Oral and long-acting antipsychotics for relapse prevention in schizophrenia-spectrum disorders: a network meta-analysis of 92 randomized trials including 22,645 participants
Abstract
According to current evidence and guidelines, continued antipsychotic treatment is key for preventing relapse in people with schizophrenia-spectrum disorders, but evidence-based recommendations for the choice of the individual antipsychotic for maintenance treatment are lacking. Although oral antipsychotics are often prescribed first line for practical reasons, long-acting injectable antipsychotics (LAIs) are a valuable resource to tackle adherence issues since the earliest phase of disease. Medline, EMBASE, PsycINFO, CENTRAL and CINAHL databases and online registers were searched to identify randomized controlled trials comparing LAIs or oral antipsychotics head-to-head or against placebo, published until June 2021. Relative risks and standardized mean differences were pooled using random-effects pairwise and network meta-analysis. The primary outcomes were relapse and dropout due to adverse events. We used the Cochrane Risk of Bias tool to assess study quality, and the CINeMA approach to assess the confidence of pooled estimates. Of 100 eligible trials, 92 (N=22,645) provided usable data for meta-analyses. Regarding relapse prevention, the vast majority of the 31 included treatments outperformed placebo. Compared to placebo, “high” confidence in the results was found for (in descending order of effect magnitude) amisulpride-oral (OS), olanzapine-OS, aripiprazole-LAI, olanzapine-LAI, aripiprazole-OS, paliperidone-OS, and ziprasidone-OS. “Moderate” confidence in the results was found for paliperidone-LAI 1-monthly, iloperidone-OS, fluphenazine-OS, brexpiprazole-OS, paliperidone-LAI 1-monthly, asenapine-OS, haloperidol-OS, quetiapine-OS, cariprazine-OS, and lurasidone-OS. Regarding tolerability, none of the antipsychotics was significantly worse than placebo, but confidence was poor, with only aripiprazole (both LAI and OS) showing “moderate” confidence levels. Based on these findings, olanzapine, aripiprazole and paliperidone are the best choices for the maintenance treatment of schizophrenia-spectrum disorders, considering that both LAI and oral formulations of these antipsychotics are among the best-performing treatments and have the highest confidence of evidence for relapse prevention. This finding is of particular relevance for low- and middle-income countries and constrained-resource settings, where few medications may be selected. Results from this network meta-analysis can inform clinical guidelines and national and international drug regulation policies.
Schizophrenia-spectrum disorders are considered to be major drivers of the global burden of disease, as measured in prevalence, disability-adjusted life-years, and years lived with disability. More than 50% of diagnosed individuals have long-term, intermittent symptoms of psychosis, and around 20% have chronic symptoms and disability1. According to currently available evidence, regular pharmacological treatment since the early phases of disease may represent a key element for preserving neurocognitive abilities, preventing structural brain changes, and hindering the progression towards chronic functional deterioration2-4.
Many randomized controlled trials (RCTs) have compared oral antipsychotics for the treatment of acute symptoms of schizophrenia and related disorders5, while fewer studies are available for long-term, maintenance treatment6-8. According to a recent Cochrane review9 and a network meta-analysis (NMA) on long-acting injectable antipsychotics (LAIs)10, maintenance treatment with antipsychotics prevents relapse to a significantly greater extent than placebo for up to two years of follow-up, although long-term adverse effects must be carefully monitored over time11, 12.
Current guidelines agree in recommending maintenance treatment for at least one year after the first episode of psychosis, while intermittent treatment is discouraged13, 14. However, it has been estimated that up to one half of individuals suffering from schizophrenia may not take their medications as prescribed and even less are fully adherent to antipsychotic treatment15, 16, and that non-adherence is among the most important predictors of relapse17-19. For this reason, an earlier and wider use of LAIs has been suggested in order to prevent discontinuation, relapse and hospitalization since the earliest phases of disease10, 20-22. Still, individuals who begin antipsychotic treatment are usually prescribed oral formulations, as they allow easier titration, as well as more rapid tapering and discontinuation in case of adverse events. At such an early illness phase, future levels of adherence are difficult to predict, and switching to an LAI formulation might be needed without delay if the issue of non-adherence arises. Thus, it is of clinical relevance to identify which antipsychotics, including those available in both oral and LAI formulations, are the most tolerable, effective, and supported by the highest certainty of evidence.
