Boldness towards novelty and translocation success in captive-raised, orphaned Tasmanian devils
Abstract
Translocation of endangered animals is common, but success is often variable and/or poor. Despite its intuitive appeal, little is known with regards to how individual differences amongst translocated animals influence their post-release survival, growth, and reproduction. We measured consistent pre-release responses to novelty in a familiar environment (boldness; repeatability = 0.55) and cortisol response in a group of captive-reared Tasmanian devils, currently listed as “Endangered” by the IUCN. The devils were then released at either a hard- or soft-release site within their mothers' population of origin, and individual growth, movement, reproduction (females only), and survival across 2–8 months post-release was measured. Sex, release method, cohort, behavior, and cortisol response did not affect post-release growth, nor did these factors influence the home range size of orphan devils. Final linear distances moved from the release site were impacted heavily by the release cohort, but translocated devils' movement overall was not different from that in the same-age wild devils. All orphan females of reproductive age were subsequently captured with offspring. Overall survival rates in translocated devils were moderate (∼42%), and were not affected by devil sex, release method, cohort, release weight, or pre-release cortisol response. Devils that survived during the study period were, however, 3.5 times more bold than those that did not (effect size r = 0.76). Our results suggest that conservation managers may need to provide developmental conditions in captivity that promote a wide range of behaviors across individuals slated for wild release. Zoo Biol. 33:36–48, 2014. © 2013 Wiley Periodicals Inc.
INTRODUCTION
Translocation of threatened or endangered species is an important tool used by many conservation programs to supplement already existing populations or to return animals to sites where they were previously extirpated. Unfortunately, survival rates of translocated animals (especially carnivores) raised in captivity are usually low [Beck et al., 1994; Fischer and Lindenmayer, 2000; Jule et al., 2008; Molinari-Jobin et al., 2010]. Factors such as pre-release conditioning [Dobson and Lyles, 2000], time spent in captivity [Fernández-Morán et al., 2004; McPhee, 2004; Devineau et al., 2011], release method [Ewen and Armstrong, 2007], cohort effects [Rödel et al., 2009], age [Aaltonen et al., 2009], sex [Wedekind, 2002], and social factors [Gelling et al., 2010; Shier and Swaisgood, 2012] may all have an impact on translocation success, but for many endangered or threatened carnivores, if and when these different factors impact on success (or interact) are generally unknown. In cases where conservation programs implement translocation on new species, use of adaptive management to refine future decisions can be implemented [Rout et al., 2009]. Given uncertainty surrounding a new translocation strategy, adaptive management involves systematic collection of information on released individuals and outcomes of their success to determine the effectiveness of different management methods [Sarrazin and Barbault, 1996].
Of course, information-gathering by conservation programs is often restricted logistically and/or financially, and it can be unclear in cases where new species are translocated as to which components of a translocation strategy should be considered. In addition, working with an endangered species may necessarily restrict sample sizes available to test multiple putative factors of interest. In cases such as these, in addition to considering more obvious (and easily measured) features such as an individual's sex [Letty et al., 2000] or its history of temporally-specific social and environmental conditions [e.g., cohort effects: Linklater et al., 2012], variation in pre-release behavior and physiology between captive animals may need to be considered, as these are thought to be the most likely predictors of an individual's post-release health, survival, and reproduction [Kleiman, 1989; Sarrazin and Barbault, 1996; Sarrazin and Legendre, 2000; Teixeira et al., 2007; Jule et al., 2008; Dickens et al., 2010]. Individual variation in behavior and physiology is ubiquitous in nature [i.e., “personalities,” “coping” styles, and “behavioral syndromes”: Koolhaas et al., 1999; Wingfield, 2003; Sih et al., 2004]. Individual variation in behavior and/or stress physiology may largely affect an individual's foraging behavior [Rands et al., 2003; van Oers et al., 2005], habitat choice [Stamps and Swaisgood, 2007; Stamps et al., 2009], antipredator response [Sih et al., 2003; Quinn and Cresswell, 2005], social strategy [Sinervo, 2001; Sih and Watters, 2005], and survival [Dingemanse et al., 2004]. In other words, individual differences in behavior and stress physiology in a release group may be the salient feature when considering translocated animals' welfare, reproduction, and mortality, and thus overall translocation success [Teixeira et al., 2007]. Unfortunately, there is a dearth of information currently available on intrinsic individual-level variation and subsequent translocation success in endangered carnivores [Bremner-Harrison et al., 2004; Watters and Meehan, 2007].
The Tasmanian devil (Sarcophilus harrissii), the world's largest extant carnivorous marsupial, is restricted in its range to the island state of Tasmania, where it has faced significant population declines since the 1990s [Hawkins et al., 2006] due to a contagious fatal disease, Devil Facial Tumor Disease [DFTD; McCallum and Jones, 2006; McCallum, 2008]. DFTD, a transmissible cancer, has had significant impacts on population structure [Lachish et al., 2007; 2009], life history [Jones et al., 2008; Lachish et al., 2011], and resulting population dynamics. In some local populations devil numbers have decreased by 90% since the late 1990s [McCallum et al., 2009], resulting in a listing of the species by the IUCN as “Endangered” (http://www.iucnredlist.org/). Management approaches aimed at the recovery of devil populations in the wild are currently limited, but include reintroduction following extinction in the wild from an “insurance” metapopulation and genetic restoration involving translocations [Jones et al., 2007]. An insurance metapopulation of Tasmanian devils currently exists in a number of captive facilities, including intensive captive pens in wildlife parks and zoos, in free-range enclosures, and in a semi-wild population on an island [Jones et al., 2007; Sinn et al., 2010a]. These populations are managed with the idea of maintaining 95% genetic diversity in captivity for 50 years, but to date, little has been quantified concerning how captive devils may behave, reproduce, and survive upon translocation to the wild.
