Antipsychotic augmentation vs. monotherapy in schizophrenia: systematic review, meta-analysis and meta-regression analysis
Abstract
Antipsychotic polypharmacy in schizophrenia is much debated, since it is common and costly with unclear evidence for its efficacy and safety. We conducted a systematic literature search and a random effects meta-analysis of randomized trials comparing augmentation with a second antipsychotic vs. continued antipsychotic monotherapy in schizophrenia. Co-primary outcomes were total symptom reduction and study-defined response. Antipsychotic augmentation was superior to monotherapy regarding total symptom reduction (16 studies, N=694, standardized mean difference, SMD=–0.53, 95% CI: −0.87 to −0.19, p=0.002). However, superiority was only apparent in open-label and low-quality trials (both p<0.001), but not in double-blind and high-quality ones (p=0.120 and 0.226, respectively). Study-defined response was similar between antipsychotic augmentation and monotherapy (14 studies, N=938, risk ratio = 1.19, 95% CI: 0.99 to 1.42, p=0.061), being clearly non-significant in double-blind and high-quality studies (both p=0.990). Findings were replicated in clozapine and non-clozapine augmentation studies. No differences emerged regarding all-cause/specific-cause discontinuation, global clinical impression, as well as positive, general and depressive symptoms. Negative symptoms improved more with augmentation treatment (18 studies, N=931, SMD=–0.38, 95% CI: −0.63 to −0.13, p<0.003), but only in studies augmenting with aripiprazole (8 studies, N=532, SMD=–0.41, 95% CI: −0.79 to −0.03, p=0.036). Few adverse effect differences emerged: D2 antagonist augmentation was associated with less insomnia (p=0.028), but more prolactin elevation (p=0.015), while aripiprazole augmentation was associated with reduced prolactin levels (p<0.001) and body weight (p=0.030). These data suggest that the common practice of antipsychotic augmentation in schizophrenia lacks double-blind/high-quality evidence for efficacy, except for negative symptom reduction with aripiprazole augmentation.
Management options for patients with schizophrenia remain suboptimal, as indicated by insufficient symptom control in a sizable subgroup of patients and low response rates, frequently leading to functional impairment1-5. Recommendations after inadequate antipsychotic response include waiting for a delayed response, dose adjustment, switching to another antipsychotic, and – in case of treatment resistance to at least two adequate antipsychotic trials – clozapine treatment6-11.
Another adopted strategy is antipsychotic polypharmacy12. Limited data on clinicians’ reasoning suggest various motivations for this strategy, including attempts to increase/speed up efficacy, treat residual positive symptoms, or reduce adverse effects allowing dose reduction of the first antipsychotic13. Antipsychotic polypharmacy has been reported as a common clinical practice12, 14, 15, sometimes implemented by clinicians before or instead of trying clozapine13, 16. Although the frequency of antipsychotic polypharmacy varies according to patient, illness, setting and provider variables17, rates in schizophrenia commonly range between 10 and 30%12, 14, 17-19.
Despite common use, the evidence for the efficacy and tolerability of antipsychotic polypharmacy is weak20-22. In fact, guidelines reserve augmentation with a second antipsychotic as a last-stage treatment option after clozapine failure, intolerability or rejection6-10. Additionally, concerns about antipsychotic polypharmacy include the potential for drug-drug interactions, decreased adherence due to complex drug regimes, higher cost23-25, and increased adverse effects10, 22, 26-29.
Meta-analyses aggregate the information of conceptually similar studies and consolidate their quantitative outcomes using statistics. The derived pooled estimates of treatment efficacy and safety are more robust compared to primary study results. Moreover, meta-analyses enable researchers to contrast results from multiple studies and to identify patterns of common effects across studies, or reasons for outcome variability. However, to facilitate informative results and meaningful subgroup and meta-regression analyses, the study methodology should be as homogeneous as possible; study quality should be taken into account; and the total population studied should be sufficiently large (≥1000 subjects)30.
Although four meta-analyses examined the efficacy of antipsychotic polypharmacy, either irrespective of the antipsychotics used20 or restricted to clozapine-treated patients21, 31, 32, their results remained somewhat inconclusive, possibly influenced by: a) mixing together antipsychotic augmentation (adding a second antipsychotic after non-response to the first) and co-initiation (combination of two antipsychotics from the beginning) strategies20; b) lack of separating lower from higher quality studies20, and c) the relatively low number of available studies and patients treated in an augmentation paradigm20, 21, 31, 32.
In one of those meta-analyses, polypharmacy was associated with significantly greater response than monotherapy, with a number-needed-to-treat of 720. However, the improved response was moderated by studies lasting at least ten weeks, conducted in China, examining co-initiation and involving clozapine. Further, that meta-analysis only included six studies of antipsychotic augmentation (N=197), and did not assess symptom reduction due to lacking data.