Systematic reviews of studies assessing the comparative effectiveness and tolerability of both oral antipsychotics and LAIs vs. placebo and head-to-head for the prevention of relapse are relatively sparse. One systematic review and meta-analysis each compared the long-term effectiveness of first- vs. second-generation antipsychotics8 and of second-generation antipsychotics between each other7, and one NMA attempted to pool together both formulations6. However, several new studies have been conducted since then, and some existing studies were not included9, 10. Furthermore, prior meta-analyses mixed together studies where patients were randomized during the acute exacerbation with studies where patients were randomized after clinical stabilization had occurred, which could have yielded biased results due to differential rates of stabilization across treatment arms.
This study aimed to assess the differential effectiveness and tolerability of oral antipsychotics and LAIs for the maintenance treatment of schizophrenia-spectrum disorders by applying a NMA approach, eliminating trials where randomization had occurred during the acute phase.
METHODS
This study was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines specific for NMA24. The study protocol was registered in advance in the Open Science Forum (https://osf.io/3nb4s).
Study selection and data extraction
We searched for RCTs including adults (≥18 years old) diagnosed with schizophrenia-spectrum disorders (including schizophrenia, schizoaffective disorder, schizophreniform disorder, delusional disorder, and psychotic disorders not otherwise specified), according to validated diagnostic systems (DSM or ICD), and requiring antipsychotic maintenance treatment. We considered only studies randomizing clinically stable patients at baseline. Whenever this was not clearly stated by the study authors, clinical stability was ascertained on the basis of the mean score on a rating scale at baseline, according to validated cut-offs for severity – i.e., Brief Psychiatric Rating Scale (BPRS): ≤44; Positive and Negative Syndrome Scale (PANSS): ≤78; Clinical Global Impression-Severity (CGI-S): ≤425, 26.
All available oral antipsychotics and LAIs, according to the Anatomical Therapeutic Chemical with Defined Daily Dose (ATC/DDD) classification (https://www.whocc.no/atc_ddd_index), were eligible. Studies comparing an antipsychotic with a mix of different antipsychotics were excluded. We excluded RCTs lasting <12 weeks, as previously suggested27.
We searched without time or language restrictions the Medline, EMBASE, PsycINFO, Cochrane Central Register of Controlled Trials (CENTRAL), and Cumulative Index to Nursing and Allied Health Literature (CINAHL) electronic databases. We performed additional searches in databases of regulatory agencies (e.g., US Food and Drug Administration, European Medicines Agency), online trial registers (e.g., clinicaltrials.gov; controlled-trials.com; World Health Organization (WHO)‘s International Clinical Trials Registry Platform) and websites of pharmaceutical companies producing antipsychotics. We searched records from database inception to June 8, 2021 (for full search strategy, see supplementary information).
Two authors independently assessed titles, abstracts and full texts of potentially relevant articles, and two others extracted data following recommendations of the Cochrane Handbook for Systematic Reviews of Interventions28. Two authors assessed the methodological quality of included studies using the Cochrane Risk of Bias version 2 (RoB2) tool29. Disagreements were resolved by discussion and consensus with a third author.
Outcomes
Two co-primary outcomes were analyzed: relapse (i.e., the number of participants experiencing at least one relapse by the end of the trial, as a proportion of the total of randomized participants) and tolerability (i.e., the number of participants who dropped out by the end of the trial because of an adverse event, as a proportion of the total of randomized participants). The definition of relapse provided by each study was considered. If data were not available, the number of relapses was imputed according to commonly used cut-off scores on validated rating scales measuring psychopathology (i.e., PANSS increase ≥25%; BPRS increase ≥30%; CGI-S increase ≥2 points)30-32, using a validated methodology33.
Secondary outcomes included: a) mean change score on validated rating scales measuring psychopathology at the end of the trials (“efficacy”); b) number of participants who dropped out by the end of the trial for any cause; c) number of participants who were admitted to hospital for psychiatric relapse by the end of the trial; d) mean change score on validated rating scales measuring quality of life at the end of the trial; e) mean change score on validated rating scales measuring the level of functioning at the end of the trial; f) common antipsychotic-related adverse events, including sedation, insomnia, QTc prolongation, anticholinergic symptoms, weight gain, hyperprolactinaemia, extrapyramidal symptoms, akathisia, and tardive dyskinesia.
Statistical analysis
We performed a standard pairwise, random-effects meta-analysis for every comparison, and, for each outcome, we also conducted a NMA with a random-effects model in a frequentist framework, using the R software34 netmeta package and the Stata35 mvmeta package. For dichotomous outcomes, we calculated and pooled relative risks (RRs) with 95% confidence intervals (CIs). For continuous outcomes, we pooled the mean differences (MDs) between treatment arms at the end of the study if all trials used the same rating scale; otherwise, we pooled standardized mean differences (SMDs).