Our overall aim in this paper is to explore whether pre-release behavior and physiology, sex, cohort, and release method influence the post-release growth, movement, reproduction, and survival of translocated captive-reared Tasmanian devils. A secondary aim is to compare patterns of stress physiology, sex, movement, reproduction, and survival of the translocated orphan devils with similar aged and sex devils from the wild. Our available sample size of orphan devils for translocation was limited in this endangered species conservation program, necessitating rigorous reduction of the number of independent variables prior to analysis and careful interpretation of results. This paper represents the first exploratory analysis of the factors that may influence translocation success of Tasmanian devils, a key component of the effective conservation of this species.
METHODS
Subjects
We had access to two different cohorts of orphaned Tasmanian devils that were slated for release in their mothers' population of origin, as well as a small same-age wild cohort of devils. Twenty-eight subadult devils contributed data to our study. Four subjects (two males and two females) were selected from the wild population of devils in the release area on the Forestier Peninsula in southeast Tasmania (42° 03′ 53″ S, 148° 17′ 14″ E). Twenty-four subjects had been hand-reared within the Department of Primary Industries, Parks, Water, & Environment (DPIPWE) orphan care program, Tasmania, Australia, following removal as pouch young from diseased females that were euthanized as part of a disease suppression trial on the Forestier Peninsula [see Lachish et al., 2010]. Hand-reared orphaned devils were from two different cohorts. The first cohort (birth year 2006) consisted of 13 devils (six females, seven males) from seven different litters. The 2006 cohort came into orphan care during July 2006—August 2006, where they were hand-reared (bottle-fed) together in litters or groups of no less than two individuals. The 2006 cohort was moved from individual carers to four adjacent group-holding pens at a wildlife park (Tasmanian Devil Conservation Park, Taranna, TAS) in April 2007, where they remained in mixed groups of three to four individuals until release. The 2007 cohort came into orphan care during July 2007–August 2007, and consisted of 11 devils (six females, five males) from five different litters. The 2007 cohort differed from the 2006 one in that they did not spend time in the mixed group-holding pens. The 2007 cohort was released at a younger age directly from individual orphan care and so data were unavailable for pre-release behavioral and cortisol responses (see Data Analysis section). Since sample sizes were small, we used all available data from the two orphan cohorts where possible. All devils were uniquely tagged with individual microchip transponders (Allflex©, Palmerston North, New Zealand) implanted subcutaneously under the skin to allow for individual identification.
Release Sites and Methods
Release of the 2006 cohort occurred from 29 November 2007 to 10 December 2007, when individuals were approximately 20 months old. All 2007 cohort devils were released from 11 January to 19 May 2008 directly from orphan care, when devils were 10–14 months of age. Typically, male and female devil offspring are weaned and disperse from their natal home at 9 months of age [Lachish et al., 2011]. Orphaned devils were released at three different sites, all within DFTD-affected areas but also within the orphans' population of origin (Fig. 1). The Forestier Peninsula site offered several additional useful features: (a) it is large (160 km2); (b) it has a high degree of natural isolation being surrounded by water on all sides and connected to “mainland” Tasmania only by a narrow road bridge across a boating channel and to the Tasman Peninsula to the south by a narrow isthmus; and (c) there was an already existing intensive quarterly trapping program over the entire peninsula [Lachish et al., 2010]. The northern third of the Forestier peninsula comprises a mixture of dry eucalypt forest and improved pasture on a large livestock property that supports a high-density devil population. All three release sites were in the northern third of the peninsula. The lower two-thirds of the peninsula are primarily forests managed for timber harvest which grade from dry into wet eucalypt forest at the southern end that supports lower densities of devils [Pukk, 2005].
Translocated orphan devils were given either a “soft-” or “hard-release.” Hard release involved transporting the devils in traps to the release site in the late afternoon. Traps were placed within 100 m of each other and left open for the devils to leave on their own free will. The hard release site was located within forest in the northeastern part of the peninsula just south of the main area of pasture, and was chosen to maximize the distance (5 km) from the main highway on the peninsula. Soft release involved holding devils in pens on-site for 1 week prior to release, and providing supplemental food after release. Supplemental feeding involved placing carcasses of native herbivores at several sites within 1 km from the release site, three times a week for 4 weeks, then twice a week for 4 weeks, followed by once a week for 4 weeks. The soft release site for the 2006 cohort was located in a mosaic of pasture and forest in the northwestern part of the peninsula along a drainage system that channeled animal movement away from the highway (1 km distant), and was chosen for logistical reasons (i.e., staff access for supplemental feeding). For the 2007 cohort, a new soft release site was chosen on the pastured at the eastern edge of the peninsula, 5 km north of the hard release site and 8 km from the highway.
Five individuals from the 2006 orphan cohort showed stereotypical pacing of pen walls and were retained in the wildlife park as display animals. The remaining devils from the 2006 cohort were assigned to hard or soft release sites after consideration of relatedness, sex, and size of individuals. Eight individuals (four males, four females) were equally split across hard and soft release sites with related individuals and rearing groups (2006 cohort) being split among treatments. The majority of the 2007 cohort was released at the soft release site (four males, five females); two individuals (one male, one female) from the 2007 cohort were released at the hard release site (Table 1).