The three remaining meta-analyses focused on combination treatments with clozapine, either mixing co-initiation and augmentation studies together21, or focusing on augmentation studies but analyzing only individual drug combinations32, or focusing only on symptom reduction and not response rates31. One meta-analysis found clozapine co-treatment to be superior to clozapine monotherapy, but this finding was only apparent in open-label studies21. In one other meta-analysis, augmentation of clozapine with a second antipsychotic was associated with a small benefit (effect size = 0.239, p=0.028), but only 14 trials and 714 patients provided data, and higher versus lower quality studies were not analyzed separately31.
Due to the limitations of those prior meta-analyses, the frequent use of antipsychotic polypharmacy in ordinary practice, and the recent publication of many additional studies, we conducted a new systematic review and meta-analysis comparing the efficacy and adverse effects of antipsychotic augmentation vs. monotherapy. Based on the prior literature20, 21, 31-34, we hypothesized that antipsychotic augmentation would not be superior to monotherapy regarding efficacy (measured as total and specific symptom reduction as well as response/remission/relapse) when focusing on augmentation trials and those with higher quality, but that antipsychotic augmentation might confer a higher risk of adverse effects (except for reduction of specific adverse effects when adding a partial D2 agonist to D2 antagonists).
METHODS
The systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) standard35, 36. At least two independent authors searched PubMed/MEDLINE, PsycINFO, Chinese Journal Net, Wangfan, and China Biology Medicine databases from inception until May 25, 2015, without language restrictions, supplemented by a manual review of reference lists from eligible publications and relevant reviews. Authors were contacted for additional information if needed.
We included randomized controlled trials with samples consisting of at least 20 adults with a diagnosis of schizophrenia or schizoaffective disorder; in which patients were assigned to augmentation of the current antipsychotic with a different antipsychotic versus augmentation with placebo (in blinded studies) or continuation of existing antipsychotic monotherapy; and in which meta-analyzable data were reported, including symptomatic/functional or adverse effect outcomes. We excluded studies comparing antipsychotic monotherapy versus two antipsychotics started concurrently, as well as those comparing antipsychotic augmentation with antipsychotic switch instead of continuation of the original antipsychotic monotherapy.
Co-primary outcomes were total symptom reduction, as assessed by the Positive and Negative Syndrome Scale (PANSS)37 or the Brief Psychiatric Rating Scale (BPRS)38, and study-defined treatment response. Secondary outcomes were all-cause and specific-cause discontinuation (inefficacy, intolerability); reduction of positive symptoms (as assessed by the PANSS positive, the BPRS positive, or the Scale for the Assessment of Positive Symptoms, SAPS39), of negative symptoms (as assessed by the PANSS negative, the BPRS negative, or the Scale for the Assessment of Negative Symptoms, SANS40), and of general symptoms (as assessed by the PANSS general); reduction of global illness severity (as assessed by the Clinical Global Impression Scale - Improvement, CGI-I41); reduction of depressive symptoms (as assessed by the PANSS/BPRS anxiety/depression, the Hamilton Scale for Depression, HAM-D42, or the Calgary Depression Scale for Schizophrenia, CDSS43); improvement of functioning (as evaluated by the Global Assessment of Functioning Scale, GAF44); and frequency and severity of adverse effects.
Data of each study were independently identified, checked and extracted by at least two authors, including information relevant for the Cochrane risk-of-bias tool45. Inconsistencies were resolved by consensus/involvement of a third reviewer.
We conducted a random effects46 meta-analysis of outcomes using Comprehensive Meta-Analysis V3 (www.meta-analysis.com). Study heterogeneity was explored using I2 statistics and chi-square test of homogeneity, with I2>50% and p<0.05 indicating significant heterogeneity. All analyses were two-tailed with alpha=0.05, without adjustments for multiple comparisons.
For “total” and “specific” psychopathology (except depression and negative symptoms) and for inefficacy-related discontinuation, all studies except those focusing on the amelioration of adverse effects were analyzed. The reason for using this restricted data set was that studies focusing on the amelioration of adverse effects could have included treatment responders, leaving little or no room for improvement. In contrast, for depression and negative symptoms and for individual adverse effects, all-cause discontinuation and intolerability-related discontinuation, all available data were analyzed, including studies focusing on the reduction of adverse effects.
Group differences in continuous outcomes were analyzed as the pooled standardized mean difference (SMD) in either change from baseline to endpoint (preferred) or endpoint scores (only preferred if change score results were skewed, i.e., SD > twice the mean). Additionally, weighted mean difference (WMD) was calculated for weight change in kilograms. Dichotomous data were analyzed calculating the pooled risk ratio (RR). Intention-to-treat (ITT) data were always preferred, but observed cases (OC) data were also allowed.
All outcomes were analyzed for the pooled sample and for high-quality studies separately. The latter were defined a priori as double-blind studies using ITT/last-observation-carried-forward (LOCF) analyses, as opposed to open-label studies and those using OC data. In two studies with more than one active augmentation arm47, 48, the number of patients in the monotherapy group was divided by the number of active study arms to avoid double-counting of control subjects.