We calculated dichotomous data on a strict intention-to-treat (ITT) basis, considering the total number of randomized participants as the denominator. For continuous variables, we applied a modified ITT analysis, whereby participants with at least one post-baseline measurement were represented by their last observation carried forward (LOCF). When a study included different arms of the same antipsychotic (oral or LAI) at different doses, we pooled these arms into a single one28, provided that they were administered within a therapeutic dose range36, 37. Very low doses of antipsychotics were considered as pseudo-placebo, as endorsed by regulatory agencies38, and pooled together with placebo in the analysis. Furthermore, following a pragmatic approach and considering their pharmacological similarity39, fluphenazine enanthate and decanoate, as well as clopenthixol and zuclopenthixol decanoate, were pooled together in the analysis.
We asked trial authors to supply missing data or, alternatively, we imputed them with validated statistical methods28. Particularly, we calculated missing standard deviations (SDs) based on the standard error (SE), t-statistics or p values40. If this was not possible, we substituted missing SDs with a weighed mean of SDs reported in the other included trials41. As a last option, we used the SD of the mean baseline score.
For pairwise meta-analyses, we assessed heterogeneity by visual inspection of forest plots, and by the I-squared statistics. For the NMA, common heterogeneity across all comparisons was assumed and estimated in each network42, 43.
We assessed global heterogeneity by using the 𝜏2 and the I2 statistics. As previously suggested23, we compared the common 𝜏2 to the empirical distributions of heterogeneity found in meta-analyses of pharmacological treatments for mental health outcomes, showing a median of the 𝜏2 distribution of 0.049 and an inter-quartile range (IQR) of 0.010 to 0.24244, and considered heterogeneity low when the estimated 𝜏2 was below the 25% quartile, moderate between the 25% and the 50% quartile, and high above 50% quartile. The I2 statistics was interpreted according to the Cochrane handbook: 0-40%: might not be important; 30-60%: may represent moderate heterogeneity; 50-90%: may represent substantial heterogeneity; 75- 100%: considerable heterogeneity29.
According to the assumption of transitivity, effect modifiers should be equally distributed across the comparisons. We extracted the key study characteristics judged to be potential effect modifiers, i.e. sample size, year of publication, follow-up duration, blinding (double-blind vs. open-label), industry sponsorship, placebo relapse rate, overall dropout rate, mean age, percentage of female participants, mean score of overall psychopathology at baseline, and dose of medication (expressed as a ratio between prescribed daily dose and defined daily dose)45. By comparing the distribution of these possible effect modifiers across treatments included in the NMA using the Kruskal-Wallis test, and assessing their actual impact on the treatment effect through meta-regression analyses, we formulated a judgment on whether distribution differences were large enough to threaten the validity of the analysis46.
We evaluated the presence of inconsistency by comparing direct and indirect evidence within each closed loop by applying the separating indirect from direct evidence (SIDE) approach47, 48. We further compared the goodness of fit for a NMA model assuming consistency with a model allowing for inconsistency in a “design-by-treatment interaction model” framework49-51, using the decompose.design function in R package netmeta 52.
For the co-primary outcomes, we calculated the probability of each treatment of being at each possible rank, and produced a treatment hierarchy by means of surface under the cumulative ranking curve (SUCRA) and mean ranks with the R gemtc package53.
If ≥10 studies were included in a primary outcome, we assessed publication bias by visually inspecting the funnel plot, testing for asymmetry with the Egger's regression test54, and investigating possible reasons for funnel plot asymmetry.
For each co-primary outcome, we assessed the confidence of evidence by using the Confidence in Network Meta-Analysis (CINeMA) methodology55, 56 and its web-based application (http://cinema.ispm.ch).
For the co-primary outcomes, we conducted sensitivity analyses excluding trials: a) not employing double-blind design; b) with overall high risk of bias according to RoB2; c) for which information about clinical stability was assumed based on mean rating scale scores at baseline; d) with follow-up duration <1 year; e) where treatment effectiveness was not the primary outcome; and f) placebo-controlled.