| Devil | Cohort | Sex | Release date | Release method | Behavioral assay (Y/N) | ACTH challenge (Y/N) | Radiocollared (Y/N) | Survival to July 08 |
|---|---|---|---|---|---|---|---|---|
| Captive-raised orphan devils that were released | ||||||||
| Blinky | 2006 | M | 10/12/07 | Soft | Y | Y | Y | N |
| Pixie | 2006 | F | 29/11/07 | Hard | Y | Y | Y | N |
| Buzzy | 2006 | M | 1/11/07 | Soft | Y | Y | Y | N |
| Fanou | 2006 | F | 10/12/07 | Soft | Y | Y | Y | N |
| Dutchess | 2006 | F | 29/11/07 | Hard | Y | Y | Y | N |
| Victor | 2006 | M | 29/11/07 | Hard | Y | Y | Y | Y |
| Joe | 2006 | M | 29/11/07 | Hard | Y | Y | Y | Y |
| Queenie | 2006 | F | 10/12/07 | Soft | Y | Y | Y | Y |
| Mozart | 2007 | M | 11/01/08 | Soft | N | N | N | Y |
| Tosca | 2007 | M | 11/01/08 | Soft | N | N | N | N |
| Sweetie Pie | 2007 | F | 1/03/08 | Soft | N | N | N | Y |
| Hamburger | 2007 | M | 23/02/08 | Soft | N | N | N | N |
| Freddy | 2007 | M | 24/02/08 | Soft | N | N | N | N |
| Minty | 2007 | F | 26/03/08 | Soft | N | N | N | Y |
| Murf | 2007 | F | 26/03/08 | Soft | N | N | N | Y |
| Myrna | 2007 | F | 4/04/08 | Hard | N | N | N | Y |
| Levi | 2007 | M | 4/04/08 | Hard | N | N | N | N |
| Lyrra | 2007 | F | 19/05/08 | Soft | N | N | N | N |
| Ashka | 2007 | F | 19/05/08 | Soft | N | N | N | N |
| Captive-raised orphan devils retained in captivity | ||||||||
| Orion | 2006 | M | N/A | N/A | Y | Y | N | N/A |
| Draco | 2006 | M | N/A | N/A | Y | Y | N | N/A |
| Lady | 2006 | F | N/A | N/A | Y | Y | N | N/A |
| Wilma | 2006 | F | N/A | N/A | Y | Y | N | N/A |
| Derek | 2006 | M | N/A | N/A | Y | Y | N | N/A |
| Wild devils | ||||||||
| Masikus | 2006 | F | N/A | N/A | N | Y | Y | Y |
| Julia | 2006 | F | N/A | N/A | N | Y | Y | Y |
| BHoliday | 2006 | M | N/A | N/A | N | Y | Y | Y |
| B Bevan | 2006 | M | N/A | W | N | N | Y | N |
Pre-Release Behavioral Assay
Behavioral assays were performed on the 13 devils from the 2006 cohort prior to release; assays were not performed on the 2007 cohort due to logistics. The test arena for behavioral observations was a holding pen that devils had already been exposed to during group housing at the wildlife park, 5 m × 5 m in size, which consisted of a 1.2 m high perimeter fence made of corrugated iron placed on natural ground, skirted with 0.5 m wide chicken wire to discourage digging near fences. The test arena was modified by fixing a large pink exercise ball (1.2 m diameter) with rope at one end of the enclosure and a mirror (0.8 m × 1.4 m) at the other. None of the devils had been exposed to these objects previously; thus, we considered their reactions to these objects to be “boldness” with regards to novelty (the beach ball and mirror) in a semi-familiar environment [Réale et al., 2007]. Each devil was given the behavioral assay twice; once during the second week of November 2007 and then again 6 days later. Testing order of individuals on each test day was haphazard (i.e., groups of devils were targeted for testing on a particular day, but the order of testing individuals within target groups was randomized). Due to logistic reasons (i.e., volunteers were only able to feed devils during the day), the 2006 cohort devils were day active at the time of the behavioral assays, so assays were given during daylight hours (0800–1700). Preliminary observations indicated that after a short acclimation period, devils did not react to the presence of human observer standing outside the test arena as long as the observer remained quiet and restricted their movements. At the start of each behavioral assay, the subject was placed in a standardized location in the test arena in a hessian sack, and was allowed to emerge from the sack in its own time. A 30 min observation period began when the devil emerged from the sack (all devils climbed out of sacks unassisted, mean time = 67.8 sec, SD = 83.6 sec).
Five behaviors were recorded during behavioral assays: latency to approach within 1 min of the mirror (s), latency to approach within 1 min of the beach ball (s), number of times the subject devil touched the mirror (frequency count), number of times the subject devil touched the beach ball (frequency count), and total time spent moving (sec). For frequency counts, we used an a priori “5 second rule,” whereby a behavior was scored as a multiple frequency if there was at least a 5 sec break between occurrences. For example, if a subject touched the beach ball, subsequent touches were counted only if they occurred 5 sec after the previous touch. The first author performed all behavioral observations. During the second week of testing a second observer independently recorded behaviors: inter-observer agreement (Pearson's r) on four of the five recorded behaviors was higher than 0.53. Pearson's r between observers for total time spent moving was low (0.12), this variable was therefore removed from further analyses. Only behavioral observations made by DS were used in further statistical analysis.
Pre-Release Physiological Assay
The 2006 orphan cohort and four wild same-age cohort devils were given an ACTH challenge to test the functionality of the hypothalamic pituitary axis, and to provide pre-release measures of the capacity of the animals to mount an appropriate cortisol response to a standard stressor. The ACTH challenge was a single dose of Synacthen Depot (Novartis), 1 mg/ml. One milligram Synacthen corresponds to approximately 100 international units of ACTH so a 5 kg devil receiving 0.5 ml Synacthen receives a dose equivalent to 10 iu/kg body weight. The ACTH challenge was given to the captive orphans during the fourth week of November 2006, after the second behavioral assay, but prior to release. The wild devils were tested similarly in January 2008 during a trapping trip. Captive and wild animals were trapped and blood sampling occurred before 12:00 hr. The subject devil was gently removed from the trap to a hessian sack; 600 µl of blood was obtained by pricking the peripheral ear vein with a lancet and collecting the blood into heparinized microhematocrit tubes. The devil was weighed and an intramuscular injection of ACTH was given in the rump or thigh at a dosage rate of 20 μl per kilogram body weight; the animal was then returned to the trap. At 30 min after the ACTH injection, a second blood sample was taken following the same procedure. Blood samples were stored on cold packs in the field and centrifuged that night; the plasma component was separated and stored at −20C until assayed.