For meta-regression analyses, the baseline BPRS total scores were converted to PANSS total scores using equipercentile linking49. Exploratory subgroup and meta-regression analyses were added post-hoc for negative symptom change (the only overall significant outcome in both low- and high-quality studies) in studies using partial D2 agonists.
We inspected funnel plots, used Egger's regression test50 and the Duval and Tweedie's trim and fill method51 to quantify whether publication bias could have influenced the results.
RESULTS
The initial search resulted in 17,653 hits. Altogether, 17,427 studies were excluded at the title/abstract level. Of the remaining 226 references, 195 were excluded after full text review, yielding 31 studies that were included in the meta-analysis (Figure 1).
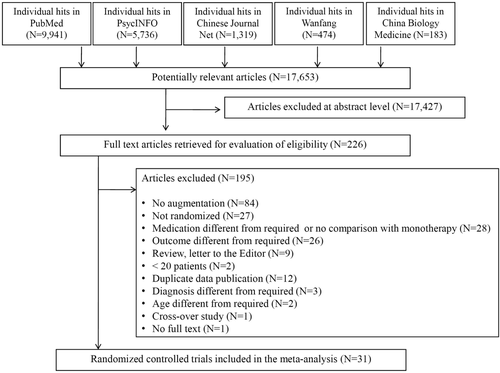
PRISMA diagram of the literature search
Efficacy of antipsychotic monotherapy vs. augmentation (efficacy data set)
Details on the 22 meta-analyzed studies with efficacy as the primary outcome (N=1,342) are provided in Table 1. They included 13 double-blind and ITT/LOCF “high-quality” studies and 9 open-label and/or OC “low quality” ones.
| Study | Agents | No. patients |
Risk of biasa |
Blinding | Primary outcome | Analysis | Trial duration (weeks) | Setting | Monotherapy dose, mg/d: mean (range) | Augmentation group dose, mg/d: mean (range) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Clozapine + first-generation antipsychotic | |||||||||||
| Liu et al52 (China) | CLZ + FLU | T: 60 M: 30 A: 30 | 1 | OL | Efficacy | ITT | 24 | Inpatients | CLZ: NR (375-500) | CLZ: NR (375-500) | FLU: NR (25-50) |
| Friedman et al53 (US) | CLZ + PIM | T: 53 M: 28 A: 25 | 3 | DB | Efficacy | ITT | 12 | Inpatients (64.2%) and outpatients (35.8%) | CLZ: 478.1 (NR) | CLZ: 518.8 (NR) | PIM: 6.48 (2.0-8.9) |
| Gunduz-Bruce et al54 (US) | CLZ + PIM | T: 28 M: 14 A: 14 | 4 | DB | Efficacy | ITT | 12 | Outpatients | CLZ: NR (NR) | CLZ: NR (NR) | PIM: 4 (fixed) |
| Clozapine + second-generation antipsychotic | |||||||||||
| Chang et al55 (Korea) | CLZ + ARI | T: 61 M: 32 A: 29 | 5 | DB | Efficacy | ITT | 8 | Inpatients and outpatients (% NR) | CLZ: 290.6 (NR) | CLZ: 304.3 (NR) | ARI: 15.5 (5-30) |
| Fan et al56 (US) | CLZ + ARI | T: 38 M: 18 A: 20 | 2 | DB | Adverse effects | OC | 8 | Outpatients | CLZ: 400 (NR) | CLZ: 397 (NR) | ARI: 15 (fixed) |
| Fleischhacker et al57 (Europe, South Africa) | CLZ + ARI | T: 207 M: 99 A: 108 | 4 | DB | Adverse effects | ITT | 16 | Outpatients | CLZ: 363 (163-900) | CLZ: 384 (200-900) | ARI: 11.1 (5-15) |
| Guan58 (China) | CLZ + ARI | T: 60 M: 30 A: 30 | 1 | OL | Efficacy | ITT | 16 | Inpatients | CLZ: NR (300-500) | CLZ: NR (200-300) | ARI: NR (20-30) |
| Muscatello et al59 (Italy) | CLZ + ARI | T: 40 M: 20 A: 20 | 5 | DB | Efficacy | OC | 24 | Outpatients | CLZ: 341.2 (200-450) | CLZ: 310.7 (200-450) | ARI: 12.5 (10-15) |