We performed meta-regression analyses to assess if the following covariates acted as moderators of treatment effect: sample size, year of publication, follow-up duration, blinding (double-blind vs. open-label), industry sponsorship, placebo relapse rate, overall dropout rate, mean age, percentage of female participants, mean score of overall psychopathology at baseline, and dose of medication. In particular, for each potential effect modifier, we first tested the hypothesis of equality of parameters related to interaction terms between the covariate and treatment indicators; then, in case of non-rejection of such hypothesis, we evaluated statistical significance of the common covariate parameter; otherwise, we assessed the global significance of each covariate-treatment interaction.
RESULTS
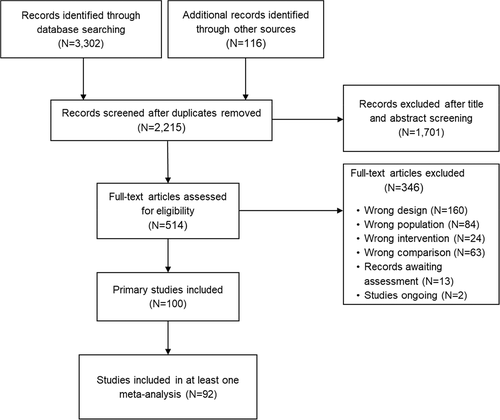
We identified 3,418 records after database and hand-search. After removing duplicates and examining titles and abstracts, we selected 514 records for full-text assessment. Of these, 100 primary studies were eligible for inclusion (corresponding to 99 full-text articles57-155, as one paper reported on two trials). Of these, 92 studies, including 22,645 participants, provided data for ≥1 outcome of interest (see Figure 1). The list of included and excluded studies, and the detailed characteristics of included studies, are provided in the supplementary information.
The mean sample size of included studies was 274 individuals (range: 49 to 1,098; median: 134), with 42 studies (45.6%) including ≤50 participants. The mean age of included participants was 39.2 years (range: 21.5 to 69.6; median: 39.7). Four studies included only males. In the remaining studies, the mean proportion of included women was 38.1% (range: 8 to 74%; median: 39%). According to the RoB2, 34.1% of the studies had an overall high risk of bias for the outcome relapse, and 16.7% for the outcome tolerability (see supplementary information).
Table 1 describes the characteristics of studies included in the two primary analyses, and Figures 2 and 3 show the corresponding network plots. Figures 4 and 5 show the forest plots comparing each antipsychotic with placebo for the two primary outcomes. Results were grouped according to the level of confidence as assessed by CINeMA. The transitivity assumption was not violated for any of the potential effect modifiers analyzed (see supplementary information).
| Relapse network | Tolerability network | |
|---|---|---|
| Number of studies | 89 | 81 |
| Number of individuals included | 22,275 | 21,504 |
| Age (years, mean±SD) | 39.0±11.9 | 38.9±11.9 |
| Gender (% women) | 36.4 | 37.7 |
| Mean follow-up (% studies) | ||
| 12 to 26 weeks | 37.1 | 37.0 |
| 27 to 52 weeks | 44.9 | 44.4 |
| 53 weeks or more | 18.0 | 18.6 |
| Blinding (% studies) | ||
| Double-blind | 73.0 | 74.1 |
| Open-label | 27.0 | 25.9 |
| Year of publication (% studies) | ||
| Until 1989 | 28.1 | 25.9 |
| 1990 to 2009 | 33.7 | 34.6 |
