Importance of cell wall mannoproteins for septum formation in Saccharomyces cerevisiae
Abstract
The mannosyltransferase mutants mnn9 and mnn10 were isolated in a genetic screen for septation defects in Saccharomyces cerevisiae. Ultrastructural examination of mutant cell walls revealed markedly thin septal structures and occasional failure to construct trilaminar septa, which then led to the formation of bulky default septa at the bud neck. In the absence of a functional septation apparatus, mnn10 mutants are unable to complete cytokinesis and die as cell chains with incompletely separated cytoplasms, indicating that mannosylation defects impair the ability to form remedial septa. We could not detect N-linked glycosylation of the β(1,3)glucan synthase Fks1p and mnn10 defects do not change the molecular weight or abundance of the protein. We discuss a model explaining the pleiotropic effects of impaired N-linked protein glycosylation on septation in S. cerevisiae. Copyright © 2005 John Wiley & Sons, Ltd.
Introduction
The cell wall of Saccharomyces cerevisiae is a complex and dynamic structure composed of glycoproteins and polysaccharides. The current understanding of the wall architecture is that a β(1,3)-glucan molecule is synthesized first, to which β(1,6)-glucan chains are added (Cabib et al., 2001; Roh et al., 2002). Heavily glycosylated proteins are linked to β(1,6)-glucan via the GPI anchor (van der Vaart et al., 1996). The last component to be added to the cell wall is chitin [β(1,4) N-acetylglucosamine], which is linked to both β(1,3) and β(1,6)-glucan.
In S. cerevisiae, three chitin synthases supply chitin for different processes (Shaw et al., 1991). At cytokinesis, chitin synthase 2 activity constructs a primary septum at the bud neck to separate mother and daughter cells. Chitin synthase 3 activity forms a chitin ring around the bud neck early in the cell cycle and supplies the chitin that is interwoven in the peripheral cell wall. A third function for chitin synthase III activity is to reinforce the cell wall when its integrity is threatened. In a pioneering study, Osmond et al. (1999) isolated a series of mutants that relied on chitin synthase III activity for survival. Among those were mutants with a defect in N-linked glycosylation. This finding led the authors to propose that, following hypoglycosylation of the cell wall glycoproteins, cells counteract cell wall weakness by increasing the chitin content
In S. cerevisiae, N-linked sugar structures are comprised of a long backbone of α (1,6)-linked mannoses to which short branches of α(1,2) and α (1,3)-linked mannoses are attached (Herscovics and Orlean, 1993). The α (1,6) chains contain about 50 residues and are attached to a conventional core structure (Figure 1). The first two mannoses of the side chains are α (1,2)-linked and the final mannose is attached via α (1,3) linkage. Phospho-mannoses are substituted on the α (1,2) mannose side chains (Jigami and Ogami, 1999). The synthesis of the mannan structure occurs in the Golgi apparatus, where first an α (1,6)-linked mannose is attached to all N-linked core oligosaccharides by the Och1p mannosyltransferase (Nakayama et al., 1992). After this initial modification, a subset of N-linked glycans is extended by two protein complexes with α (1,6)-transferase activity (Jungmann and Munro, 1998). The first complex, M-pol I, contains Mnn9p and Van1p, and the second complex, M-pol II, contains Mnn9p, Mnn10p, Mnn11p, Hoc1p and Anp1p (Jungmann and Munro, 1998). Loss of any of these proteins results in hypoglycosylation of secreted and cell wall proteins. Mutations in M-pol I components cause a complete loss of the α (1,6) mannan backbone, whereas mutations in M-pol II components result in a shortening of the α (1,6) mannan backbone from 50 to about 10–15 residues (Jungmann et al., 1999).