| Sun et al60 (China) | CLZ + ARI | T: 62 M: 30 A: 32 | 1 | OL | Efficacy | ITT | 6 | Inpatients | CLZ: 368.2 (200-450) | CLZ: 168 (75-300) | ARI: 21.6 (10-30) |
| Lin et al61 (China) | CLZ + PAL | T: 70 M: 35 A: 35 | 3 | DB | Efficacy | ITT | 12 | Inpatients | CLZ: 217.9 (NR) | CLZ: 231.7 (NR) | PAL: 8.2 (6-12) |
| Freudenreich et al62 (US) | CLZ + RIS | T: 24 M: 11 A: 13 | 2 | DB | Efficacy | ITT | 6 | Outpatients | CLZ: 456 (200-700) | CLZ: 456 (200-700) | RIS: 4 (NR) |
| Anil Yagcioglu et al63 (Turkey) | CLZ + RIS | T: 30 M: 14 A: 16 | 6 | DB | Efficacy | ITT | 6 | Inpatients (20.0%) and outpatients (80.0%) | CLZ: 414.3 (300-900) | CLZ: 515.6 (300-900) | RIS: 5.1 (NR) |
| Honer et al64 (International) | CLZ + RIS | T: 68 M: 34 A: 34 | 6 | DB | Efficacy | ITT | 8 | Inpatients (38.2%) and outpatients (61.8%) | CLZ: 487 (NR) | CLZ: 494 (NR) | RIS: 3 (NR) |
| Josiassen et al65 (US) | CLZ + RIS | T: 40 M: 20 A: 20 | 3 | DB | Efficacy | ITT | 12 | Inpatients (26.1%) and outpatients (73.9%) | CLZ: 403 (NR) | CLZ: 529 (NR) | RIS: 4.4 (NR) |
| Hu66 (China) | CLZ + RIS | T: 60 M: 30 A: 30 | 1 | OL | Efficacy | ITT | 12 | Inpatients | CLZ: 253.6 (NR) | CLZ: 126.3 (NR) | RIS: 2.9 (2-6) |
| Weiner et al67 (US) | CLZ + RIS | T: 69 M: 36 A: 33 | 2 | DB | Efficacy | ITT | 16 | Inpatients (26.1%) and outpatients (73.9%) | CLZ: NR (NR) | CLZ: NR (NR) | RIS: 4 (fixed) |
| Nielsen et al68 (Denmark) | CLZ + SER | T: 50 M: 25 A: 25 | 6 | DB | Efficacy | ITT | 12 | Outpatients | CLZ: 435 (NR) | CLZ: 394 (NR) | SER: 16 (fixed) |
| Shiloh et al69 (Israel) | CLZ + SUL | T: 28 M: 12 A: 16 | 5 | DB | Efficacy | ITT | 10 | Inpatients | CLZ: 446 (NR) | CLZ: 403 (NR) | SUL: NR (100-600) |
| Jiang et al70 (China) | CLZ + ZIP | T: 24 M: 12 A: 12 | 2 | OL | Efficacy | ITT | 12 | NR | CLZ: 597.2 (75-600) | CLZ: 489.7 (75-600) | ZIP: NR (20-160) |
| Muscatello et al71 (Italy) | CLZ + ZIP | T: 40 M: 20 A: 20 | 6 | DB | Efficacy | ITT | 16 | Outpatients | CLZ: 462.5 (350-600) | CLZ: 428.7 (350-600) | ZIP: 80.0 (fixed) |
| First-generation + second-generation antipsychotic | |||||||||||
| Shim et al72 (US, Korea) | HAL + ARI | T: 54 M: 28 A: 26 | 2 | DB | Adverse effects | ITT | 8 | NR | HAL: 24.8 (NR) | HAL: 20.7 (NR) | ARI: 22.5 (15-30) |
| Second-generation + second-generation antipsychotic | |||||||||||
| Chen et al47 (China) | RIS + ARI | T: 119 M: 30 A: 89 | 3 | DB | Adverse effects | ITT | 8 | Inpatients and outpatients (% NR) | RIS: 4.93 (NR) | RIS: 4.63 (NR) | ARI: 5 (fixed) |
| RIS: 4.79 (NR) | ARI: 10 (fixed) | ||||||||||
| RIS: 5.07 (NR) | ARI: 20 (fixed) | ||||||||||
| Kane et al73 (US) | QTP/RIS + ARI | T: 323 M: 155 A: 158 | 1 | DB | Efficacy | ITT | 16 | Outpatients | QTP/RIS: 516/4.8 (400-800/4-8) | QTP/RIS: 513/4.6 (400-800/4-8) | ARI: 10.3 (2-15) |
| Lee et al74 (Korea) | RIS + ARI | T: 35 M: 18 A: 17 | 2 | DB | Adverse effects | ITT | 12 | Inpatients | RIS: 3 (NR) | RIS: 3 (NR) | ARI: 10 (fixed) |
| Liu et al48 (China) | RIS + ARI | T: 86 M: 27 A: 59 | 1 | DB | Adverse effects | ITT | 4 | Inpatients | RIS: NR (>4) | RIS: NR (>4) | ARI: 5 (fixed) |
