Synthesis, Structure, and Disorder in Na36Sn5Pn18 (Pn = P, Sb)
Abstract
Solid ion conducting materials are important components for all-solid-state batteries. Recently, solid lithium-ion-conducting materials with tetrahedral EP4 units are discovered for E = Si, Ge, Sn, Al, Ga, and In. The increasing demand for high-performance and low-cost battery technology promotes the search for new sodium-ion-conducting materials, since sodium holds promise to be a cheap and abundant alternative for lithium. In this work, the synthesis and characterization of the two new compounds Na36Sn5Pn18 (Pn = P, Sb) are reported. Both phases are obtained via ball-milling procedures and subsequent annealing processes. Crystallographic investigations based on single-crystal X-ray diffraction data reveal that both compounds crystallize in the space group I41/amd (no. 141). The structure can be described based on a distorted cubic closed packing of Pn atoms with Sn atoms located in tetrahedral voids. Additionally, a dimeric ethane analogue Sn2Pn6 unit is present that can be understood as a Sn2 dumbbell that occupies an octahedral void. In the P derivative, an additional disorder is observed which reveals trigonal planar SnP3 beside SnP4 and Sn2P6 units. Both compounds correspond to electron-precise Zintl phases.
1 Introduction
Replacing liquid electrolytes in ion batteries and establishing all-solid-state batteries (ASSBs) as viable alternatives are a forefront topic for the development of new technologies. Currently, lithium-based ion conductor materials are primarily focused in the development of such ASSBs.[1] However, in order to obtain long-term sustainability, a more abundant material than lithium is needed. Sodium is already considered a cheap, abundant alternative for large-scale energy storage in renewable energies.[2] Similar chemical/electrochemical characteristics as lithium furthermore allow for substitution of lithium by sodium in already established technologies. Therefore, extensive effort was made in the investigation of solid-state electrolyte materials that exhibits suitable properties for the application in ASSBs. Herein, a better understanding of the origin of material properties, such as the ionic conductivity, is essential. Investigation of structure-property relationship through comparison of crystalline materials with diverse structural characteristics respectively linked to their electronic properties allows for successive improvement of destined material attributes. This way, not only the search for high performance ionic conductors seems viable as investigation of materials with lower conductivities also unveils structure-property relationships responsible for favorable or unfavorable properties.
The system of lithium phosphidotrielates[3] and tetrelates[4] has been proven to be very well-suited for such investigations on structure-property relationships in a single material class. It offers a great variety of compounds that differ structurally and in their respective properties, while all of them can be related trough crystallographic symmetry. Among these compounds are not only fast ion conduction materials but also materials with semiconducing properties or electronic conductors.[5] We recently expanded this system by replacing lithium with its heavier homologue sodium and reported on the compounds Na8GeP4[6] and two polymorphs in Na8SnP4,[7] where the crystal structures are built from isolated tetrahedral [TtP4] units just like in their related lithium counterparts and sodium-ion conductivity is observed in the high-temperature modification of Na8SnP4. Other sodium compounds in this system can also exhibit ionic conductivity, as in supertetrahedral phosphidosilicates,[8] or form structures with condensed cage-like infinite anionic units that result in semiconducting properties or electronical conduction as in Na2Ge3P3, Na5Ge7P5, or Na3Ge2P3.[9]
Here, we present on the synthesis and characterization of the compounds Na36Sn5Pn18 (Pn = P, Sb) that contain dimeric anionic units alongside isolated tetrahedral units as building blocks in its crystallographic structure and thus represent a very close but slightly deviation neighbor of the phases Na8SnP4 and Na8SnSb4.[7, 10] The two new compounds Na36Sn5Pn18 (Pn = P,Sb) are readily accessible in gram scale and high purity. Characterization was performed with single-crystal X-ray diffraction (XRD) analysis as well as Rietveld refinement conducted on powder XRD data at room temperature.