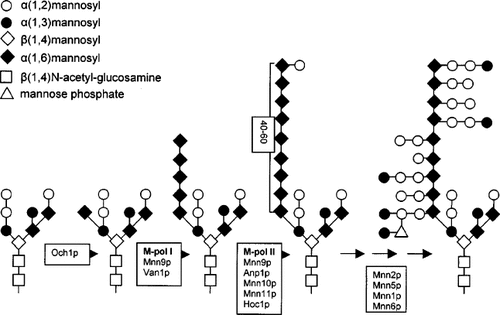
Synthesis of N-linked mannoses in the Golgi apparatus. First, an α(1,6)-linked mannose is added to the core oligosaccharide in an Och1p-dependent reaction. The M-pol I and M-pol II complexes extend the structure by adding 40–60α(1,6)-linked mannoses. The α(1,6) mannose chain is branched by short α(1,2)-linked mannoses side chains, which terminate in an α(1,3)-linked mannose. Mannose phosphate is found substituted on the α(1,2)-linked mannoses side chains
Since mannosylated proteins make up about 40% of the yeast cell wall, glycosylation defects can be expected to impair cell wall integrity. Protein glycosylation may also affect cell wall integrity by influencing folding, trafficking and activity of proteins providing for the synthesis of other cell wall components (Herscovics and Orlean, 1993; Chavan et al., 2003).
The isolation of mnn9 and mnn10 mutants in a screen for cytokinesis mutants led us to investigate the effect of N-linked glycosylation on septum formation.
Materials and methods
Strains and growth conditions
All yeast strains used in this study are listed in Table 1. Media and growth conditions were as described in Burke et al. (2000).
| Strain | Genotype | Source |
|---|---|---|
| BY4742 fks1Δ::KANR | MATa his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 fks1Δ::KANR | Open Biosystems |
| ECY46-4-1B | MATa ura3-52 lys2-801 ade2-101 trp1-Δ63 his3-Δ200 leu2-Δ1 chs3::LEU2 | Crotti et al., 2001 |
| YMS11 | MATa ura3-52 lys2-801 ade2-101 trp1-Δ63 his3-Δ200 leu2-Δ1 chs2::TRP1 | Schmidt et al., 2002 |
| YMS401 | MATa ura3-52 lys2-801 ade2-101 trp1-Δ63 his3-Δ200 leu2-Δ1 chs3::LEU2 mnn9 mnn10::URA3 [pEC28] | This study |
| YMS404 | MATa ura3-52 lys2-801 ade2-101 trp1-Δ63 his3-Δ200 leu2-Δ1 chs3::LEU2 mnn10::URA3 [pEC28] | This study |
| YMS405 | MATa ura3-52 lys2-801 ade2-101 trp1-Δ63 his3-Δ200 leu2-Δ1 mnn10::URA3 | This study |
| YMS406 | MATa ura3-52 lys2-801 ade2-101 trp1-Δ63 his3-Δ200 leu2-Δ1 chs2::TRP1 mnn10::URA3 [pMS52] | This study |
| YMS432 | MATα ura3-52 lys2-801 ade2-101 trp1-Δ63 his3-Δ200 leu2-Δ1 mnn10::TRP1 | This study |
| YMS433 | MATa ura3-52 lys2-801 ade2-101 trp1-Δ63 his3-Δ200 leu2-Δ1 chs2::TRP1 mnn10::HIS3 [pMS23] | This study |
| YMS450 | MATa ura3-52 lys2-801 ade2-101 trp1-Δ63 his3-Δ200 leu2-Δ1 chs3::LEU2 mnn9 [pEC28] | This study |
| YMS451 | MATa ura3-52 lys2-801 ade2-101 trp1-Δ63 his3-Δ200 leu2-Δ1 chs3::LEU2 mnn10 [pEC28] | This study |
| YPH499 | MATa ura3-52 lys2-801 ade2-101 trp1-Δ63 his3-Δ200 leu2-Δ1 | Sikorski and Hieter, 1989 |
Plasmid and strain construction
All DNA manipulations were performed as described by Ausubel et al. (2003). Plasmids used in this study are listed in Table 2. Plasmid pMS7 was constructed by inserting a 500 bp MET3 promoter fragment in front of the CHS3 start codon in plasmid pHV8 (Valdivieso et al., 1991) to yield vector pHV8MET3. The 4.7 kb MET3P-CHS3 fragment was cut from pHV8MET3 and inserted into pRS314, using SalI–EcoRI restriction sites. For the construction of plasmid pMS23, plasmid YEp352 XhoI CHS2, carrying an engineered XhoI site in front of the CHS2 ATG, was digested with XhoI and HindIII, removing the CHS2 promoter. A 500 bp MET3 promoter fragment with engineered HindIII and SalI sites was amplified by PCR and cloned in front of the CHS2 reading frame to yield pMS23.