| ARI: 10 (fixed) | |||||||||||
| Ou et al75 (China) | OLZ + ARI | T: 70 M: 35 A: 35 | 1 | OL | Efficacy | OC | 8 | Inpatients | OLZ: 18.2 (NR) | OLZ: 17.8 (NR) | ARI: 10 (fixed) |
| Yasui-Furukori et al76 (Japan) | RIS/OLZ + ARI | T: 36 M: 18 A: 18 | 1 | DB | Adverse effects | OC | 12 | Outpatients | RIS/OLZ: 5.0/12.5 (3-8/5-20) | RIS/OLZ: 5.9/12.1 (2-12/2.5-20 | ARI: 15.2 (6-30) |
| Zhao77 (China) | RIS + ARI | T: 56 M: 28 A: 28 | 1 | OL | Adverse effects | NR | 12 | Inpatients and outpatients (% NR) | RIS: NR (3-8) | RIS: NR (3-8) | ARI: 10 (fixed) |
| Zhou et al78 (China) | RIS + ARI | T: 100 M: 50 A: 50 | 0 | OL | Adverse effects | NR | 24 | Inpatients | RIS: NR (4-6) | RIS: NR (4-6) | ARI: 5 (fixed) |
| Liang & Liu79 (China) | ARI + CLZ | T: 65 M: 33 A: 32 | 1 | OL | Efficacy | ITT | 8 | NR | ARI: NR (20-30) | ARI: NR (20-30) | CLZ: NR (25-100) |
| Kotler et al80 (Israel) | OLZ + SUL | T: 17 M: 8 A: 9 | 2 | OL | Efficacy | ITT | 8 | Inpatients | OLZ: 22.5 (20-30) | OLZ: 22.2 (20-30) | SUL: 600 (fixed) |
- a number of low risk judgements, T – total, M – monotherapy, A – augmentation, OL – open label, DB – double blind, ITT – intent to treat, OC – observed cases, CLZ – clozapine, FLU – fluphenazine, PIM – pimozide, ARI – aripiprazole, PAL – paliperidone, RIS – risperidone, SER – sertindole, SUL – sulpiride, ZIP – ziprasidone, HAL – haloperidol, QTP – quetiapine, OLZ – olanzapine, NR – not reported
Antipsychotic augmentation was superior to monotherapy regarding total symptom reduction (16 studies, N=694, SMD =–0.53, 95% CI: −0.87 to −0.19, p=0.002), but only in open-label (n=6, N=285, SMD=–0.81, 95% CI: −1.18 to −0.43, p<0.001) and low-quality (n=7, N=316, SMD=–0.83, 95% CI: −1.16 to −0.50, p<0.001) studies, not in double-blind (n=10, N=409, SMD=–0.37, 95% CI: −0.83 to 0.10, p=0.120) and high-quality (n=9, N=378, SMD=–0.30, 95% CI: −0.78 to 0.19, p=0.226) ones (Figures 2 and 3). The funnel plots and Egger's test did not indicate publication bias (p=0.320).
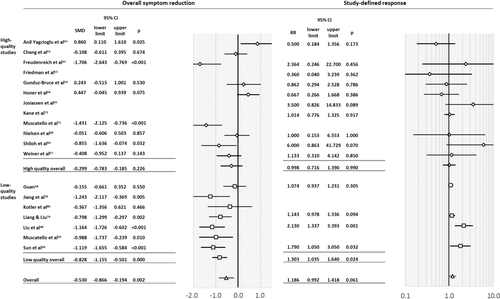
Forest plots of overall symptom reduction and study-defined response. SMD – standardized mean difference, RR – risk ratio
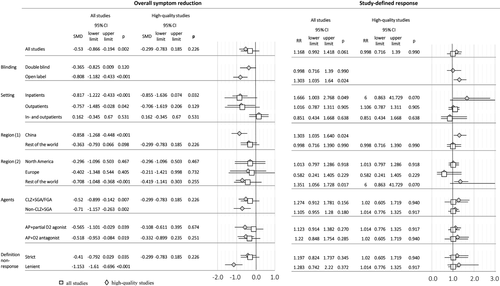
Primary outcomes, subgroup analyses and meta-regression in studies with efficacy as primary outcome. SMD – standardized mean difference, RR – risk ratio, CLZ – clozapine, SGA – second generation antipsychotic, FGA – first generation antipsychotic, AP – antipsychotic
In subgroup analyses, antipsychotic augmentation was superior in certain settings (only inpatients: n=6, N=316, SMD=–0.82, 95% CI: −1.22 to −0.43, p<0.001; only outpatients: n=5, N=247, SMD=–0.76, 95% CI: −1.49 to −0.03, p=0.042) and regions (China: n=5, N=269, SMD=−0.86, 95% CI: −1.27 to −0.45, p<0.001; non-North American/European countries: n=8, N=374, SMD=–0.71, 95% CI: −1.05 to −0.37, p<0.001). However, superiority in these subgroups was not apparent in high-quality studies (Figure 3).