2 Results and Discussion
2.1 Synthesis of the Compounds Na36Sn5P18 and Na36Sn5Sb18
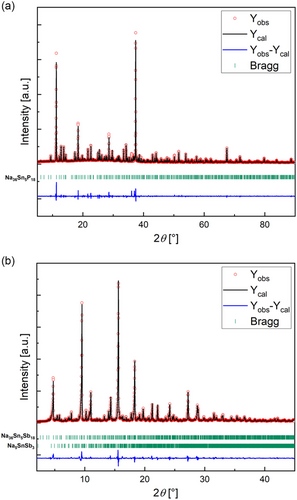
Na36Sn5P18 and Na36Sn5Sb18 were synthesized via two-step procedure from the elements. First, a reactive mixture was produced using an established ball-milling procedure. According to X-ray powder diffraction, this reactive mixture already contains small amounts of the desired compounds in case of the phosphorus compound and more significant amounts in case of the antimony compound. Subsequently, the reactive mixture was annealed in sealed niobium ampules at 500 °C for 24 h, and afterward a highly flammable polycrystalline black powder in case of the phosphorus compound and a shiny silver powder for the antimony compound was obtained. The compounds resemble a very closely related compound to the recently found polymorphs in Na8SnP4[7] as well as the respective antimony analogue.[10] For structural characterization, single crystals of both compounds were synthesized directly utilizing a one-step synthesis with a slow cooling rate was performed; as such, a procedure has proven to yield better and larger crystallites. For this, the respective elements were heated to 600 or 500 °C for the phosphorous and antimony compound, respectively, and after annealing time slowly cooled to room temperature with a rate of 0.1 K min−1. Differential scanning calorimetry (DSC) measurements prove that the compounds show melting points around 600 °C for the P-containing phase and 500 °C for the Sb-containing phase.
2.2 Crystal Structure of Na36Sn5P18 and Na36Sn5Sb18
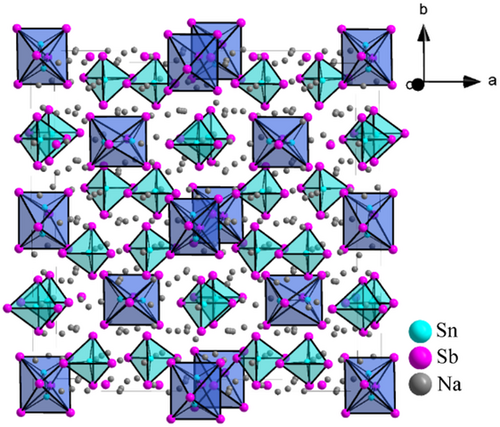
The compounds Na36Sn5P18 and Na36Sn5Sb18 crystallize in their own structure type in the space group I41/amd (no. 141) with unit cell parameters of a = 28.723(4) Å and c = 13.532(3) Å as well as a = 31.190(4) Å and c = 14.703(3) Å for the P and Sb phase, respectively (Figure 2). Details on the single-crystal refinement data at 150 K are given in Table 1. Atomic coordinates as well as anisotropic displacement parameters are given in the Supporting Information. Rietveld refinement of powder data confirm the results obtained from single crystal data (Figure 1). The data reveal disorder for some units in Na36Sn5P18 and no disorder for Na36Sn5Sb18. Hence, in the following discussion, the ordered structure parts are jointly discussed. For Na36Sn5P18, the main component of the disordered part with an occupation of 86.6(1)% is equivalent to the fully ordered structure of Na36Sn5Sb18, whereas atoms with an occupation of 13.4(1)% differ. This disordered substructure is separately discussed.