| 2010 to 2019 | 38.2 | 39.5 |
| Type of studies (% studies) | ||
| Placebo-controlled | 33.7 | 33.3 |
| Only active comparator | 66.3 | 66.7 |
| Including oral formulation | 73.0 | 72.8 |
| Including LAI formulation | 49.4 | 49.4 |
| Setting (% studies) | ||
| Inpatients | 20.2 | 18.5 |
| Outpatients | 56.2 | 55.6 |
| Mixed | 23.6 | 25.9 |
- LAI – long-acting injectable antipsychotic
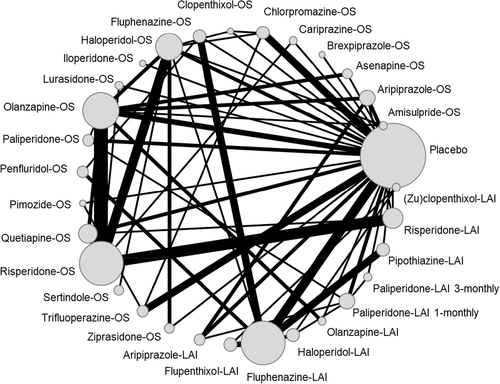
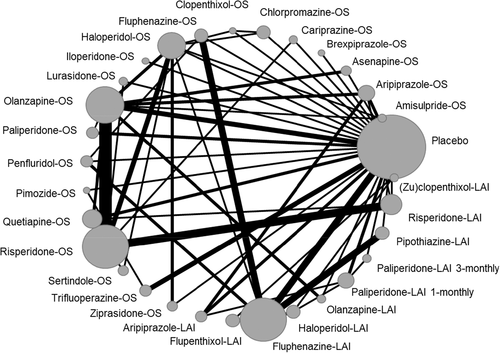
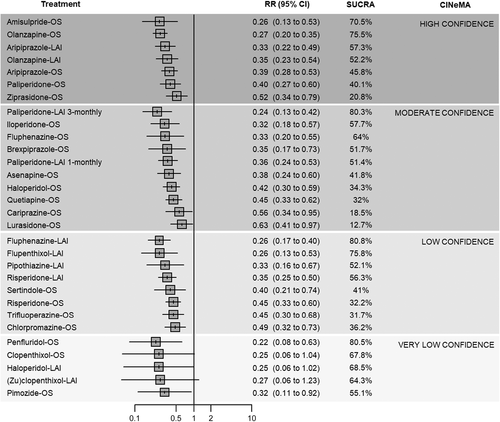
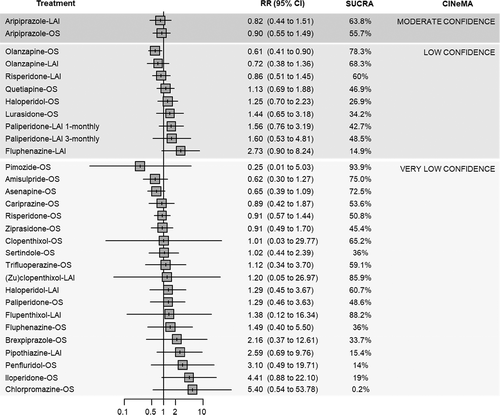
In terms of relapse prevention, all antipsychotics – with the exception of clopenthixol-oral (OS), haloperidol-LAI and (zu)clopenthixol-LAI – were significantly more effective than placebo. “High” confidence was found for the following antipsychotics (ordered from the largest to the smallest point estimate): amisulpride-OS, olanzapine-OS, aripiprazole-LAI, olanzapine-LAI, aripiprazole-OS, paliperidone-OS, and ziprasidone-OS. “Moderate” confidence was found for the following antipsychotics (ordered from the largest to the smallest point estimate): paliperidone-LAI 1-monthly, iloperidone-OS, fluphenazine-OS, brexpiprazole-OS, paliperidone-LAI 1-monthly, asenapine-OS, haloperidol-OS, quetiapine-OS, cariprazine-OS, and lurasidone-OS. For the remaining antipsychotics, the confidence in the estimate was “low” or “very low” (see Figure 4).
Head-to-head comparisons showed relatively few statistically significant differences between antipsychotics. Among those with moderate-to-high confidence according to CINeMA, aripiprazole-LAI was more effective than lurasidone-OS; olanzapine-OS than cariprazine-OS, chlorpromazine-OS, haloperidol-OS and lurasidone-OS; paliperidone-LAI 3-monthly than cariprazine-OS, chlorpromazine-OS, lurasidone-OS and ziprasidone-OS; risperidone-LAI than lurasidone-OS (see supplementary information).
In the pairwise meta-analyses, moderate heterogeneity (i.e., I2>50%) was detected for the following pairwise comparisons: aripiprazole-OS, olanzapine-OS, quetiapine-OS and trifluoperazine-OS vs. placebo; olanzapine-OS vs. asenapine-OS. Substantial heterogeneity (i.e., I2>75%) was detected for risperidone-OS vs. quetiapine-OS. Overall, the NMA showed low-to-moderate heterogeneity (𝜏2=0.056; I2=32.8%, 95% CI: 9.8% to 49.9%), and no overall incoherence emerged according to the global approach (design-by-treatment test, p=0.089), while the local SIDE approach showed significant inconsistency of two comparisons (placebo vs. pimozide-OS; pimozide-OS vs. trifluoperazine-OS).
Fluphenazine-LAI, penfluridol-OS, paliperidone-LAI 3-monthly, flupenthixol-LAI, olanzapine-OS and amisulpride-OS ranked best according to the mean SUCRA. However, only for paliperidone-LAI 3-monthly, olanzapine-OS and amisulpride-OS the confidence in the evidence was “moderate” or “high” compared to placebo. In most cases, “low” or “very low” estimates were due to incoherence and within-study bias (see Figure 4 and supplementary information).