Plasma cortisol in blood samples was measured using a specific radioimmunoassay as in [Jones et al., 2005] but using a different antiserum. Briefly, 25 µl plasma was extracted with 1 ml absolute ethanol (A.R. grade); 100 µl extract was assayed in duplicate and commercial control sera were included to confirm accuracy. Standards were set to 12.5–800 pg authentic cortisol in 50 µl absolute ethanol. Fifty microliters of tritiated cortisol (∼4,500 cpm: Amersham, diluted in absolute ethanol) were added to each sample or standard tube and dried down before 200 µl of antiserum (Bioquest SiroSera C-3368, diluted 1:20,000 in phosgel buffer) was added to all tubes except the non-specific binding tubes. The assay was incubated overnight at 4°C. Aliquots of 300 µl supernatant were counted in 2.5 ml scintillation fluid (Ecolite®) for 5 min using a Beckman LS 5801 liquid scintillation counter. Cortisol concentrations were calculated using a log-logit plot, and corrected for the extraction efficiency of 80%. Assay accuracy and precision were assured by including three levels of a commercially available human control serum (CON-6®; Diagnostic Products Corporation, Los Angeles, California, USA) in each assay.
Post-Release Monitoring of Survival, Growth, Reproduction, and Movement
The Forestier Peninsula site had been trapped as three contiguous trapping regions up to four times a year since January 2006. For this study, we examined trapping data (GPS location and body weight (kg) for all individuals, and reproductive activity in released females) from four trips to the region in December 2007, and January, April, and July 2008. Trapping periods typically included 40 traps set for 10 nights in each trapping region simultaneously, except for December 2007 when a single trip was performed for seven nights using 35 traps. During field trips, PVC pipe traps (diameter 315 mm × length 875 mm) were placed approximately 250 m apart at landscape features in locations that would increase the likelihood of capture success. Traps were baited with native herbivore prey (wallaby Macropus rufogriseus and pademelon Thylogale billardierii) sourced from private culling programs on site, and checked daily commencing in the early morning. DFTD transmission was minimized by following a protocol developed by veterinary practitioners; this involved the sterilization of all equipment and traps with Virkon™ (Antec International, Du Pont Animal Health Solutions, Sudburg, UK), an antibacterial, antiviral, antifungal, DNA denaturing solution, and subsequent burning of hessian sacks and any remaining bait.
We used trapping records to measure survival to July 2008, post-release growth of orphaned devils ((last known weight − release weight)/number of days between measures (kg/day)), and to observe whether orphaned female devils successfully reproduced through observations of female pouches. We considered devils that were not found post-mortem or were not trapped after release as “unsuccessful”; we assumed these individuals had most likely died. It is possible that devils that were not found had left the reintroduction area, but we consider this unlikely given the size and geographic isolation of the Forestier Peninsula.
Data on devil movements were also collected using GPS data loggers (Televilt electronics) attached to leather collars that were placed on the animals prior to release (eight captive-raised individuals from the 2006 cohort) or on wild individuals from the same age cohort (four wild individuals, the same wild individuals that experienced the ACTH challenge). All collars were removed or collected by January 2008. All GPS collars had an inbuilt magnetic pre-programmed time-release; collars were timed to release and fall off devils during the night to decrease the likelihood collars would be lost underground in dens. Unfortunately, we obtained reliable data from collars for only one captive raised orphaned devil and three wild individuals due to a combination of collar malfunction, loss, and difficulty of obtaining fixes. Therefore, we combined all available radio collar, trapping location, and location of mortality data (Table 2) to calculate two animal movement metrics using ArcView GIS 3.3: the linear distance from the release site to last known location (from collar or trapping data), and the individuals' 95% minimum convex polygon (MCP) using all available data points. Final linear distance was not calculated for the four wild-caught devils, but 95% MCP home range metrics were.
| Group | Radiotelemetry | Trapping | Mortality | Total |
|---|---|---|---|---|
| Soft release | ||||
| Male (n = 6) | 0 | 16 | 2 | 18 |
| Female (n = 7) | 3 | 20 | 0 | 23 |
| Hard release | ||||
| Male (n = 3) | 2 | 52 | 2 | 56 |
| Female (n = 3) | 0 | 15 | 1 | 16 |
| Wild | ||||
| Male (n = 2) | 12 | 14 | 0 | 26 |
| Female (n = 2) | 54 | 12 | 0 | 66 |
| Total | 71 | 129 | 5 | 205 |
- The two separate cohorts of devils are collapsed here.
Data Analyses
Due to our small sample size and large number of factors, we first attempted to reduce the number of potential predictor variables used in subsequent analyses. For each of the four observed behaviors, we averaged observations across weeks within individuals [Epstein, 1983; Fleeson, 2001; 2004] and examined the subsequent correlation matrix of the four averages (N = 13 for all pairwise comparisons; Table 3). We also performed principal components analysis [PCA, N = 13: Tabachnick and Fidell, 1996] on the standardized average behaviors. The four behaviors (latency to touch mirror, latency to touch ball, (−) frequency of mirror touches mirror, (−) frequency of ball touches) were strongly inter-correlated (averaged r using Fisher's r to z transformation = 0.78, L95% CI = 0.40, U95% CI = 0.93), and loaded together on a single principal component explaining 84.3% of the variation (Table 4).
| Behavior | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Latency to touch mirror (1) | 1.00 | |||
| Latency to touch ball (2) | 0.65 | 1.00 | ||
| Frequency of mirror touches (3) | −0.83 | −0.80 | 1.00 | |
| Frequency of ball touches (4) | −0.80 | −0.65 | 0.85 | 1.00 |
- Spearman's rank coefficients are given.
| Behavior | PCA 1—“Boldness” |
|---|---|
| Latency to touch mirror | −0.91 |
| Latency to touch ball | −0.89 |
| Frequency of mirror touches | 0.93 |
| Frequency of ball touches | 0.94 |
| KMO | 0.86 |
| Bartlett's test of sphericity | 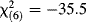 , P < 0.0001 , P < 0.0001 |
- The Kaiser–Meyer–Olkin (KMO) statistic compares the observed correlations and partial correlations among the original variables; correlation matrices with KMO < 0.5 are inappropriate for PCA, those with KMO below 0.6–0.7 should be treated with caution, and those with KMO > 0.70 are adequate [Budaev, 2010]. Bartlett's test of sphericity has the null hypothesis that all correlations are zero.