| Sum formula | Na35.7(1)Sn4.99(2)P18 | Na35.3(5)Sn4.97(1)Sb18 |
|---|---|---|
| Molar mass [g mol−1] | 1932.57 | 3605.03 |
| Crystal system | Tetragonal | Tetragonal |
| Space group | I41/amd (no. 141) | I41/amd (no. 141) |
| Radiation λ [Å] | 0.71073 (Mo–Kα) | 0.71073 (Mo–Kα) |
| Crystal size [mm3] | 0.15 × 0.15 × 0.2 | 0.1 × 0.1× 0.05 |
| Crystal color/shape | Black, chunk | Silver, tetragon |
| Cell parameters [Å] |
a = 28.713(4) b = 28.713(4) c = 13.532(3) |
a = 31.190(4) b = 31.190(4) c = 14.703(3) |
| Cell parameters [°] |
α = β = 90 γ = 90 |
α = β = 90 γ = 90 |
| Cell volume [Å3] | 11 157(4) | 14 304(5) |
| Z | 8 | 8 |
| ρ (calc.) [g cm−3] | 2.301 | 3.348 |
| μ [mm−1] | 3.003 | 8.595 |
| F(000) | 7152 | 12 485 |
| Temperature [K] | 150(2) | 150(2) |
| Reflections collected | 21 560 | 32 955 |
| Independent reflections | 3348 | 4277 |
| Reflections with I > 2σ(I) | 2064 | 3705 |
| Observed hkl |
–19 ≤ h ≤ 37 –37 ≤ k ≤ 40 –17 ≤ l ≤ 17 |
–30 ≤ h ≤ 40 –32 ≤ k ≤ 40 –19 ≤ l ≤ 15 |
| Measuring range θmin/max [°] | 2.838/27.480 | 2.399/27.496 |
| Goodness-of-fit on F02 | 0.944 | 1.041 |
| R int/Rσ | 0.0625/0.0714 | 0.0155/0.0324 |
| R indices (F02 ≤ 2σ(F02)) | R1 = 0.037, wR2 = 0.057 | R1 = 0.033, wR2 = 0.085 |
| R indices (all data) | R1 = 0.085, wR2 = 0.064 | R1 = 0.041, wR2 = 0.089 |
| Largest diff. peak/hole [Å3] | 0.831/–0.723 | 2.675/–2.042 |
| Depository Nr. | CSD-2416108 | CSD-2416109 |
The crystal structure of the ordered part contains four Sn, six respectively P or Sb, and 14 Na crystallographically independent positions (Figure 2). Herein, the pnictide orders in a strongly distorted cubic closed packing (ccp) arrangement where Sn1, Sn3, and Sn4 are situated in tetrahedral voids of the pnictide atom arrangement forming isolated [SnPn4]8− tetrahedra. The Sn2 position forms a SnSn dumbbell and can be interpreted as the SnSn vector situated around the center of a (strongly distorted) octahedral void, thus being better described as a trigonal antiprismatic void (Figure 3a). Consequently, the dumbbell forms an isolated dimeric [Sn2Pn6]12− unit with the six pnictide atoms. The unit can also be interpreted as two tetrahedras that intersect and are connected via a homoatomic SnSn bond (Figure 3b). Such ethane-like units also occur as motifs in condensed structures like Na3Ge2P3[6] or GeP[11] in the related germanium system but has to the best of our knowledge not been observed in Sn compounds. Sodium atoms are situated surrounding the Sn building units on 14 crystallographic independent positions which are located in 11 tetrahedral and three octahedral voids formed by the P/Sb atoms. Including Sn1, Sn3, Sn4, and Na1 to Na10 as well as Na14 atoms, an overall occupation of 85.6% of all tetrahedral voids realized. Considering Na11 to Na13 as well as Sn2 of the dumbbell octahedral void occupation reaches 50%.
