Structure and Chemical Bonding of ScNiB4
Georg Eickerling
Institut für Anorganische und Analytische Chemie, Universität Münster, Corrensstrasse 30, 48149 Münster, Germany
Search for more papers by this authorCorresponding Author
Wolfgang Scherer
Institut für Anorganische und Analytische Chemie, Universität Münster, Corrensstrasse 30, 48149 Münster, Germany
Wolfgang Scherer, Institut für Anorganische und Analytische Chemie, Universität Münster, Corrensstrasse 30, 48149 Münster, Germany, Fax: +49-251-83-36002
Rainer Pöttgen, Institut für Physik, Universität Augsburg, Universitätsstrasse 1, 86159 Augsburg, Germany, Fax: +49-821-598-3227
Search for more papers by this authorThomas Fickenscher
Institut für Physik, Universität Augsburg, Universitätsstrasse 1, 86159 Augsburg, Germany
Search for more papers by this authorUte Ch. Rodewald
Institut für Physik, Universität Augsburg, Universitätsstrasse 1, 86159 Augsburg, Germany
Search for more papers by this authorCorresponding Author
Rainer Pöttgen
Institut für Physik, Universität Augsburg, Universitätsstrasse 1, 86159 Augsburg, Germany
Wolfgang Scherer, Institut für Anorganische und Analytische Chemie, Universität Münster, Corrensstrasse 30, 48149 Münster, Germany, Fax: +49-251-83-36002
Rainer Pöttgen, Institut für Physik, Universität Augsburg, Universitätsstrasse 1, 86159 Augsburg, Germany, Fax: +49-821-598-3227
Search for more papers by this authorGeorg Eickerling
Institut für Anorganische und Analytische Chemie, Universität Münster, Corrensstrasse 30, 48149 Münster, Germany
Search for more papers by this authorCorresponding Author
Wolfgang Scherer
Institut für Anorganische und Analytische Chemie, Universität Münster, Corrensstrasse 30, 48149 Münster, Germany
Wolfgang Scherer, Institut für Anorganische und Analytische Chemie, Universität Münster, Corrensstrasse 30, 48149 Münster, Germany, Fax: +49-251-83-36002
Rainer Pöttgen, Institut für Physik, Universität Augsburg, Universitätsstrasse 1, 86159 Augsburg, Germany, Fax: +49-821-598-3227
Search for more papers by this authorThomas Fickenscher
Institut für Physik, Universität Augsburg, Universitätsstrasse 1, 86159 Augsburg, Germany
Search for more papers by this authorUte Ch. Rodewald
Institut für Physik, Universität Augsburg, Universitätsstrasse 1, 86159 Augsburg, Germany
Search for more papers by this authorCorresponding Author
Rainer Pöttgen
Institut für Physik, Universität Augsburg, Universitätsstrasse 1, 86159 Augsburg, Germany
Wolfgang Scherer, Institut für Anorganische und Analytische Chemie, Universität Münster, Corrensstrasse 30, 48149 Münster, Germany, Fax: +49-251-83-36002
Rainer Pöttgen, Institut für Physik, Universität Augsburg, Universitätsstrasse 1, 86159 Augsburg, Germany, Fax: +49-821-598-3227
Search for more papers by this authorAbstract
The ternary polyboride ScNiB4 was synthesized from the elements by arc-melting and subsequent annealing for crystal growth. The structure of ScNiB4 was investigated by X-ray diffraction on powder and a single crystal (high-resolution data up to 2θ = 140°, Mo-Kα radiation): YCrB4 type, Pbam, a = 578.7(2), b = 1120.7(3), c = 327.2(1) pm, R1 = 0.0477 (1286 F2 values with I ≥ 2(I)), wR2 = 0.1137 (all 2225 F2 values), and 38 variables. The four crystallographically independent boron sites build up planar layers which consist of almost regular pentagons and heptagons which sandwich the nickel and scandium atoms, respectively. Within the two-dimensional [B4] networks each boron atom has slightly distorted trigonal planar boron coordination with B–B distances in the range 171–181 pm. A topological analysis of the electron density reveals the differences between the bonding situation of the scandium atoms in the heptagonal prismatic environment on the one hand and the Ni atoms in the pentagonal prismatic environment on the other hand.
References
- 1 J. K. Burdett , S. Lee , T. J. McLarnan , J. Am. Chem. Soc. 1985 , 107 , 3083 .
- 2 G. J. Miller
, Eur. J. Inorg. Chem.
1998
, 523
.