| Plasmid | Features | Source |
|---|---|---|
| pEC28 | pRS412 CHS3 | Schmidt et al., 2002 |
| pHV8 | YEp352 CHS2 | Valdivieso et al., 1991 |
| pHV8MET3 | YEp352 MET3P-CHS3 | This study |
| pMS7 | pRS314 MET3P-CHS3 | This study |
| pMS23 | YEp352 MET3P-CHS2 | This study |
| pMS52 | pRS412 CHS2 | Schmidt et al., 2002 |
| pRS316CDC3GFP | URA3 CDC3-GFP | M. Longtine |
| YEp352 XhoI CHS2 | URA3 CHS2 | T. Drgon |
For deletion of the MNN10 gene, mnn10::HIS3, mnn10::URA3 and mnn10::TRP1 deletion cassettes were amplified by PCR with primers OMS427 (GGA GAG AAA AGC TCT CTA TTT ATT TTT ATA AGG AAT AAT TGT GCA TGT ACA ACT ATA CAA ATT GTA CTG AGA GTG CAC CA) and OMS428 (ATT AAA AAG AAA AAT AAA AGC TTG TAA ATG CTT CTC TTT ACA ATA TTG ATA ACT TCC ATT GCG GTA TTT CAC ACC GCA GG) from plasmids pRS313, pRS306 and pRS304, respectively (Sikorski and Hieter, 1989). Deletions were verified by PCR.
Cloning of mnn9 and mnn10 mutations as synthetic lethal with chs3
Isolation and cloning of chs3 synthetic lethal mutations was achieved as described earlier (Osmond et al., 1999; Schmidt et al., 2003) with some modifications: Mutations were generated by ethyl methane sulphonate treatment in chs3 deletant strain ECY46-4-1B with CHS3-bearing plasmid pEC28. The mutagenized cells were grown in synthetic medium overnight to express the clumping phenotype characteristic for septation defects. The overnight culture was filtered through an 11 µm filter (Millipore, Billerica, USA) to retain clumping mutants. Cells retained in the filter were plated on appropriately supplemented synthetic media and incubated for 5 days at 30 °C. Synthetic lethality with chs3 was assessed by the inability of the mutants to survive without plasmid pEC28. Since pEC28 also complements a genomic ade2 mutation, the presence of a chs3 synthetic lethal mutation leads to growth of white colonies devoid of the red sectors that appear in indicator strain colonies due to plasmid segregation. All strains bearing a chs3-synthetic lethal mutation were grown in synthetic medium to mid-logarithmic phase of growth and inspected under the microscope for a cell separation defect. chs3-synthetic lethal mutations were cloned by complementation from a pRS200-based genomic library (ATCC77164).
Detection of synthetic lethality with chs2
The indicator strain for synthetic lethality with chs2 was constructed by transforming strain YMS11, carrying a chs2 deletion, with plasmid pMS52, bearing CHS2 (Schmidt et al., 2002).
Chs2p and Chs3p withdrawal
To assess the effects of Chs2p withdrawal, a mnn10::HIS3 deletion was introduced into strain YMS11 carrying plasmid pMS23. Effects of Chs3p withdrawal were examined after exchanging pEC28 for pMS7 in the original mnn10 point mutant YMS451. After the plasmid exchange, the mutant strain was transformed with pRS316CDC3GFP to assess the integrity of the septation apparatus. To terminate transcription of MET3-promoter constructs, cells were grown for 20 h in synthetic medium supplemented with 0.4 mg/ml methionine.
Vital staining
For determination of viability, cells were washed with water and incubated for 5 min at RT in 2 mg/ml methylene blue in 50 mM KH2PO4. Under these conditions, inviable cells accumulate the blue pigment.
Cell wall digestion
Cell wall digestion of fixed cells was performed to assess completion of cell separation (Cabib and Schmidt, 2003). Cells were grown overnight in synthetic medium. 5 ml cells were fixed by addition of formaldehyde to a final concentration of 5%. After overnight incubation at 4 °C, fixed cells were washed once with PBS and suspended in 1 ml citrate/phosphate buffer (40 mM Na2HPO4 and 20 mM citric acid mixed 39/61 to obtain pH 6.5, 0.8 M Sorbitol, 1 mM EDTA, 50 mM β-mercaptoethanol). 100 µl Glusulase (Perkin-Elmer Life Sciences, Boston, USA) was added to the fixed cells and cell walls were digested at 30 °C for 90 min with gentle shaking (60 r.p.m.), carefully avoiding mechanical stress. Spheroplast-like appearance of the digested cells indicated complete cell wall removal.