Aggregate behavior scores were computed for each devil for each test time by summing the four observed standardized measurements (“latency to touch mirror” [reverse keyed], “latency to touch ball” [reverse keyed], “frequency of mirror touches” and “frequency of ball touches”). We conducted standardization according to the mean and SD of the global behavior observed at both time periods (i.e., N = 26, each individual occurred in two rows), allowing for variation in scores between time points. Higher boldness scores describe devils that had shorter latencies to initially touch novel objects and touched them more frequently during test periods; lower scores described devils with larger latencies and higher overall frequencies of touching the novel objects. Boldness scores were repeatable from time 1 to time 2 (intraclass correlation coefficient: 0.55, F(12,13) = 3.093, P = .03), so we summed the first and second standardized boldness scores to arrive at a single sum boldness score per devil. Variation in between-individual aggregate responses to novel objects in familiar environments is commonly considered an index of “boldness” in nonhuman animals [Réale et al., 2007]; we adopt this terminology here. Only the single sum boldness score was used in subsequent analyses, hereafter referred to as “boldness.”
In a further attempt to reduce the number of factors needed for prediction we tested whether boldness was expressed in a sex-specific manner using a t-test (N = 13), and examined the correlation matrix of boldness, the three cortisol measures from the ACTH challenge, and initial release body weight (N for all pairwise comparisons = 13). Basal cortisol concentrations were non-normally distributed (Shapiro–Wilk normality test: W = 0.82, P = 0.004), so we used Spearman's rank for correlations that included this variable.
We tested whether the ACTH challenge was successful using a one-way ANOVA with sex as the between-subjects factor and time (basal and 30 min post-treatment concentrations) as the within-subjects factor (N = 17). Due to sample sizes and unbalanced design we did not include “source” (i.e., wild or orphan) in this linear model. Instead, we graphically compared means and 95% confidence intervals of basal cortisol and 30 min cortisol responses between orphan and wild devils [Cumming et al., 2007].
Standardized values of boldness, basal cortisol, and release weight were independent of one another (Table 5). Given this, we used main effects models to examine how sex, cohort, release method, boldness, and basal cortisol impacted on growth, movement, and survival. We tested whether sex, release method, and cohort predicted post-release growth using a general linear model (GLM; N = 16). We then examined the relationship between post-release growth, boldness, and basal cortisol (N = 7 for both comparisons) using Spearman-rank coefficients (post-release growth was not normally distributed, Shapiro–Wilk W = 0.67, P = 0.0001).
| Boldness | Basal cortisol | 30 min cortisol | Release weight | |
|---|---|---|---|---|
| Boldness | 1 | |||
| Basal cortisol | 0.00 | 1 | ||
| 30 min cortisol | 0.42 | −0.32 | 1 | |
| Release weight | −0.03 | 0.10 | 0.10 | 1 |
- N = 13 in all comparisons. Correlation coefficents are Pearson's r except for those comparisons that involved basal cortisol circulating concentrations, in which case they are Spearman's rank coefficients. None of the estimates reached statistical significance.
We tested whether sex, release method, and orphan cohort predicted the final linear distance animals traveled using a GLM; we also tested whether these same three independent variables predicted 95% MCP home range of translocated devils using a GLM (N = 9 for both models). Using the 2006 cohort data only, we examined the relationship between boldness, basal cortisol, and the two movement metrics using Pearson's and Spearman-rank correlations (N = 6 for all tests). Finally, we examined means and 95% confidence intervals to examine whether 95% MCP homes ranges were different between reintroduced orphan devils and their wild counterparts (N = 10 for orphans, N = 4 for wild-caught devils).
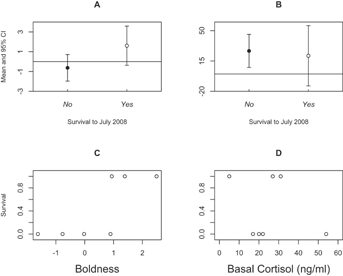
For survival, we first fit a binary logistic model with survival to July 2008 as the dependent variable, with sex, release method, cohort, and release weight as independent predictors (N = 19). Binary logistic models with boldness and basal cortisol from the 2006 cohort would not converge, likely due to complete separation in the data (Fig. 2, Panel C) and/or sample size (N = 8). Instead, we examined mean-level differences in boldness and basal cortisol concentrations in 2006 cohort devils that survived to July 2008 and those that did not, using two separate two-tailed t-tests (N = 8 for each test).
All analyses were performed using R version 3.0.1. For all GLMs we assessed linearity and homogeneity of residuals [Field et al., 2012]; no violations were found.
RESULTS
Variable Reduction and Relationships Amongst Predictors
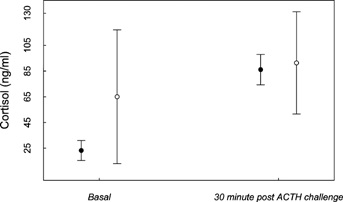
There was no effect of sex on boldness (t(10.63) = 0.02, P = 0.98), nor for sex on circulating cortisol (F(1) = 0.06, P = 0.81) or a time by sex interaction on ACTH response (F(1) = 0.37, P = 0.55) in the 2006 cohort. However, there was an overall strong 30 min response in circulating cortisol in response to the ACTH challenge (F(1) = 57.0, P < 0.001; Cohen's d = 2.79). Examination of means and 95% confidence intervals of cortisol concentrations in wild and orphan devils revealed that orphaned devils did not have a significantly different pattern of response to the ACTH challenge than wild devils did, but wild devil baseline readings tended to be higher than those observed in orphans (P ≈ 0.05; Fig. 3).
Growth
None of the three independent variables (sex, release method, and cohort) successfully predicted post-release growth of captive-raised devils (R2 = 0.11, F(3,11) = 0.45, P = 0.72), and there was no relationship between an individual's boldness (Spearman's r(7) = −0.03, P = 0.96), basal cortisol (Spearman's r(7) = −0.31, P = 0.50), and post-release growth.