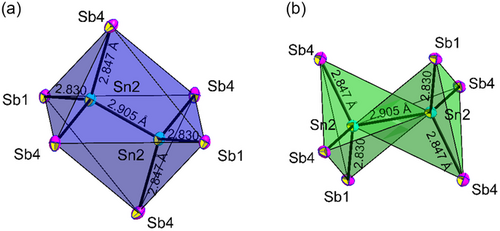
In the ordered part of the compound with crystallographically determined composition Na35.7(1)Sn4.99(2)P18, the Sn-P distances range from 2.537(1) to 2.542(1) Å which matches with known compounds such as Na10Sn2P6 (2.50–2.59 Å)[12] or Na8SnP4 (2.58(1) Å–2.92(1) Å).[7] The SnSn bond formed by two symmetry equivalent Sn2 in the dimeric unit exhibits a bond length of 2.8449(9) Å which is slightly shorter than in Ba3Sn2 that contains a Sn2 dumbbell with a SnSn distance of 2.9518 Å and formal charge of 6-.[13] The tetrahedron around the Sn4A position exhibits noticeably longer bonds with a SnP bond length of 2.648(1) Å which is likely caused by a high degree of disorder at this site as well as by defective occupation leading to smaller mean bond distances between Sn4A and P atoms at this position. Na atoms are located in either tetrahedral or octahedral voids, where the NaP bond distances are in the range of 2.875(2) Å–3.451(2) Å for tetrahedrally coordinated sites and in the range of 2.93(2) Å–3.454(3) Å for octahedrally coordinated sites matching literature known compounds like Na10Sn2P6.[10]
For the compound with crystallographically determined composition Na35.3(5)Sn4.97(1)Sb18, all atoms are ordered. Herein, Sn1 and Sn3 bond lengths to neighboring atoms are between 2.8152(5) and 2.8725(6) Å and are well within the characteristic range of covalent SnSb distances as found in related compounds such as Na8SnSb4 (2.843 Å)[10] or Na5SnSb3 (2.805(1)–2.945(1) Å).[14] The tetrahedral coordination tetrahedron around the Sn4 positions exhibits noticeably longer bonds with a SnSb bond length of 2.9761(6) Å which matches the observation in the related P derivative as described above and is accordingly most likely caused by Sn defects (site occupancy factor 94%). The SnSn bond length of 2.9050(9) Å is slightly longer than in the related P derivative which originates from larger Sb atoms. All Na atoms are located in tetrahedral or octahedral voids, where the NaSb bond distances for the residual tetrahedrally coordinated sites are in the range of 3.043(2) Å–3.554(2) Å and for the residual octahedrally coordinated sites in the range of 3.208(2) Å–3.872(27) Å. This is in good accordance with known compounds like Na8SnSb4.[10]
Single-crystal diffraction data refinement of Na35.7(1)Sn4.99(2)P18 reveals structural disorder. As a result, the main part of the structure is equivalent to the fully ordered structure of the Sb derivative, whereas other parts reveal a disorder in which 13.4% show different structural motifs. Thus, the structure exhibits a superposition of two structure motifs involving the atoms Sn2, Sn4, and P6 (Figure 4). Structure motif 1 (Figure 4a) is equivalent to the ordered part. The Sn2 split position Sn2A forms the SnSn dumbbell located in a distorted octahedral void (or trigonal antiprism of P1 and P4 atoms), and the Sn4 split position Sn4A is correspondingly located at the center of a tetrahedral void spanned by four P6A positions (Figure 4c). The reduced occupancy at the Sn4A position with 46% is related to the symmetry equivalence of the four surrounding Sn4B positions with each heaving a probability of 13.4%. This part is observed at 86% probability.
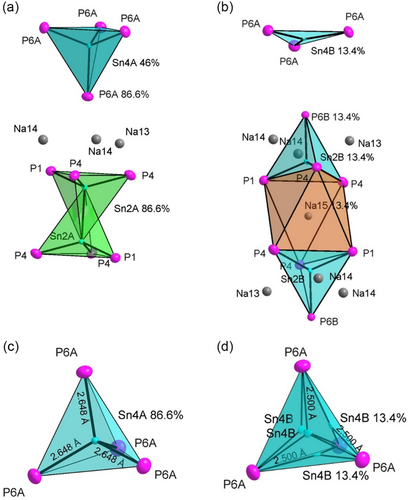
For structure motif 2 (Figure 4b), the split position Sn2B centers an SnP4 tetrahedron which shares a face with the octahedron. The latter is now centered by Na15. The tetrahedral coordination of Sn2B is formed by one P1, two P4s, and one P6B. The positions Na15, Sn2B, and P6B show each an occupancy of 13.4%. In consequence to the filling of the P4 tetrahedron with Sn2B, the neighboring vertex-sharing tetrahedron formed by P6B and three P6As filled with Sn4B is strongly distorted. The Sn4B atom is shifted toward the triangular plane of the tetrahedron spanned by three P6A, thus forming an almost planar SnP3 unit. Notice, due to the symmetry, this applies to all four symmetrically equivalent triangular planes of the Sn4 tetrahedron (Figure 4d). Refinement of the single crystal data revealed an observed probability of the expected 13.4% for this case.