10.1002/(SICI)1099-0682(199805)1998:5<523::AID-EJIC523>3.0.CO;2-L CAS Web of Science® Google Scholar
- 3 X. Rocquefelte
, S. E. Boulfelfel
, M. Ben Yahia
, J. Bauer
, J.-Y. Saillard
, J.-F. Halet
, Angew. Chem.
2005
, 117
, 7714
.
10.1002/ange.200503080 Google Scholar
- 4 G. J. Miller
, Z. Anorg. Allg. Chem.
2006
, 633
, 2078
.
10.1002/zaac.200670006 Google Scholar
- 5 T.-S. You , M.-K. Han , G. J. Miller , Inorg. Chim. Acta 2008 , 361 , 3053 .
- 6 R.-D. Hoffmann , R. Pöttgen , W. Jeitschko , J. Solid State Chem. 1992 , 99 , 134 .
- 7 B. Rohrmoser , G. Eickerling , M. Presnitz , W. Scherer , V. Eyert , R.-D. Hoffmann , U. Ch. Rodewald , C. Vogt , R. Pöttgen , J. Am. Chem. Soc. 2007 , 129 , 9356 .
- 8 C. Vogt , R.-D. Hoffmann , U. Ch. Rodewald , G. Eickerling , M. Presnitz , V. Eyert , W. Scherer , R. Pöttgen , Inorg. Chem. 2009 , 48 , 6436 .
- 9 W. Scherer , C. Hauf , M. Presnitz , E.-W. Scheidt , V. Eyert , G. Eickerling , R.-D. Hoffmann , U. Ch. Rodewald , A. Hammerschmidt , C. Vogt , R. Pöttgen , Angew. Chem. Int. Ed. 2010 , 49 , 1578 .
- 10 L. V. Zavalii , Yu. B. Kuz'ma , S. I. Mikhalenko , Izv. Akad. Nauk SSSR, Neorg. Mater. 1988 , 24 , 1814 ; L. V. Zavalii , Yu. B. Kuz'ma , S. I. Mikhalenko , Izv. Akad. Nauk SSSR, Neorg. Mater. 1988 , 24 , 1814 ;
- 11 Yu. B. Kuz'ma , Kristallografiya 1970 , 15 , 372 ; Yu. B. Kuz'ma , Kristallografiya 1970 , 15 , 372 ;
- 12 R. Pöttgen , Th. Gulden , A. Simon , GIT Labor Fachzeitschrift 1999 , 43 , 133 .
- 13 D. Niepmann , Yu. M. Prots' , R. Pöttgen , W. Jeitschko , J. Solid State Chem. 2000 , 154 , 329 .
- 14 K. Yvon , W. Jeitschko , E. Parthé , J. Appl. Crystallogr. 1977 , 10 , 73 .
- 15 P. Blaha, K. Schwarz, G. K. H. Madsen, D. Kvasnicka, J. Luitz, WIEN2k, An Augmented Plane Wave + Local Orbitals Program for Calculating Crystal Properties, Technische Universität Wien, Wien, Austria, 2001.
- 16 J. P. Perdew , K. Burke , M. Ernzerhof , Phys. Rev. Lett. 1997 , 78 , 1396 .
- 17 J. P. Perdew , K. Burke , M. Ernzerhof , Phys. Rev. Lett. 1996 , 77 , 3865 .
- 18 A. Otero-de-la-Roza , M. A. Blanco , A. M. Pendás , V. Luaña , Comput. Phys. Commun. 2009 , 180 , 157 .
- 19 W. Humphrey , A. Dalke , K. Schulten , J. Mol. Graphics 1996 , 14 , 33 .
- 20 G. M. Sheldrick, SHELXL-97 – Program for Crystal Structure Refinement, University of Göttingen, Germany, 1997.
- 21 G. M. Sheldrick , Acta Crystallogr., Sect. A 2008 , 64 , 112 .
- 22 P. Villars, K. Cenzual, Pearson's Crystal Data: Crystal Structure Database for Inorganic Compounds, Release 2008/9, ASM International®, Materials Park, OH, USA, 2008.
- 23 I. Veremchuk , T. Mori , Yu. Prots , W. Schnelle , A. Leithe-Jasper , M. Kohout , Yu. Grin , J. Solid State Chem. 2008 , 181 , 1983 .