Findings regarding symptom reduction were replicated in augmentation studies of clozapine with a second generation antipsychotic (SGA) or a first generation antipsychotic (FGA) (n=14, N=612, SMD=–0.52, 95% CI: −0.90 to −0.14, p=0.007), clozapine with a SGA (n=12, N=528, SMD=–0.52, 95% CI: −0.93 to −0.11, p=0.012), and non-clozapine SGA with a SGA (n=2, N=82, SMD=–0.71, 95% CI: −1.16 to −0.26, p=0.002); studies augmenting with a partial D2 agonist (n=4, N=214, SMD=–0.57, 95% CI: −1.10 to −0.03, p=0.039), and those augmenting with D2 antagonists (n=12, N=480, SMD=–0.52, 95% CI: −0.95 to −0.08, p=0.019). Results persisted independent of the non-response definition (strict, ≥2 adequate trial failures vs. lenient, ≥1 adequate trial failure): respectively, n=13, N=542, SMD=–0.41, 95% CI: −0.79 to 0.03, p=0.035; and n=2, N=86, SMD=–1.15, 95% CI: −1.61 to −0.70, p<0.001). However, again, differences were non-significant when analyzing only high-quality studies (Figure 3).
In meta-regression analyses, a higher augmentation-to-monotherapy ratio of chlorpromazine equivalent dose (p=0.019) and higher baseline PANSS/converted BPRS scores (p=0.011) were associated with less symptom improvement, while studies with high risk of bias near-significantly moderated greater improvement with antipsychotic augmentation (p=0.050). The influence of the PANSS/converted BPRS scores was replicated in high-quality studies (p=0.033), whereas the other factors were non-significant.
Response, as defined by ≥20% PANSS/BPRS reduction (n=10), ≥25% PANSS reduction (n=3), and ≥20% PANSS reduction or CGI-I of 1 or 2 (n=1), did not differ between antipsychotic augmentation and monotherapy (n=14, N=938, RR=1.19, 95% CI: 0.99 to 1.42, p=0.061). In subgroup analyses, antipsychotic augmentation was superior in open-label/low-quality studies (n=4, N=245, RR=1.30, 95% CI: 1.04 to 1.64, p=0.024), but not in double-blind/high-quality ones (n=10, N=693, RR=1.00, 95% CI: 0.72 to 1.39, p=0.990) (Figure 2). The funnel plots and Egger's test did not indicate publication bias (p=0.508).
Antipsychotic augmentation was again superior in inpatient only studies (n=4, N=207, RR=1.67, 95% CI: 1.00 to 2.77, p=0.049), Chinese studies (n=4, N=245, RR=1.30, 95% CI: 1.04 to 1.64, p=0.024) and non-North American/European studies (n=5, N=273, RR=1.35, 95% CI: 1.06 to 1.73, p=0.017) (Figure 3). In these subgroups, the number of high-quality studies was ≤1, not allowing for separate analyses. There was no advantage of any specific antipsychotic combination, or depending on non-response definition. No significant moderator of treatment response emerged. No between-group differences were observed regarding inefficacy-related discontinuation (n=6, N=596, RR=1.08, 95% CI: 0.44 to 2.67, p=0.870), global clinical impression (n=8, N=403, SMD=–0.01, 95% CI: −0.32 to 0.30, p=0.947), positive symptoms (n=14, N=604, SMD=–0.25, 95% CI: −0.66 to 0.16, p=0.230), general symptoms (n=4, N=144, SMD=–0.73, 95% CI: −1.91 to 0.46, p=0.229), and functioning (n=2, N=80, SMD=–0.36, 95% CI: −1.19 to 0.47, p=0.389).
Efficacy and tolerability of antipsychotic monotherapy vs. augmentation (complete data set)
The complete data set (efficacy-focused plus adverse effect-focused studies) included 31 trials (N=2,073) (see Table 1). The mean PANSS/converted BPRS score was higher in efficacy-focused studies (total sample = 79.7 ± 10.8, clozapine studies = 79.3 ± 9.6, non-clozapine studies = 81.7 ± 15.9) than in adverse-effect focused ones (total sample = 67.4 ± 9.2, clozapine studies = 71.5, non-clozapine studies = 66.6 ± 9.9).
All-cause discontinuation (n=22, N=1,482, RR=1.13, 95% CI: 0.90 to 1.42, p=0.284), and intolerability-related discontinuation (n=11, N=949, RR=0.87, 95% CI: 0.50 to 1.50, p=0.611) did not differ between antipsychotic augmentation and monotherapy.