Sensitivity analyses suggested that placebo-controlled studies might have been responsible for most of the observed heterogeneity. Removing studies with high risk of bias, those for which stability was imputed, those with less than one year of follow-up, and placebo-controlled studies reduced the observed local and global inconsistency. Despite this, effect estimates from sensitivity analyses did not change significantly compared to the primary analysis (see supplementary information).
Meta-regression analyses showed that only the clinical severity at baseline was a statistically significant effect modifier, with studies randomizing more severely ill individuals showing a smaller effect size. However, results of a post-hoc sensitivity analysis excluding people who were markedly ill at baseline were not significantly different from the primary analysis (see supplementary information).
Compared to placebo, none of the antipsychotics included showed significant differences in terms of tolerability (dropouts due to adverse events), with the only exception of olanzapine-OS, which was more tolerable than placebo. However, only for aripiprazole-LAI and aripiprazole-OS the confidence according to the CINeMA assessment was “moderate”, while it was “low” or “very low” for all remaining treatments (see Figure 5).
Head-to-head analyses showed olanzapine-OS to be more tolerable than haloperidol-OS, iloperidone-OS and lurasidone-OS; and olanzapine-LAI to be more tolerable than iloperidone-OS and fluphenazine-LAI.
Substantial heterogeneity (i.e., I2>75%) was detected for two pairwise comparisons (olanzapine-OS vs. placebo; ziprasidone-OS vs. haloperidol-OS). Overall, the NMA showed moderate heterogeneity (𝜏2=0.078; I2=20.9%, 95% CI: 0% to 42.8%). Incoherence was detected according to the global approach (design-by-treatment test, p=0.01), while the local SIDE approach showed significant inconsistency between placebo and asenapine-OS, fluphenazine-LAI and haloperidol-OS, olanzapine-OS and quetiapine-OS. Pimozide-OS, flupenthixol-LAI, (zu)clopenthixol-LAI, olanzapine-OS and amisulpride-OS ranked best according to the mean SUCRA. However, for all of these comparisons, the confidence in the evidence was “low” or “very low”. In most cases, “low” or “very low” estimates were due to incoherence, imprecision and within-study bias (see Figure 5 and supplementary information).
Sensitivity analyses suggested that placebo-controlled studies were the main source of the observed heterogeneity. Local and global inconsistency was notably reduced when removing studies with less than one year of follow-up (global approach: from p=0.09 to p=0.51; local SIDE approach: from two to zero inconsistent comparisons) and placebo-controlled studies (global approach: from p=0.09 to p=0.88; local SIDE approach: from two to zero inconsistent comparisons). Despite this, effect estimates from sensitivity analyses did not change significantly compared to the primary analysis (see supplementary information).
With regard to efficacy-related secondary outcomes, in descending ranking order of effect as compared to placebo, sertindole-OS, olanzapine-LAI, risperidone-LAI, olanzapine-OS, paliperidone-LAI 3-monthly, risperidone-LAI and fluphenazine-LAI showed lower risk of hospitalization for psychiatric relapse; brexpiprazole-OS, lurasidone-OS, pimozide-OS, sertindole-OS, ziprasidone-OS, iloperidone-OS, olanzapine-OS, asenapine-OS, risperidone-OS, cariprazine-OS, paliperidone-OS, risperidone-LAI, aripiprazole-OS, olanzapine-LAI, haloperidol-OS, aripiprazole-OS, paliperidone-LAI 3-monthly, paliperidone-LAI 1-monthly and quetiapine-OS showed larger reduction of mean rating scale scores at study endpoint; (zu)clopenthixol-LAI, pimozide-OS, olanzapine-OS, aripiprazole-LAI, trifluoperazine-OS, paliperidone-LAI 3-monthly, haloperidol-LAI, olanzapine-LAI, amisulpride-OS, asenapine-OS, aripiprazole-OS, fluphenazine-LAI, haloperidol-OS and risperidone-OS showed lower risk of total dropouts.