Determination of chitin content
Chitin content of isolated cell walls was determined according to Roberts et al. (1983).
Determination of chitin and glucan synthase activities
Glucan synthase activity was determined following a protocol from Mol et al. (1994). Membrane preparations were incubated for 30 min at 30 °C in 40 µl reaction mixture containing 8% glycerol (contained in the membrane preparation), 75 mM Tris–Cl, pH 7.5, 0.75% BSA, 25 mM KF, and 5 mM 14C UDP-glucose (5E8 cpm/mmol, Amersham Radiochemicals, Uppsala, Sweden). For maximum activity, GTP-χS was added to samples to a final concentration of 20 µM. After the incubation, reactions were stopped by adding 1 ml 10% trichloroacetic acid. Samples were filtered through glass fibre filters (Gelman Type A/E, Pall, East Hills, USA). Radioactivity retained in the washed and dried filters was quantified in a scintillation counter.
Chitin synthase activity was determined according to Choi and Cabib (1994). Total chitin synthase activity was measured in the presence of 5 mM magnesium acetate with activation by trypsin.
Fluorescence microscopy
The integrity of septin rings was analysed by observation of GFP-tagged Cdc3p with a Zeiss Axioskop II fluorescence microscope using a standard FITC filter set (Zeiss, Oberkochen, Germany).
Electron microscopy
Electron microscopy was performed as described in Schmidt (2004). Cells were grown in 100 ml YPD media to a titre of 1–3 × 107 cells/ml, harvested, washed once with water and resuspended in 1 ml 3% glutaraldehyde, 0.1 M cacodylate containing 5 mM CaCl2 and 5 mM MgCl2. Cells were embedded in agarose, cooled and cut into blocks. Blocks were fixed in 4% KMnO4 for 1 h at RT, washed with water and incubated with 0.5% sodium meta-periodate for 15 min at RT. After washing with 50 mM KPO4 (pH 7.4), the blocks were incubated in 50 mM ammonium phosphate (pH 7.4) for 15 min. After washing twice with water, the blocks were placed overnight in 2% uranyl acetate (pH 4.5) at RT in the dark. Blocks were dehydrated through a graded series of ethanol solutions (50–100% v/v at 40 °C) and left overnight in fresh 100% ethanol at RT. They were then washed twice with 100% ethanol and twice with propylene oxide before being embedded in Spurr resin. Sections were cut from these blocks and post-stained with lead citrate for 2–5 min.
Results and discussion
Mannosylation defects impair cytokinesis
Cytokinesis defects of mutants were deduced from synthetic lethality with chs3, the occurrence of overly large cells and a clumping phenotype—morphological characteristics of the archetypical septation mutant chs2 (Shaw et al., 1991). Of five cytokinesis mutants, four could be complemented with library plasmids containing MNN10 and one with a plasmid containing MNN9. The following experiments focused on mnn10 mutants, since they were more abundant. A mnn10::URA3 deletion was introduced into ECY46-4-1B [pEC28], preventing loss of pEC28 and proving synthetic lethality with chs3 (strain YMS404). The mnn10 mutants form cell clumps that break apart during mild sonication, indicating that cell aggregation may be due in part to cell surface adhesion. It should be noted, however, that even cell clumps of chs2 mutants break apart upon mild sonication (Shaw et al., 1991). Introducing the mnn10::URA3 deletion into the mnn9 mutant YMS450 did not aggravate the cytokinesis phenotype (strain YMS401), showing epistasis of mnn10 and mnn9 mutants.
A mnn10 mutation affects N-linked protein glycosylation in the Golgi apparatus (Dean and Poster, 1996; Jungmann et al., 1999). As a result of this defect, mnn10 mutants have severely truncated N-linked glycans on secreted and cell wall proteins. Probably because of its impact on the large fraction of heavily glycosylated mannoproteins, a mnn10 mutation causes instability of the yeast cell wall (Osmond et al., 1999).
