Open label, interventional, single arm multicentric clinical study to analyze the potency of VELNEZ nasal pack, post-nasal surgery
Abstract
Background
Nasal packs are central to nasal surgeries. Primarily, these packs function by controlling bleeding, modulating pain and reducing adhesions postsurgery. However, the major setback of the currently used conventional nasal packs is the unbearable pain the patient undergoes upon removal of these packs. To overcome this shortcoming a variety of biodegradable packs have emerged. This study was aimed at evaluating the safety, efficacy and tolerability of VELNEZ nasal packs. VELNEZ, a patented Datt Mediproducts Pvt. Ltd. nasal pack, is one of its kind biodegradable composite that fragments within a few days of application.
Methods
Eighty patients were included in an open label, interventional, single arm clinical study using clinical endpoints to investigate the safety and efficacy of nasal pack VELNEZ. The patients were questioned using a visual analog scale from discharge day to 28th postoperative day (9 follow-up visits) at regular intervals. The standardized questionnaires for hemorrhage control, relief from postoperative pain, moderate obstruction, and pain were used.
Results
A total of 76 patients were enrolled in the study and 74 patients completed the study. VELNEZ nasal pack played a significant role in controlling hemorrhage and reducing postoperative pain. The average hemorrhage control time was 7.49 ± 3.90 min with only 34.24% of population complaining of pain on the sixth day of surgery (follow-up 4). Forteen days postsurgery only 10.95% of subject population complained of postoperative pain. This biodegradable composite has an average fragmentation time of 4.7 days in the nasal cavity. In addition, this study did not observe any postoperative adverse events or serious adverse events.
Conclusion
VELNEZ, a fragmentable nasal pack, is comfortable, safe, and effective against postsurgery bleeding and pain.
Key points
-
VELNEZ is a safe and tolerable nasal pack.
-
Hemostasis is achieved within 20 min.
-
Postoperative pain alleviation.
-
Minimal Subject discomfort.
INTRODUCTION
Septoplasty or nasal surgery is a common procedure used by otorhinolaryngologists to correct nasal septum anomalies. The primary function of this procedure is to reduce nasal obstruction and improve breathing.1 Septoplasty can be done alone or in combination with procedures like functional endoscopic sinus surgery (FESS), rhinoplasty or turbinoplasty. Nasal packs, an integral part of this procedure, are commonly used in controlling pain, bleeding and adhesions triggered by septoplasty.2 The use of nasal packing post-surgery dates back to 1847.1 Since then, a wide variety of material have been used as a nasal packing material.2, 3
Common postoperative complications of nasal surgery are bleeding and infection. Over the first few weeks, the patients can develop crusting and adhesions between the middle turbinate and the lateral nasal wall. The latter can lead to further blockage and a recurrence of the symptoms treated for. There is no consensus treatment for the prevention of these complications in the literature. However, among the recommended methods, nasal packing has been suggested to achieve hemostasis and prevent middle meatal adhesions when placed as a spacer lateral to the middle turbinate.4, 5
Currently in India, patients undergoing nasal surgery are conventionally packed with petroleum jelly impregnated gauze for achieving postoperative hemostasis. However, postsurgery nasal packing becomes a difficult task, particularly if the patient has recovered from anesthesia and is conscious. Nasal pack removal is one of the most painful procedure for the patient.4 In addition, mucosal injury, discomfort, pressure, and adhesions are all the risk-associated with the packing and removal of nasal packs. This problem can be overcome by the use of biodegradable and fragmentable nasal packs.
VELNEZ nasal pack manufactured and marketed by Datt Mediproducts Pvt. Ltd. is a biodegradable composite that fragments within a few days of application. The pain associated with the traditional nasal pack removal is completely eliminated with VELNEZ, since removal procedure becomes irrelevant. VELNEZ fragments completely and separates itself from the compromised mucosal surfaces. Furthermore, VELNEZ reduces fibrosis and promotes healing and clotting.
The current study was done to evaluate the safety and tolerability of VELNEZ as a nasal pack in subjects undergoing planned nasal surgery.
MATERIALS AND METHODS
This study was conducted at Mahatma Gandhi Memorial Hospital, Warangal, India and KLE's Dr. Prabhakar Kore Hospital & Medical Research Centre, Belagavi, Karnataka, India, following approval by Internal institutional Ethics Committee (CTRI/2018/12/016535). It was performed in accordance with ICH E6 (R2) (Step 5) Guideline for good Clinical Practice, New Drugs and Clinical Trials Rules 2019 (G.S.R.227(E)), ICMR guidelines (2017).