With regard to tolerability-related secondary outcomes, in descending ranking order of effect as compared to placebo, risperidone-LAI, paliperidone-OS, lurasidone-OS and risperidone-OS showed significantly higher risk of sedation; aripiprazole-OS, olanzapine-LAI, olanzapine-OS, paliperidone-LAI 1-monthly and paliperidone-LAI 3-monthly showed significantly higher risk of weight gain; haloperidol-OS, fluphenazine-LAI and pipothiazine-LAI showed significantly higher risk of extrapyramidal symptoms; haloperidol-OS, haloperidol-LAI and trifluoperazine-OS showed significantly higher risk of akathisia; olanzapine-OS, olanzapine-LAI, paliperidone-LAI 1-monthly, paliperidone-LAI 3-monthly, risperidone-LAI, risperidone-OS and paliperidone-OS showed significantly higher risk of hyperprolactinaemia; olanzapine-OS, olanzapine-LAI, asenapine-OS, paliperidone-LAI 3-monthly and risperidone-OS showed significantly lower risk of insomnia. No antipsychotics showed higher risk of QTc prolongation and tardive dyskinesia as compared to placebo, although CIs were imprecise for most comparisons. For anticholinergic symptoms, a NMA could not be carried out, as data were relatively few and the network poorly connected (four sub-networks were identified); pairwise meta-analyses showed a higher risk for risperidone-LAI and quetiapine-OS as compared to placebo (see supplementary information).
Efficacy measured with rating scales, hospitalization rates and dropouts due to any cause was generally in line with findings from the primary analysis, while data on quality of life, functioning, and some common adverse events (particularly anticholinergic symptoms, QTc change, tardive dyskinesia) were relatively scarce. Significant incoherence and high heterogeneity were not detected for any of these outcomes, with the only exception of efficacy measured with rating scales (see supplementary information).
DISCUSSION
To our knowledge, this is the largest and most updated systematic review and NMA comparing data on the maintenance treatment of individuals with schizophrenia-spectrum disorders.
Use of LAIs from the earliest phase of disease has been recommended10, 20, 22. However, in real-world practice, most individuals begin with an oral treatment for practical reasons. Thus, from a strictly clinical perspective, choosing an antipsychotic for which both oral and LAI formulation are available would be valuable, in order to facilitate a switch to the LAI when required. According to this viewpoint, our analyses suggest that olanzapine, aripiprazole and paliperidone are the most reasonable choices, as they are: a) among the best-performing treatments in terms of relapse prevention according to the effect estimate and the SUCRA ranking; b) supported by the highest confidence of evidence according to the CINeMA approach; and c) available in both oral and LAI formulation.
Regarding tolerability (dropouts due to adverse events), no antipsychotic was significantly worse than placebo, although the certainty of evidence was generally low, being “moderate” only for aripiprazole-OS and aripiprazole-LAI. Although dropouts due to intolerability reflect the overall burden of adverse events, this information alone cannot be exhaustive when tailoring the choice of antipsychotics to individual patients, for which detailed knowledge of specific adverse events might be more useful. However, analyses of common adverse events were limited and imprecise in many cases, calling for greater attention to measuring and reporting these adverse effects in maintenance/relapse prevention trials of antipsychotics.
Overall, the finding that most LAIs and oral antipsychotics are effective in preventing relapse and re-hospitalization as compared to inactive treatment (as in placebo-controlled trials) or no treatment/treatment “as usual” (as in observational studies) is consistent with existing large observational database studies22, 156 and with meta-analyses of observational and randomized studies6, 9, 157 on the maintenance treatment of schizophrenia.
Our results are generally in line with those from a previous NMA on oral antipsychotics in acutely ill individuals23. Compared with placebo, the ranking and the magnitude of effect of treatments are roughly comparable between the two NMAs, with few exceptions, such as risperidone-OS, sertindole-OS and lurasidone-OS apparently performing better in the “acute” population, and fluphenazine-OS performing better in the “maintenance” population. However, these differences are of relatively small magnitude, and the confidence of evidence for these treatments was rated as “low” or “very low” in at least one of the two NMAs. Furthermore, it needs to be recognized that differences in populations and trial design across several decades when the acute and maintenance studies were conducted could also have affected the results, limiting the indirect comparability of antipsychotic effectiveness, both within and across illness stage (acute vs. maintenance).
This NMA did not detect clear advantages of LAIs over oral antipsychotic formulations in terms of relapse and re-hospitalization. This is in line with the observation that, in general, LAIs have shown clearer advantages over oral antipsychotics in observational studies21, 22, 158, 159 rather than in randomized trials6, 21, 160. As previously suggested, observational studies might have greater external validity because of less restrictive patient selection, although the lack of blinding might increase the risk of bias (e.g., detection, performance and prescribing bias)161.