Sn2B–P and Sn4B–P distances are in the range of 2.505(3)–2.603(2) Å and 2.506(5) Å, respectively. Comparing the Sn–P distance of the disordered Sn4B site in an almost planar SnP3 unit with the ordered part where Sn is located at the Sn4A site in the center of a tetrahedron spanned by four P atoms, a significant shortening of the Sn–P distance from 2.648(1) Å (Sn4A–P) to 2.506(5) Å (Sn4B–P) is observed. Interestingly, all disordered Sn-P distances are in the same range to the ordered part, matching literature observed values.[7, 10]
3 Discussion
The crystal structure of Na36Sn5Pn18 (Pn = P, Sb) represents a novel type which features two different anionic units simultaneously contrary to related compounds such as Na8SnP4 and Na8SnSb4 exhibiting exclusively isolated tetrahedral units. The electron count of both compounds can be described with the Zintl–Klemm concept by using covalent anionic building units in which Sn atoms in tetrahedral voids form discrete [SnPn4]8− units, while the Sn sites located in the dimeric unit form a SnSn bond forming [Sn2Pn6]12− units. Besides the disorder found in the P derivative, the electron count of the ordered part is as follows: Considering the multiplicity of the Sn sites, the overall eight (16 h site for Sn2) [Sn2Pn6]12− units and 23.75 (4b for Sn1, 16 g for Sn3, and 4a for Sn4 with S.O.F 0.94) [SnPn4]8− units are formed resulting in overall 286 negative charges per unit cell. These negative charges are charge balanced by 282(4) sodium atoms per unit cell resulting within standard deviation (3σ rule) in the electronically balanced formula (Na+)282(4)[(SnPn4)8−]23.75[(Sn2Pn6)12−]8 or Na35.25Sn4.97Pn18 with Z = 8 as found for the Sb derivative Na35.3(5)Sn4.97(1)Sb18. For the disordered part I, the P derivative [Sn2P6]12− units appear with 86.6%, however, together with corresponding 2 x [(Sn2B)P4]8− units at 13.4% probability and an additional Na15 atom. In addition, 86.6% [Sn4A(P6A)4]8− tetrahedra appear together with 4 x trigonal planar [Sn4B(P6A)3]5− units at 13.4% probability resulting in the formula (Na+)286(1)[(SnP4)8−]20{[(SnP4)8−]4}0.466 {[(SnP3)5−]16}0.134 {[(Sn2P6)12−]8}0.866 {[(SnP4)8−]16}0.134 arising within standard deviation equivalent to the crystallographic determined composition Na35.7(1)Sn4.99(2)P18 with Z = 8. Considering the standard deviation (3σ rule), the compound is with 285.9(1) and 286(1) negative and positive charges, respectively, within standard deviation charge balanced.
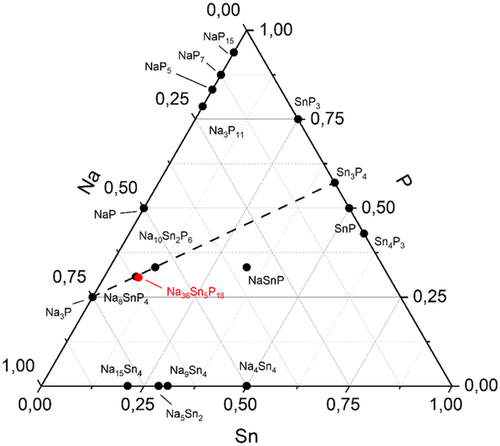
Looking at the Gibbs ternary phase triangle in Figure 5, the motif of isolated tetrahedral units is found on the line between Na3P[15] and Sn3P4. The present compounds are slightly shifted away from this line through the inclusion of a dimeric unit containing a covalent SnSn bond, which is a feature observable for compounds in the related Na–Ge–P system, where here, the presented dimeric units are found in several compounds such as Na3Ge2P3 and the binary GeP. Interestingly, the homoatomic bonds are very common for compounds in the Na–Ge–P system, but no compound in the related Na–Sn–P or Na–Sn–Sb system is reported featuring this attribute. Instead, all reported compounds feature exclusively heteroatomic bonds. Hence, the here presented compounds indicate the possibility of further compounds with unusual compositions and different structure motifs in this chemical system (Figure 5).
