyses of subgroups and interactions, and sensitivity analyses Done (page 6/7) |
Discussion |
||
Key results |
18 |
Summarize key results with reference to study objectives Done page 8 |
Limitations |
19 |
Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss both direction and magnitude of any potential bias Done, page 9 |
Interpretation |
20 |
Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies, and other relevant evidence Done page 8/9 |
Generalizability |
21 |
Discuss the generalizability (external validity) of the study results Done page 8/9 |
Other information |
||
Funding |
22 |
Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based Done, no funding (title page and last page) |