While it was known that mnn10 mutations cause a delay in bud emergence and have an impact on morphogenesis (Mondésert and Reed, 1996), the finding that they also influence cytokinesis was surprising. One simple explanation for the defect lies within the bud neck geometry: the bud neck diameter of a mnn10 point mutant (YMS451), measured as diameter of Cdc3-GFP rings, is increased by 49% over the corresponding wild-type (from 1.38 ± 0.57 µm to 2.06 ± 0.44 µm; 50 cells measured). The wider the bud neck becomes, the more difficult it is for the cell to construct a septum. Bud neck expansion beyond a certain range inhibits septation altogether and causes cell death (Schmidt et al., 2003).
An ultrastructural examination of the septum confirmed abnormalities in mnn9 and mnn10 mutants (Figure 2). In the top row, cells are shown that formed very thin primary and secondary septa. In the bottom row, cells are shown that formed bulky structures resembling default septa. Of 22 mnn9 mutant septa analysed, 11 (50%) appeared thin and four (18%) resembled remedial septa. Of 17 analysed mnn10 mutant septa, seven (41%) appeared thin and three (18%) resembled remedial septa. It also became evident that the thickness of the general cell wall is reduced in mnn9 and mnn10 mutants. An analysis of the cell wall in electron micrographs showed a thickness of 204 ± 51 nm in YPH499 (n = 67), a thickness of 147 ± 43 nm in YMS450 (mnn9, n = 21) and a thickness of 144 ± 65 nm in YMS451 (mnn10, n = 20).
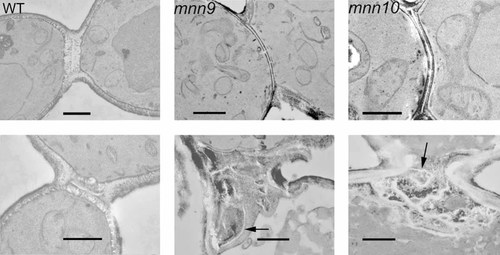
Ultrastructure of normal and mutant septa. Left column, wild-type (ECY46-4-1B[pEC28]); middle column, mnn9 mutant; right column: mnn10 mutant. Two kinds of abnormalities are observed: some finished septa are very thin (top), whereas others are the product of remedial septation (bottom). In mnn9 and mnn10 mutants, a chitin primary septum can be observed as an electron-translucent line (indicated by arrows in the bottom panel). The bar represents 1 µm
We propose the following explanation. Abnormally wide mutant bud necks together with a reduced cell wall mannose content complicate the formation of septa. Sometimes primary septum closure fails, requiring the synthesis of bulky default septa. In these structures, the unfinished chitin primary septa can be detected as electron-translucent layers.
Mannosylation defects prevent remedial septum formation
To further characterize the influence of N-linked protein glycosylation on septation, the formation of remedial septa in mnn10 deletion mutants was examined. Remedial septa become essential in the absence of a functional primary septum (reviewed recently by Cabib, 2004) and, consequently, defects in remedial septum formation are expected to show synthetic lethality with a chs2 mutation. We constructed a mnn10::HIS3 deletion in YMS11[pMS52] and detected synthetic lethality of mnn10 and chs2 by the lack of sectoring of the resulting strain YMS406.
Building on the synthetic lethality between chs2 and mnn10, we went on to show that simultaneous loss of Mnn10p and Chs2p leads to cells dying with a septation defect (Figures 3, 4). After 16 h of methionine-induced shutdown of CHS2 expression, viability of strain YMS433[pMS23] declined from 78 ± 5% to 33 ± 12% (n = 2, minimum 735 cells counted). The change in viability of the control strain YMS11[pMS23] was insignificant under these conditions (79 ± 4% to 77 ± 10%, n = 2, minimum 1686 cells counted). In order to examine whether the loss of viability is a consequence of incomplete cytokinesis, we enzymatically removed the cell wall after fixation of the cells. We found that shutdown of CHS2 expression increases the number of incompletely separated cells in the mnn10 mutant YMS433[pMS23], whereas the control strain YMS11[pMS23] is only moderately affected (Figure 4).