4 Conclusions
We recently reported on the synthesis and characterization of the new sodium-ion conductor material Na8SnP4, which is the first ion conductor material where the structural principles reported for the system of lithium phosphidotetrelates with high ionic conductivities could be adapted to sodium. With the compounds Na36Sn5Pn18 (Pn = P, Sb), we now present two phases closely related to the Na8SnP4 and Na8SnSb4 phases, respectively, which exhibit the same structural feature of well-known isolated tetrahedral [SnPn4]8− units and dimeric [Sn2Pn6]12− units.
Both compounds can be readily synthesized by ball milling the elements and subsequent annealing procedure of the obtained reactive mixture. The crystal structures were analyzed by refinement of single-crystal XRD data, and the results were confirmed by Rietveld refinement of powder data. The compounds crystallize isotypic in the space group I41/amd (no. 141) in their own structure type where interestingly the P-containing compound shows disorder in the anionic structural units, while the Sb-containing structure shows complete ordering. In both compounds, the pnictide forms a distorted ccp wherein Sn is either located in a tetrahedral void forming a [SnPn4]8− unit or located as SnSn dumbbell located in an octahedral void forming a [Sn2Pn6]12− unit. The observed disorder occurs at the dimeric unit where the Sn position can be shifted away into a neighboring tetrahedral void to create two isolated tetrahedral units and a new octahedral void that is filled by sodium. Further disorder is observed in the neighboring SnP4 unit where the central Sn atom can be shifted toward a triangular plane creating an almost planar SnP3 unit. The polyanion [SnP3]5− is isostructural to carbonate CO32−. It appears for the lighter Sn homologs in Cs5SiP3, Rb5GeP3, and Cs5GeP3[16] and has also been predicted to exist in Rb5SnP3.[17]
These interesting structural characteristics together with close similarities with structural motifs found in the recently published sodium-ion conductor materials Na8SnP4 make these new compounds valuable targets for electrochemical investigation on sodium-ionic conductivity.
5 Experimental Section
All steps of synthesis and sample preparation were carried out inside an argon-filled glove box (MBraun, p(H2O), p(O2) < 0.1 ppm). Prior to use, sodium (Na, rods, Merck Schuchardt, >99%) was cleaned from oxide layers. Tin (Sn, powder, Alfa Aesar, 99.8%), phosphorus (P, powder, Sigma-Aldrich, 97%), and antimony (Sb, powder, Alfa Aesar, 99.5%) were used without any further purification.
Synthesis of the Compounds Na36Sn5P18 and Na36Sn5Sb18
Na36Sn5Pn18 compounds were synthesized from the elements via ball milling and subsequent annealing procedure. For the synthesis of Na36Sn5P18, 3–5 g of a stoichiometric mixture of sodium, tin, and phosphorous with the nominal composition of “Na8.2Sn0.95P4” were prepared. This mixture was ball-milled (Retch PM 100 planetary mill) for 18 h at 350 rpm in intervals of 10 min with direction reversal and subsequent 5-min resting using a tungsten carbide milling set (50 mL jar with 3 balls with a diameter of 15 mm each). The obtained black powder (“reactive mixture”) was filled into niobium tubes (8 mm diameter) in batches of 200 mg. The tubes were sealed in an electric arc furnace (Edmund Bühler MAM1), enclosed in a silica reaction container under vacuum and subsequently heated with 4 K min−1 up to 500 °C and dwelled for 24 h in a tube furnace (HTM Reetz Loba 1200-42-600-1-OW with a EUROTHERM S 14083 temperature controller) and cooled down at a rate of 1 K min−1. After grinding, a black highly flammable powder was obtained. The synthesis of Na36Sn5Sb18 was performed accordingly with batches of 3–5 g mixtures of sodium, tin, and antimony in the stoichiometric composition of “Na36Sn5Sb18.” After synthesis, a shiny silver powder was obtained.