Movement
In total, 205 location fixes were obtained from trapping, mortality location, and GPS collar fixes (Table 2). In the main effects model sex, cohort, and release method successfully predicted final linear distance from release site (R2 = 0.91, F(3,5) = 16.16, P = 0.005). However, cohort was the only significant parameter, with devils from the 2007 cohort traveling almost five times greater distance from their release sites than devils from the 2006 cohort (Table 6; Fig. 4). For the captive-raised 2006 cohort, there was no relationship between boldness and final linear distance (Pearson's r(5) = 0.28, P = 0.64). The rank-order estimate for the relationship between basal cortisol concentrations and final linear distance was negative and moderate, but not statistically significant (Pearson's r(5) = −0.61, P = 0.27).
| Parameter | Estimate | Standard error | t | P-value |
|---|---|---|---|---|
| Intercept | 765.7 | 1474.2 | 0.52 | 0.63 |
| Sex | 2254.9 | 1002.7 | 2.25 | 0.07 |
| Cohort | 5540.2 | 1124.7 | 4.93 | 0.004 |
| Release method | −433.8 | 1194.6 | −0.36 | 0.73 |
- Parameter estimates for sex is females relative to males, for cohort is 2006 relative to 2007, and for release method is soft-relative to hard-release.
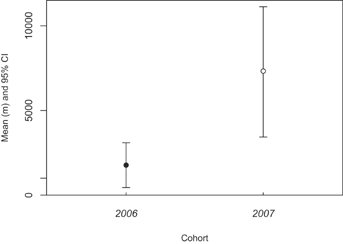
There was no relationship between sex, cohort, and release method on 95% MCP home ranges (R2 = 0.31, F(3,5) = 0.75, P = 0.57). There was a trend for bolder devils to have larger 95% MCP ranges, but this relationship did not reach statistical significance (Pearson's r(6) = 0.68, P = 0.14). The relationship between 95% MCP range and basal cortisol was strong and positive (Spearman's r(10) = 0.68, P = 0.03). Mean 95% MCP ranges between captive raised (5.71 ± 3.74 km2) and the four wild devils (6.64 ± 5.02 km2) had a large degree of overlap of 95% confidence intervals.
Reproduction
Of the captive raised released females that survived to July 2008 (two from the 2006 cohort, three from the 2007 cohort), the two females of typical reproductive age (i.e., 2-year-old age class) from the 2006 cohort were both found to be carrying four pouch young each in July 2008. None of the three 2007 cohort females had reproduced by July 2008. Both wild females used as the comparison females also had four pouch young detected during the April 2008 trip.
Survival
Of the 19 orphan devils released from the 2006 and 2007 cohorts, eight devils were alive as of July 2008, six were confirmed dead (five confirmed roadkill), and five individuals were not trapped or located by radiotelemetry after release (these individuals were presumed dead), resulting in an overall survival rate of 42.1%. During the same period of time, there was a 75% survival rate for the four wild-caught individuals, although in July 2008 one wild individual included in our study was found with signs of DFTD (M.E. Jones, unpublished data). No released orphans were found with any signs of DFTD as of July 2008. From the 2006 cohort, three individuals were alive as of July 2008, three were confirmed dead (all roadkill), and two individuals were not recaptured, resulting in a known survival rate of 37.5%. From the 2007 cohort, five individuals were alive as of July 2008, three confirmed dead (two from roadkill), and three individuals were not recaptured or found, resulting in a known survival rate of 45.4%.
None of the four independent variables (sex, release method, cohort, and release weight) successfully predicted survival of the captive-raised orphans to July 2008 over and above the constant-only logistic model (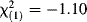 , P = 0.90). There was complete separation of boldness scores between 2006 cohort devils that survived and those that did not (Fig. 2), with devils having survived until July 2008 being, on average, more bold towards novelty than those that did not survive (t(5.50) = −3.35, P = 0.02; effect size r = 0.76). There were no mean-level differences in basal cortisol concentrations between successful and unsuccessful reintroduced devils (t(4.72) = 0.53, P = 0.62; Fig. 2).
, P = 0.90). There was complete separation of boldness scores between 2006 cohort devils that survived and those that did not (Fig. 2), with devils having survived until July 2008 being, on average, more bold towards novelty than those that did not survive (t(5.50) = −3.35, P = 0.02; effect size r = 0.76). There were no mean-level differences in basal cortisol concentrations between successful and unsuccessful reintroduced devils (t(4.72) = 0.53, P = 0.62; Fig. 2).
DISCUSSION
Here we provide a first exploratory report of several putative factors that may have an impact on translocation of captive-reared Tasmanian devils. We measured responses to novel objects in a familiar environment [“boldness”: Réale et al., 2007] and hormonal response to an adrenocorticotropic hormone (ACTH) challenge prior to release in hand-raised orphaned juvenile devils. Subjects were then released at either a hard- or soft-release (i.e., food supplemented) site within their mothers' population of origin, and we monitored release sites and measured individual survival, growth, reproduction (females only), and movement of the released animals. In addition, we monitored survival, reproduction (females only), hormonal response to an ACTH challenge, and animal movement of a same age cohort of wild devils to compare with wild fitness at release sites [Mathews et al., 2005]. Overall, we found little evidence for an effect of an individual's sex, cohort, and release method on its subsequent post-release growth, movement, and survival. There was also no detected relationship between an individuals' sex, its pre-release boldness, cortisol, and release weight. Orphans and wild devils had similar cortisol profiles in response to the ACTH challenge, but wild devils had higher baseline cortisol concentrations overall. The younger cohort of orphaned devils (2007) moved greater distances than the older cohort of orphans (2006), but there were no differences in measured home range sizes between orphan cohorts and wild devils. Orphaned devils with higher baseline cortisol concentrations had larger resultant home ranges during our study. Female orphan devils of reproductive age were all found to be carrying pouch young. Overall, survival of orphans during our study was moderate (∼42%), and again, neither sex, cohort, release method, or cortisol predicted survival. Instead, we found that devils that survived during our study were three and a half times as bold in pre-release behavioral assays than those than did not. Below we discuss these results in light of our sample sizes and subsequent translocation efforts in this and other endangered carnivore translocation studies.