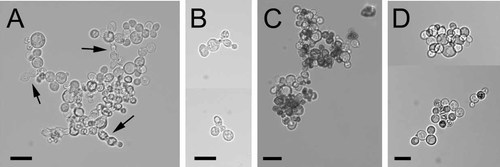
Effect of Chs2p and Chs3p withdrawal on viability and cytokinesis of a mnn10 mutant. (A) Phase contrast image of YMS433[pMS23] after 16 h in methionine-containing medium. Note the large clump size and the cell chains formed under CHS2-repressing conditions (arrows). (B) Composite picture of cell chains with incompletely separated cytoplasms in YMS433[pMS23] after 16 h in methionine-containing medium. Cell walls have been enzymatically removed. (C) Viability stain of YMS451 with pMS7 after 24 h growth with methionine. Cell viability declines as compared to the control without methionine without the formation of cell chains. (D) Composite picture of strain YMS11[pMS23] after 16 h in methionine-containing medium. Cells were stained for viability. The cells exhibit the cell separation defect that is typical for chs2 mutants, without forming the cell chains observed in chs2 mnn10 double mutants in (A). The bar represents 10 µm

Cell aggregate size after enzymatic cell wall removal (summary of two independent experiments, minimum 216 clumps analysed in each). Open bars, without methionine (with CHS2 expression); solid bars, with methionine (without CHS2 expression); top, control strain YMS11[pMS23]; bottom, YMS433[pMS23]. The failure to form a septum in YMS433[pMS23] + methionine leads to the formation of cell chains with connected cytoplasms. Chains containing up to eight cells could be observed. Note that the experiment under-represents the actual number and length of cell chains, since the cytoplasmic stalks connecting the cells are prone to breakage during cell wall removal (Cabib and Schmidt, 2003). Also note that 22% of YMS433[pMS23] cells were non-viable at the start of the experiment and thus will not form chains at all
The absence of cytoplasmic connections in a mnn10 mutant (YMS433[pMS23] grown without methionine) shows that the clumping phenotype is not due to a failure to form a septum (Figure 4B). To a minor extent, this is also true for the chs2 mutant (YMS11[pMS23] grown with methionine), where only a few cell aggregates can be observed after cell wall removal (Figure 4A).
We also examined the effect of Chs3p withdrawal on a mnn10 mutant. This became necessary in order to distinguish if mnn10 mutants depend on the chitin ring, the chitin deposited into remedial septa or the ‘stress chitin’ interwoven into the peripheral cell wall, all of which are structures constructed by chitin synthase III activity (Cabib and Schmidt, 2003). The original mnn10 mutant was transformed with pMS7, pEC28 was segregated and the cells were grown in methionine-containing media for 24 h. Chs3p withdrawal following MET3 promoter shutdown results in a decrease in cell viability from 78% to 51% (minimum 1000 cells counted). The mutant forms clumps of cells without the chains that can be observed after Chs2p withdrawal (Figure 3C; cf. chains indicated by arrows in Figure 3A). The assembly of the septin scaffold at the bud neck was unchanged under these conditions: Abnormal and mislocalized Cdc3-GFP rings were observed in 8% of cells before and 7.6% of cells after the shutdown (data not shown, minimum 330 cells analysed). The diameter of the septin rings changed only marginally upon Chs3 withdrawal, from 2.19 ± 0.48 to 2.3 ± 0.46 µm (minimum 50 rings analysed). In accordance with the interpretation by Osmond and co-workers (1999), we reached the conclusion that, in mnn10 mutants, Chs3p is required for the synthesis of ‘stress chitin’, since CHS3 withdrawal does not exacerbate the cytokinesis defect.
Taken together, the results show that perfect glycosylation of cell wall mannoproteins aids in the construction of wild-type primary septa. In situations where remedial septum formation is required, complete glycosylation of cell wall mannoproteins is much more important and mannosylation defects prevent septum formation altogether.
mnn10 mutations do not affect Fks1p
There is evidence that the activity of some cell wall synthesizing enzymes depends on glycosylation (Chavan et al., 2003). We thus examined glycosylation and activity of the β(1,3)-glucan synthase catalytic subunit Fks1p in mnn10 mutants. Western blotting was performed using anti-Fks1p antibody y-N19 (Santa Cruz Biotechnology, Santa Cruz, USA). Membranes were isolated according to Roberts et al. (1983) from wild-type YPH499 and mnn10 deletants YMS405 and YMS432. N-linked sugars were removed by Endo-H treatment according to the manufacturer's instructions (New England Biolabs, Beverly, USA). We were not able to observe differences in Fks1p molecular weight in mnn10 mutants compared to the wild-type, neither did deglycosylation change the Fks1p molecular weight (data not shown).