Negative symptoms improved with antipsychotic augmentation (n=18, N=931, SMD=–0.38, 95% CI: −0.63 to −0.13, p=0.003), but in subgroup analyses this effect was only significant in studies augmenting D2 antagonists with a partial D2 agonist (n=8, N=532, SMD=–0.41, 95% CI: −0.79 to −0.03, p=0.036), not when combining two D2 antagonists (n=10, N=399, SMD=–0.36, 95% CI: −0.72 to 0.01, p=0.055). These findings were replicated in high-quality studies (4 trials augmenting D2 antagonists with a partial D2 agonist, N=355, SMD=–0.28, 95% CI: −0.55 to −0.009, p=0.043). In exploratory subgroup and meta-regression analyses, no relevant moderator of negative symptom improvement with a partial D2 agonist emerged.
Antipsychotic augmentation and monotherapy did not differ regarding depressive symptoms (n=10, N=351, SMD=−0.69, 95% CI: −1.42 to 0.05, p=0.066).
Few differences in adverse effects emerged. D2 antagonist augmentation was associated with less insomnia (n=3, N=169, RR=0.26, 95% CI: 0.08 to 0.86, p=0.028), but more prolactin elevation (n=2, each representing augmentation with risperidone, N=74, SMD=2.20, 95% CI: 0.43 to 3.96, p=0.015), while aripiprazole augmentation of D2 antagonists was associated with reduced prolactin levels (n=9, N=450, SMD=–1.60, 95% CI: −2.19 to −1.01, p<0.001) and body weight (n=6, N=260, WMD=–0.93, 95% CI= −1.77 to −0.09, p=0.030).
DISCUSSION
While some prior meta-analyses have examined the efficacy of combination or “polypharmacy” strategies in schizophrenia20, 21, 31, 32, this is the first meta-analysis of randomized controlled trials focusing exclusively on augmentation strategies (i.e., the addition of a second antipsychotic after non-response to the first) versus continued treatment with one antipsychotic (with addition of placebo in the blinded studies), irrespective of the baseline antipsychotic.
Although our prior meta-analysis, published in 200920, included 19 studies and 1,229 patients, merely 6 studies with only 197 patients were “augmentation” studies, which are the ones that are truly relevant, as they clinically reflect the management of refractory/non-responsive patients. In the current study, we increased the meta-analyzed data from 6 to 31 studies and 197 to 2,073 patients. This greater number of studies allowed for an evaluation of various symptom domains beyond study-defined response, plus the assessment of adverse effects and subgroup and meta-regression analyses, including examination of the effect of open versus blinded trials.
In contrast to that prior meta-analysis20, in which response rates had been significantly greater in the antipsychotic polypharmacy group that mixed co-initiation and augmentation trials (number-needed-to-treat = 7), the current meta-analysis did not provide any evidence for enhanced efficacy of antipsychotic augmentation in high-quality, blinded studies for either antipsychotic response or symptom reduction. This finding suggests that expectation and salience biases, also present in clinical care, may underlie observed improvements and decision making when augmenting one antipsychotic with a second one.
Although in efficacy-focused studies total symptoms decreased significantly more in the augmentation group, this effect was driven by open-label studies and those using OC analyses. Notably, the non-significance regarding total symptom reduction in high-quality studies was not driven by fewer studies and widening of the confidence intervals. Rather, more high-quality than low-quality studies were included (nine vs. seven), and the confidence intervals remained almost identical, whereas the between-group effect size was much smaller in high-quality studies. Furthermore, in efficacy-focused studies, no difference between antipsychotic augmentation and monotherapy was found regarding response rate, but, again, in the subgroup of low-quality studies superiority of the augmentation arm was observed.
Evidence regarding symptom improvement and treatment response was lacking for augmentation of either clozapine or non-clozapine antipsychotics (with the latter studies being surprisingly uncommon). The previously identified benefit regarding augmentation of clozapine with a second antipsychotic31 could not be confirmed in blinded trials and those using ITT data. Prior meta-analyses that focused on antipsychotic co-treatment strategies involving clozapine21, 31, 32 had much fewer studies (augmentation studies: 5-14 vs. 20 in our meta-analysis; patients: 187-734 vs. 1,112 in our meta-analysis) and in one instance21 combined antipsychotic augmentation and co-initiation trials.
Despite the overall unfavorable results in high-quality studies for total, positive and general symptoms, global clinical impression, depression, treatment response and study discontinuation, augmentation of D2 antagonists with a partial D2 agonist was associated with significantly reduced negative symptoms, a finding that was confirmed in high-quality studies. Since the treatment of negative symptoms remains a big challenge in schizophrenia81, 82, these findings, based on eight studies (including four high-quality trials) clearly require further investigation, especially comparing augmentation with a partial D2 agonist versus switching to a partial D2 agonist. Since two new partial D2 agonists, brexpiprazole83 and cariprazine84, were recently approved for schizophrenia, it will be of interest to see if the potential benefits for negative symptoms extend to these other agents.
Different from the generally held notion that antipsychotic polypharmacy carries a greater risk of adverse effects22, this was only found regarding greater prolactin elevation when combining two D2 antagonists. Rather, combination of two D2 antagonists was associated with less insomnia, whereas augmentation with the partial D2 agonist aripiprazole resulted in lower prolactin levels and reduced body weight.