We detected little relationship between sex, pre-release weight, and boldness, and as well, these factors along with cohort and release method did not predict post-release measures of growth. In addition, sex and pre-release weight were not found to impact on our measure of post-release survival. An individual's sex can often have a large impact on life history [e.g., Crews et al., 1998], physiological stress responses [e.g., While et al., 2010], growth [Mangel and Stamps, 2001], and subsequent behavior [e.g., Hedrick and Kortet, 2012]. While we consider our negative results in this regard to be preliminary and in need of further testing (see below), sex, behavior, physiology, and growth traits that are phenotypically independent of one another implies that future translocation efforts using captive Tasmanian devils may be able to select for particular univariate phenotypes without affecting other individual traits that also may be important [e.g., Veenema et al., 2003; Dickens et al., 2009]. In other words, selection of release candidates may be possible based solely on sex, behavior, size, or physiology (or another independent character thought to be salient), and not on multivariate profiles of these traits simultaneously. Clearly, future tests using larger sample sizes are needed to confirm these ideas, especially since correlated suites of traits within individuals are commonly observed [e.g., Koolhaas et al., 1999; Murn and Hunt, 2008; Pinter-Wollman, 2009; Pinter-Wollman et al., 2009; Carere et al., 2010]. Genetic studies using current insurance population captive individuals may also be useful in determining any genotypic correlations between these putative traits of interest, as phenotypic correlations can often mask actual genotypic correlations between traits [van Oers and Sinn, 2013].
Related to the idea that none of our independent predictors successfully predicted growth is the idea of hard- versus soft-release methods. Results from other translocation efforts that have compared hard- versus soft-release methods are mixed. In some cases (i.e., burrowing owls [Mitchell et al., 2011] and Canadian lynx [Devineau et al., 2011]), soft-release methods significantly improve post-release survival; in other cases (i.e., Tawny owls [Griffiths et al., 2010] and hare wallabies [Hardman and Moro, 2006]) soft-versus hard-release method has little to no influence on survival of released animals. While there is some evidence that the length of time animals are allowed access to soft-release conditions may influence survival rates [Hamilton et al., 2010; Rouco et al., 2010], evidence is also accumulating that factors such as release cohort size and release-site habitat quality may be more salient to translocation success [Robinette et al., 1995; Linklater et al., 2012; Matějů et al., 2012; Shier and Swaisgood, 2012]. In the current example, habitat quality at both our hard- and soft-release sites may have been equivalent, and resulted in the lack of effect of release method and pre-release weight on post-release growth. Our data appears to support this hypothesis: all translocated individuals regardless of sex, release method, cohort, behavior, and physiology tended to have similar post-release growth. Future translocations using soft- versus hard-release methods on larger cohorts of translocated devils are needed to clarify these issues. In addition, reliable measures of habitat quality at release sites (along with measures of temporal and spatial heterogeneity) may need to be developed if a predictive translocation strategy is desired for Tasmanian devils. In the meantime, our results suggest that hard-release methods are sufficient during translocation of Tasmanian devils, but perhaps only when combined with suitable high-quality habitats [e.g., Ewen and Armstrong, 2007; Watters et al., 2003].
One of the main aims of most translocation programs is that animals remain and settle at the release site [Christie et al., 2011; Stamps et al., 2009; Stamps and Swaisgood, 2007]. We found that 91% of the variation in linear devil movement could be explained by an individual's sex, its cohort, and release method. Specifically, devils from the 2007 cohort traveled five times farther than those from the 2006 cohort. The 2006 orphan cohort were released just 4–5 months prior to their first breeding season (2-year-old females breed about 1 month later than mature adults; breeding in devils is annual with a mean birth date across Tasmanian populations of 20 March [Hesterman, 2008]). This 2006 cohort, therefore, were at the end of their subadult year; in the wild, they would have dispersed and settled in their adult home range by this time. In contrast, the 2007 cohort were released at dispersal age, in the months around and following weaning (weaning is late Jan to mid-Feb in the wild), which may help explain their increased movement. Taken together, these results are encouraging, and suggest that the additional time spent in captivity did not impact on the 2006 cohort's ability to settle and establish home ranges in the wild. Indeed, females from the 2006 cohort successfully bred during their first breeding season after translocation (due to their age, the 2007 cohort females would not have been expected to breed in their first year in a high density population such as the Forestier Peninsula [Jones et al., 2008]).
While behavior, sex, pre-release weight, and release method did not predict final linear distances or home range size, devils that tended to have higher basal circulating cortisol pre-release also had larger 95% MCP core ranges than devils with lower circulating cortisol. In addition, orphan devils overall had lower baseline circulating cortisol concentrations than wild cohort devils. While differences in baseline cortisol between wild and orphan devils was also confounded by time (i.e., wild devil cortisol readings were taken several months after orphan cortisol measures), these results provide some preliminary evidence that captivity may be altering stress physiological phenotypes of devils [Jones et al., 2005], perhaps due to a lack of predation threat in captivity. If captive conditions are indeed altering how individuals select, settle into, and retain different habitats or home ranges via hormonal mechanisms, further study on stress physiology in both healthy wild devils as well as their captive counterparts will be necessary to fully delineate any captive adaptations in stress responses.
Previous to this study there was no scientific evidence regarding translocation survival in devils. We found that overall survival rates in our translocated devils were moderate (∼42%) and were similar to that observed in many other translocation programs [e.g., Beck et al., 1994; Jule et al., 2008]. Survival rates of male and female translocated devils were not different from one another, and were close to previously documented survival rates for wild devils of 50% for both the sexes [Lachish et al., 2007; 2009; McCallum et al., 2009]. Intuitively, we expected that spending longer periods of time in captivity (i.e., the 2006 cohort), a lack of release support (i.e., hard release devils), and lower pre-release body weights should have lowered survival rates [e.g., Letty et al., 2000]. Perhaps surprisingly then, we detected no difference in survival between release method (hard vs. soft) or a cohort effect, and no influence of pre-release weight on devil survival. As well, our measures of hormonal stress responses did not have a strong relationship to post-release survival.