The results of this NMA should be interpreted in the light of some possible limitations. First, for some studies, clinical stability was not clearly described, and we imputed this information by using baseline scores of rating scales measuring psychopathology, according to validated cut-offs. This information can be considered as a valid proxy of clinical stabilization, although it may lack precision. However, after removing these studies in a sensitivity analysis, results did not change remarkably. Second, several studies lacked relevant information, and we used imputation techniques which have been empirically validated41, but might nonetheless be imprecise.
Third, included RCTs employed different study designs and diagnostic criteria, and had different primary outcomes, settings of recruitment, and follow-up periods. Despite that, the overall coherence of the networks appeared to be preserved for the primary analyses and for most secondary outcomes. Fourth, we included placebo-controlled trials, which have possible limitations162, 163, and had probably a prominent role in introducing heterogeneity and incoherence, as shown by sensitivity analyses, which, however, did not show substantial changes of overall results.
Fifth, overall risk of bias was relevant for many studies. However, after removing these studies by means of sensitivity analyses, primary results did not change remarkably. Sixth, some secondary outcomes, such as quality of life and functioning, which might play a considerable role in helping clinicians to tailor their choice to individual patients, were insufficiently reported by the original studies, leading to poorly populated and connected networks, and imprecise results.
Seventh, effectiveness need to be put into the context of tolerability, especially during long-term treatment. However, adverse effect outcomes were only partially and inconsistently reported, not allowing a detailed benefit-to-risk assessment. Nevertheless, we used the outcome of intolerability-related discontinuation as a proxy of clinically relevant adverse effects and found similar performance of the meta-analyzable antipsychotics and no difference to placebo. Thus, although individual long-term adverse effects of antipsychotics can be potentially problematic12, 164, overall, patients do not seem to discontinue antipsychotic maintenance treatment more than those randomized to placebo. Moreover, effective long-term antipsychotic treatment facilitates healthier lifestyle choices and adherence to medical treatments prescribed to mitigate illness- and/or medication-related cardiometabolic burden165, 166.
Finally, as no comparison included ≥10 studies, the risk of publication bias could not be ruled out, although this is expected to be less relevant compared to other classes of psychotropic drugs167.
Despite these limitations, to our knowledge, this is the largest and most comprehensive meta-analysis of antipsychotics for the maintenance treatment of people with schizophrenia. As such, findings of this NMA might have significant implications for clinical practice, policy and research. Current guidelines agree in recommending long-term maintenance treatment for at least one year after the first episode13, 14, 168, 169. However, clear information on which antipsychotic to choose is lacking. According to the UK National Institute for Health and Care Excellence (NICE) guidelines, current evidence cannot guide the choice between antipsychotics in the maintenance phase170, while the recently updated American Psychiatric Association guidelines suggest using the same treatment which provided benefit in the acute phase168, as it is implicitly recommended also by the WHO mental health Gap Action Programme (mhGAP) guidelines169. Data from this NMA show that, although the magnitude of benefit is apparently similar between antipsychotics, they are not all equal, because the confidence in this estimate can largely vary, which is of paramount relevance for making evidence-based choices.
Both oral and LAI formulations of olanzapine, aripiprazole and paliperidone proved to be effective and are supported by moderate-to-high confidence of evidence, and should therefore be given priority when initiating a pharmacological maintenance treatment in people with schizophrenia-spectrum disorders, although differences in adverse effect profiles should also be considered in the decision-making process. Moreover, identifying antipsychotics allowing a switch between oral and LAI formulations might be particularly useful in low- and middle-income countries (LMICs), and in constrained-resource settings in general, where only a limited number of medications may be selected for inclusion in national formularies. Although costs might be an issue, this should not prevent the inclusion of evidence-based treatments in such contexts. From this standpoint, these data call for an effort to produce more affordable second-generation LAIs, as it has been done for other treatments in LMICs171.
Taken together, results from this NMA can inform clinical practice guidelines as well as national and international drug regulation policies, including the WHO Essential Medicines List.
ACKNOWLEDGEMENTS
The authors would like to thank Y. Zhang and G.P. Morgano (Department of Health Research Methods, Evidence and Impact, McMaster University Health Sciences Centre, Hamilton, ON, Canada) for helping with the retrieval and translation of full-text papers. C.U. Correll and C. Barbui contributed equally to this work. Supplementary information on the study is available at http://doi.org/10.23728/b2share.412cc348acb34b35950bd812b9458c36.