- 24 N. F. Chaban , G. V. Chernyak , Yu. B. Kuz'ma , Izv. Akad. Nauk. SSSR, Neorg. Mater. 1981 , 17 , 1120 ; N. F. Chaban , G. V. Chernyak , Yu. B. Kuz'ma , Izv. Akad. Nauk. SSSR, Neorg. Mater. 1981 , 17 , 1120 ;
- 25 J. Emsley , The Elements , Oxford University Press , Oxford, UK , 1999
- 26 J. Donohue , The Structures of the Elements , Wiley , New York, USA , 1974
- 27 See, for example:
- 27a L. J. Farrugia , C. Evans , D. Lentz , M. Roemer , J. Am. Chem. Soc. 2009 , 131 , 1251 ;
- 27b A. Reisinger , N. Trapp , I. Krossing , S. Altmannshofer , V. Herz , M. Presnitz , W. Scherer , Angew. Chem. Int. Ed. 2007 , 46 , 8295 –8298 ;
- 27c D. Himmel , N. Trapp , I. Krossing , S. Altmannshofer , V. Herz , G. Eickerling , W. Scherer , Angew. Chem. Int. Ed. 2008 , 47 , 7798 –7801 ;
- 27e W. Scherer , V. Herz , A. Brück , C. Hauf , F. Reiner , S. Altmannshofer , D. Leusser , D. Stalke , Angew. Chem. Int. Ed. 2011 , 50 , 2845 –2849 ;
- 27f W. Scherer , V. Herz , C. Hauf , in Electron Density and Chemical Bonding I, (Ed. D. Stalke) , Springer Berlin Heidelberg , 2012 , pp. 159 –207
- 27g W. Scherer , G. Eickerling , M. Tafipolsky , G. S. McGrady , P. Sirsch , N. P. Chatterton , Chem. Commun. 2006 , 2986 –2988 ;
- 27h W. Scherer , G. Eickerling , D. Shorokhov , E. Gullo , G. S. McGrady , P. Sirsch , New J. Chem. 2006 , 30 , 309 –312 .
- 28 R. Bader
, Atoms in Molecules
, Claredon Press
, Oxford
, 1990
10.1093/oso/9780198551683.001.0001 Google Scholar
- 29 R. F. W. Bader , P. M. Beddall , J. Chem. Phys. 1972 , 56 , 3320 –3329 .
- 30 W. Scherer , G. Eickerling , C. Hauf , M. Presnitz , E.-W. Scheidt , V. Eyert , R. Pöttgen , in Modern Charge-Density Analysis, (Eds. C. Gatti, P. Macchi) , Springer Netherlands , 2012 , pp. 359 –385
- 31 P. M. Morse , H. Feshbach , Methods of Theoretical Physics , McGraw-Hill , 1953
- 32 R. F. W. Bader , P. J. Macdougall , C. D. H. Lau , J. Am. Chem. Soc. 1984 , 106 , 1594 –1605 .
- 33 R. F. W. Bader , P. M. Beddall , J. Chem. Phys. 1972 , 56 , 3320 –3329 .
- 34 R. F. W. Bader , H. Essén , J. Chem. Phys. 1984 , 80 , 1943 –1960 .
- 35 R. P. Sagar , A. C. T. Ku , V. H. Smith , A. M. Simas , J. Chem. Phys. 1988 , 88 , 4367 –4374 .
- 36 Z. Shi , R. J. Boyd , J. Chem. Phys. 1988 , 88 , 4375 –4377 .
- 37 W.-T. Chan , I. P. Hamilton , J. Chem. Phys. 1998 , 108 , 2473 –2485 .
- 38 Furthermore, four-component calculations on spherical symmetric atoms (Au) by Kohout et al. showed that the outermost shell of elements of period 6 smight not be resolved in the second dericative of the radial density: M. Kohout , A. Savin , H. Preuss , J. Chem. Phys. 1991 , 95 , 1928 –1942 . For further information, see: M. Kohout , A. Savin , H. Preuss , J. Chem. Phys. 1991 , 95 , 1928 –1942 . N. Hebben, H.-J. Himmel, G. Eickerling, C. Herrmann, M. Reiher, V. Herz, M. Presnitz, W. Scherer, Chem. Eur. J. 2007, 13, 10078–10087 and Ref. [8].
- 39 R. F. W. Bader , R. J. Gillespie , F. Martin , Chem. Phys. Lett. 1998 , 290 , 488 –494 .
- 40 G. S. McGrady , A. Haaland , H. P. Verne , H. V. Volden , A. J. Downs , D. Shorokhov , G. Eickerling , W. Scherer , Chem. Eur. J. 2005 , 11 , 4921 –4934 .
- 41
We note that the torus shaped CC reveals a secondary fine structure resulting from the partial localization of d
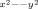 states (see the local maximum slightly above –2 eV in the pDOS depicted in Figure 2a).
states (see the local maximum slightly above –2 eV in the pDOS depicted in Figure 2a).