Instead, we found a strong effect of an individual devil's responses to novelty in a familiar environment on its post-release survival during our study. Indeed, the 2006 cohort devils that survived were three and a half times as bold as their counterparts that did not survive, and this was perhaps the strongest pre-release predictor of post-release outcomes documented here. Boldness is often a large component of an individual's fitness [Réale et al., 2007; Smith and Blumstein, 2008], and recent evidence has begun to suggest that individual variation in behavior may be a large determinant of translocation success [Bremner-Harrison et al., 2004; Mathews et al., 2005; Pinter-Wollman, 2009; Pinter-Wollman et al., 2009]. While our results are promising in terms of offering a phenotypic character that could be used to manipulate the composition of release groups, we also offer caution that the direction of the relationship between a particular type of behavior and individual fitness (i.e., the fitness landscape of particular behavioral types) is most always unknown. It is also likely that fitness landscapes of behavior change spatially and temporally [Dingemanse et al., 2004, 2011; Sinn et al., 2010b; Mathot et al., 2012]. In other words, while we found that increased boldness improved survival in one cohort during translocation, it is possible that this relationship could change given different spatial or temporal circumstances (i.e., individuals that are less bold could be favored in some cohorts in some years in some locations). Given the uncertainty of knowing which behavioral types may succeed in any given year, we emphasize that management efforts would do well to both maintain habitat variability in the wild as well as behavioral variability in the current Tasmanian devil insurance population [Watters et al., 2003; Watters and Meehan, 2007]. Our results suggest that developing measures of habitat quality at release sites along with continued measures of individual variation could be a primary focus of future translocation efforts in this and other endangered carnivores.
Inferences and Sample Sizes
Our study, similar to other work in other endangered species programs [Soulé, 1986; Kattan, 1992], was necessarily restricted by the limited sample sizes of orphan devils available for release. Due to the small sample sizes, it is worth noting that a lack of an effect of several of the putative factors tested here may indeed have an impact on translocation growth, movement, or survival, but we did not have the statistical power here to detect such effects. Similarly, our strong result of boldness on survival may have been affected by sample sizes—if our sample of orphans was not truly representative of the larger captive-reared Tasmanian devil population we may have detected an effect of boldness when in actuality there is none. Despite these caveats, public information reporting of endangered species actions is a critical component of any conservation science [Caro and Sherman, 2011, 2013], and is extremely useful for adaptive management techniques that require prior information on which to make future management decisions. Indeed, it is also a valid inference that since we detected a strong effect of boldness on survival despite our small sample sizes, the effect of variation in behavior on subsequent survival in translocated devils may indeed be very strong. Again, replication is a basic scientific inference tool, applicable to all scientific studies [Ryan, 2011], and the need for replication of our results is not unique in this respect.
Management Implications
Currently, the only option for saving Tasmanian devils from extinction is an “insurance metapopulation,” which includes maintaining a combination of disease-free populations across intensive captive situations for up to 50 years, until DFTD causes extinction in the wild or evolution of the tumor or the devil leads to coexistence of host and pathogen [Jones et al., 2007]. However, housing animals in captive conditions for such a large number of generations will invariably result in unintended genetic and phenotypic effects [McPhee, 2004; Jones et al., 2005; Kelley et al., 2006; Christie et al., 2012], with unknown consequences for reintroduction to the wild. Together, we believe a correct inference of our results is that responses to novelty (boldness) matters to translocation success in Tasmanian devils, and that the other factors measured here (sex, cortisol response, pre-release growth, cohort, and release method) may impact on translocation outcomes in Tasmanian devils, but future translocation efforts using larger sample sizes combined with longer-term monitoring are needed [Ewen and Armstrong, 2007; Burbidge et al., 2011]. Studies designed to understand the factors that promote behavioral diversity among the insurance metapopulation, such as habitat quality and variation, resource competition, and appropriate levels of stress normally experienced in the wild (such as non-lethal experiences with potential predators) are urgently needed.
CONCLUSIONS
- Factors such as stress [Teixeira et al., 2007], behavior [Bremner-Harrison et al., 2004], early experience [Stamps and Swaisgood, 2007], and release site and method [Linklater et al., 2012] continue to be common considerations for many captive-release and translocation programs, but there is a surprising lack of experimental evidence of the relative impacts of these different factors on translocation success [Mathews et al., 2005; Seddon et al., 2007; Armstrong and Seddon, 2008; Sheean et al., 2012].
- Our study provides some of the first evidence of the importance of individual-level variation in behavior, or “personalities,” on translocation outcomes.
- In the face of uncertainty regarding the fitness landscape of behaviors in the wild, conservation managers should continue to attempt to maintain habitat diversity in the wild and provide developmental conditions in captivity that promote a wide range of behaviors.
- An increased focus on measurements of habitat quality at release sites along with a focus on behavioral and physiological development in captive individuals should continue to be a primary focus of any translocation or reintroduction efforts for endangered carnivores.
ACKNOWLEDGMENTS
The authors would like to thank John Hamilton and Tom, Cynthia, and Matthew Dunbabin for allowing access to their properties during the study period. Several wildlife carers and volunteers made significant contributions to caring for orphaned devils pre-release, and the public community on the Forestier Peninsula provided invaluable information on post-release sightings of study animals. Special thanks are extended to Patsy Davies of DPIPWE's orphan care program. Clare Hawkins provided access to GPS collars, Billy Lazenby provided assistance with fitting GPS collars, and Angela Anderson provided key field assistance. All methods described here complied with all state and federal laws and DPIPWE agency policies (DPIPWE Animal Ethics Committee approval number: 9/2007-08). Regular veterinary checks were performed by Kim Skogvold on all individual devils during orphan care and during holding at Taranna (2006 cohort only).