Single crystals for the Na36Sn5P18 compound were found in a sample with the nominal composition of “Na8SnP4” prepared from 86.2 mg (3.75 mmol) Na, 55.7 mg (0.47 mmol) Sn, and 59.9 mg (1.93 mmol) P, which was heated to 600 °C, held for 48 h and subsequently slowly cooled to room temperature. Single crystals of the compound Na36Sn5Sb18 were prepared from 109 mg (4.76 mmol) Na, 86.44 mg (0.72 mmol) Sn, and 304.04 mg (2.49 mmol) Sb. The sample was heated to 500 °C, held for 96 h, and slowly cooled to room temperature.
PXRD
For powder XRD (PXRD) measurements, the samples were ground in an agate mortar and sealed inside 0.3 mm glass capillaries. PXRD measurements were performed at room temperature either on a Stadi P diffractometer (Stoe & Cie) equipped with a Ge(111) monochromator for Cu–Kα1 radiation (λ = 1.5406 Å) and a MYTHEN DCS 1K (Dectris) solid-state detector for samples with phosphorous. For samples containing antimony a Stadi P diffractometer (Stoe & Cie) equipped with a Ge(111) monochromator for Mo–Kα1 radiation (λ = 0.7093 Å) and a MYTHEN DCS 1K (Dectris) solid-state detector was used. The raw powder data were processed with the software package WinXPOW.[18]
Rietveld Refinement
Rietveld refinement of Na36Sn5P18 and Na36Sn5Sb18 were executed using the full profile Rietveld method within the FullProf program package.[19] Herein, the structural analysis of the single-crystal data was used as model. The Thomson–Cox–Hermann functional was used to model the peak profile shape. Background contribution was determined using a linear interpolation between selected data points in non-overlapping regions. Scale factor, profile shape parameters, resolution (Caglioti) parameters, and lattice parameters as well as fractional coordinates of atoms were refined freely. For Na36Sn5P18, displacement parameters of each atom sort (Sn, P, Na) were coupled and refined as one; for Na36Sn5Sb18, isotropic parameters were fixed due to interfering absorption effects.
Single-Crystal Structure Determination
Single crystals were prepared and sealed in a argon-filled glovebox (MBraun, p(H2O), p(O2) < 0.1 ppm). For data collection, capillaries were mounted on a Stoe StadiVari diffractometer (Stoe & Cie) equipped with a Ge(111) monochromator, a Mo–Kα radiation (λ = 0.71073 Å) source, and a PILATUS 3R 300 K detector (Dectris). Both structures were solved on the basis on systematic extinctions in the space group I41/amd (no. 141) by direct methods (SHELXS-2014) and refined by full-matrix against F2 (SHELXL-2014).[20] Data reduction and multiscan absorption correction were carried out with the software packages X-AREA (1.88, Stoe) and Stoe LANA.[21] Crystallographic data and structure refinement parameters are summarized in the Supporting Information. Atomic coordinates as well as anisotropic displacement parameters and selected atomic distances and angles are given in the Supporting Information.
Further details of the crystal structure investigations may be obtained from the Cambridge Crystallographic Data Centre, CCDC, 12 Union Road, Cambridge CB21EZ, UK (Fax: +44-1223-336-033; e-mail: [email protected]) on quoting the depository numbers CSD-2416108 for Na36Sn5P18 and CSD-2416109 for Na36Sn5Sb18.
DSC
For investigation of thermal behavior of the compounds, about 50 mg of the product was sealed in a niobium ampoule and measured on a DSC machine (Netzsch, DSC 404 Pegasus) under a constant gas flow of 75 mL min−1. The sample was heated to 1023 K and cooled to 423 K twice at a rate of 10 K min−1. To determine the onset temperatures of the DSC signals, the PROTEUS Thermal Analysis software was used.[22]
Acknowledgements
The authors greatly acknowledge Viktor Hlukhyy for the help on the single-crystal data refinement and the fruitful discussions on the disorder found in Na36Sn5P18.
Open Access funding enabled and organized by Projekt DEAL.
Conflict of Interests
The author declares no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.