The lack of superior efficacy of antipsychotic augmentation in high-quality studies is in contrast to common clinical belief and practice, where antipsychotic co-treatment is often implemented for non-response to antipsychotic monotherapy12. However, the clinical evaluation of improvement with antipsychotic augmentation mirrors the findings from open-label studies, suggesting that in clinical settings the expectations of patients and clinicians may translate into perceived favorable outcomes. Large pragmatic randomized controlled trials of antipsychotic augmentation strategies conducted in generalizable settings and samples are needed to confirm the lack of efficacy advantages of antipsychotic augmentation, as we cannot fully rule out a selection bias of less severely ill patients agreeing to participate in blinded trials. However, this possibility seems relatively low, since mean baseline PANSS/converted BPRS total symptom severity was around 80 in these pretreated individuals, and PANSS/converted BPRS total symptom severity did not significantly moderate the results.
In meta-regression analyses, less symptom improvement was associated with a higher chlorpromazine equivalent dose in the augmentation versus monotherapy arms, and a greater baseline symptom severity, with the latter relationship remaining significant in high-quality studies. These findings suggest that antipsychotic augmentation is even less effective in the sicker patients and those requiring higher antipsychotic doses. Alternatively, the higher total antipsychotic doses in the combination groups may be a reflection of lack of initial improvement, prompting dose escalation. This relationship might also be due to greater dopamine blockade resulting in less improvement due to secondary negative symptoms or other unfavorable effects.
Although the moderation of less efficacy by higher baseline symptom severity contradicts a recent meta-analysis85, those results pertained to acutely exacerbated patients in whom greater baseline symptom severity created more room for improvement. Conversely, in our meta-analysis, a substantial number of patients had likely benefitted to some degree from antipsychotic monotherapy in the past, so that higher residual symptom severity is probably a marker of less treatment responsiveness.
The results of this study need to be interpreted within some limitations. These include: a) the still relatively small number of double-blind studies comparing antipsychotic augmentation with monotherapy in schizophrenia, particularly for augmentation of non-clozapine antipsychotics and for specific antipsychotic co-treatment pairs; b) the heterogeneous study origin, design, definition and degree of insufficient response to monotherapy, measurements and outcomes; c) the limited number of studies reporting negative and depressive symptoms as well as adverse effects, which were often not comprehensively assessed or reported; d) the potential influence of cultural or ethnic differences (although we addressed regional effects in pre-planned subgroup analyses, yet the effect of studies from China overlapped almost 100% with an efficacy signal only detected in open-label/low-quality studies); e) the exclusion of studies focusing on adverse effects from the efficacy analyses to reduce heterogeneity (supported by a >10-point lower mean PANSS/converted BPRS symptom severity in side effect-focused versus efficacy-focused studies); f) the combination of studies augmenting clozapine and non-clozapine antipsychotics, potentially representing different patient subgroups (although mean baseline PANSS/converted BPRS total scores were comparable, and subgroup analyses replicated results in both clozapine and non-clozapine studies); g) the restriction of the distinction between high-quality and low-quality to blinding and data analysis (although risk-of-bias tool clearly confirmed quality differences without having a significant influence in the meta-regression analysis, suggesting that major influencing biases were captured through blinding/ITT categorization); h) the lack of adjustment for multiple comparisons (yet, adjustment for multiple comparisons would only have increased the level of non-significance of differences between groups); i) the potential effect of non-adherence, and j) the lack of detailed data to determine whether the effect of partial agonist augmentation was mainly on primary or secondary negative symptoms.
In summary, data from this study suggest that high-quality evidence is lacking for antipsychotic augmentation in patients with schizophrenia, which applies also to patients with inadequate response to clozapine. The clinical relevance of the negative symptom advantage of adjunctive partial D2 agonist treatment needs to be further assessed. Additionally, effects of augmentation with a partial D2 agonist versus a switch to a partial D2 agonist on negative symptoms need to be compared before these results can be considered for clinical care. Antipsychotic augmentation treatment should also be compared with high-dose antipsychotic monotherapy or augmentation with psychosocial interventions. Furthermore, non-clozapine antipsychotic augmentation strategies should be compared against a switch to clozapine or to improving adherence, including monotherapy with a long-acting injectable antipsychotic, which are each more rational choices for addressing antipsychotic non-response. Another gap is the systematic assessment of adverse effects of antipsychotic augmentation, extending also to cognition, functioning and subjective well-being. Finally, more high-quality trials are needed that examine antipsychotic augmentation in non-clozapine-treated patients across relevant outcome domains, including patients with carefully defined insufficient response to antipsychotic monotherapy.
ACKNOWLEDGEMENT
This study was supported in part by the Zucker Hillside Hospital National Institute of Mental Health (NIMH) Advanced Center for Intervention and Services Research for the Study of Schizophrenia (grant no. P30MH090590).