We also compared glucan synthase activities in the presence of GTP-χS of wild-type and mnn10 deletion mutants. No clear picture emerged from these measurements: glucan synthase activities in mnn10 mutants YMS405 and YMS432 was reduced, but not with statistical significance (data not shown). Since the glycosylation defect in mnn10 mutants does not affect post-translational modification of the β(1,3)-glucan synthase catalytic moiety, Fks1p, we conclude that the observed changes in glucan synthase activity are due to variations between the individual clones picked for analysis. These variations may reflect strain viability rather than a direct influence of mnn10 mutations on β(1,3)glucan synthesis.
It was reported previously that mutants with defects in M-pol I and M-pol II genes have an elevated chitin content (Mondésert and Reed, 1996; Osmond et al., 1999; Bulik et al., 2003). These results could be confirmed with our mutants. The mnn9 and mnn10 point mutants initially isolated from the screen contained 169% and 164% of the chitin found in the respective wild-type, ECY46-4-1B [pEC28] (n = 3). Chitin synthase activity increased in mnn10 mutants, in accordance with previously published results (Bulik et al., 2003). The chitin synthase activity of YMS405 was 166 ± 27% of the activity of the corresponding wild-type YPH499 (with activation by trypsin), giving a good explanation for the observed increase in cell wall chitin.
Model for the participation of mannoproteins in septum formation
The N-linked protein glycosylation machinery in the Golgi apparatus adds large mannose structures to the surface of many cell wall, plasma membrane and secreted proteins. Oftentimes, the activity of these proteins depends on correct glycosylation (Mrsa et al., 1987; Lipke and Ovalle, 1998). Heavily N-glycosylated proteins, termed mannoproteins, are also a major structural component of the cell wall. Mannoproteins are attached to β(1,6)-glucan via a GPI anchor, limit cell wall porosity and contribute to mechanical strength (de Nobel et al., 1990; Orlean, 1997).
Two models exist to explain the observed cell wall weakening caused by incomplete N-linked glycosylation. First, incomplete post-translational processing might directly reduce the activity of cell wall synthesis enzymes. Chavan and co-workers (2003) recently demonstrated that this is the case for β(1,6)-glucan synthesis. Our results indicate that this is not the case for β(1,3)-glucan synthesis, since the catalytic subunit Fks1p does not show N-linked glycosylation. Second, incomplete mannosylation of cell wall proteins may affect the architecture of the cell wall. In a model developed by Osmond and co-workers (1999), hypoglycosylation of the abundant cell wall mannoproteins causes a reduction in bulk cell wall material. This leads to a weakening of the cell wall and, in turn, causes an activation of chitin synthase III, which reinforces the cell wall by synthesizing ‘stress chitin’. As a result, the cell wall of hypoglycosylating mutants is rich in chitin, which appears delocalized over the cell surface (Mondésert and Reed, 1996; Osmond et al.,1999; Bulik et al., 2003).
How do mannoproteins influence septum formation? It could be observed that mnn10 mutants have larger cells with wider bud necks, which would require deposition of more cell wall material to form wild-type-like septa. The ultrastructural examination shows that such an increase does not happen: the septa of mnn9 and mnn10 mutants are often very thin and occasionally show signs of repair or remediation. The septation problems are exacerbated when remedial septum formation is required, such as in a chs2 mutant. In this case, mnn10 mutants are not capable of synthesizing enough cell wall material to form remedial septa, resulting in synthetic lethality of mnn10 and chs2. Taken together, we conclude that cell wall mannoproteins are important for yeast septum formation. A defect in mannosylation of cell wall proteins causes mild septation defects, which become insurmountable under conditions where the formation of remedial septa is required.
Acknowledgements
This project was supported by the Iowa Academy of Science, Grant ISF-04-07.