Trial design
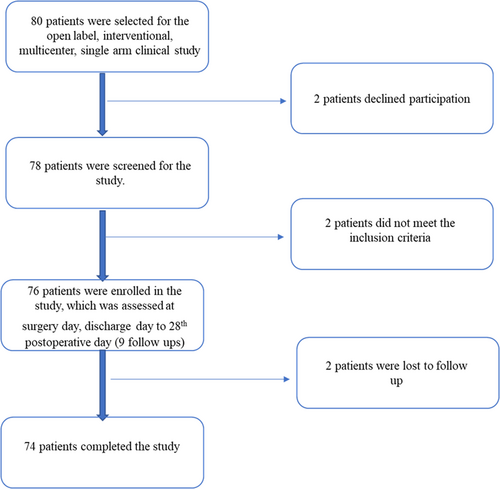
This was an open label, interventional, multicenter, single arm clinical study (Figure 1).
Participants and selection criteria
For the study 80 subjects were enrolled at two independent sites. All potential subjects of both genders of 18–60 years of age, Asian only, and patients of all socioeconomic status who met the study related inclusion and exclusion criteria were included (Table 1).
| Inclusion criteria |
| 1. Elegible to receive VELNEZ after planned nasal surgery. |
| 2. Age between 18 and 60 years. |
| 3. Can provide informed consent form in writing. |
| 4. Allowing data to be collected at follow-ups. |
| Exclusion criteria |
| 1. Subject who were unable to be treated with VELNEZ postsurgery. |
| 2. Subject who cannot provide written informed consent for data collection. |
| 3. Subject unwilling or unable to comply with the postoperative visits necessary for data collection. |
| 4. Subject with an active infection at the planned surgery site. |
| 5. Subject with a history of asthma. |
| 6. Subject who are on aspirin or antiplatelet drugs therapy. |
| 7. Hypertensive subjects. |
| 8. Subject with a history of allergic with ingredients of the device. |
| 9. Bleeding disorder. |
| 10. Medical condition unsuitable for inclusion. |
| 11. Serious rhinosinusitis. |
| 12. History of postoperative recurrent rhinosinusitis and bleeding diathesis. |
Study objective and design
The primary objective of this study was to assess the safety and tolerability of VELNEZ as a nasal pack postseptoplasty (with or without FESS). This was done by evaluating the hemorrhage control time and the extent of postoperative pain management. Secondary objectives of the present study included the assessment of adverse events and severe adverse events associated with VELNEZ, success of VELNEZ as a nasal pack and the Global assessment for VELNEZ effectiveness by Investigators based on surgeon's validated questionnaire.
This 28-day study consisted of nine follow-ups, on the following days—discharge, 2nd, 4th, 6th, 8th, 10th, 14th, 21st, and 28th day postnasal surgery. Telephonic follow-up was done with the enrolled subject by the investigator or authorized site personnel on each follow-up visits in case subject did not turn up for the visit.
Study material
The study material, VELNEZ, was a composite sterile nasal dressing that fragmented within a few days of application. It was manufactured in a cleanroom of class 10,000 under GMP certified manufacturing unit for standardized production and packing which maintained the quality and stability of VELNEZ.
Outcome
All the patients enrolled in the study were evaluated on an intent treat basis. The collected data were analyzed using Microsoft Excel. The primary endpoint of this study was to evaluate the proportion of population having failed to control hemorrhage and relief from postoperative pain. Secondary endpoints were analyzed on the proportion of population having moderate pain, moderate obstruction, and infection at the site of application and absence of adhesions.
Nasal obstruction, relief from moderate pain, nasal discharge, difficulty in swallowing, and sleep disturbance were analyzed with the aid of a visual analog scale (VAS) for nasal symptoms. VAS value ranges from 0 to 10, where “0” means no symptoms present, “5” stands for moderate symptoms; “10” means severe symptoms.
RESULTS
Eighty subjects were screened for the test, 78 subjects were included for the test and 74 subjects completed the study. Of the 78 subjects that were included, 52 were males and 26 were females, with ages ranging from 18 to 60 years (median 26.56) (Table 2).
| Patients enrolled | Age (years) | Gender, n (%) | Height (cm) | Weight (kg) | Smoker, n (%) | Race, n (%) | ||
|---|---|---|---|---|---|---|---|---|
| Male | Female | Yes | No | Asian | ||||
| 78 | 26.61 ± 8.30a | 52 (66.66) | 26 (33.34) | 162.91 ± 7.72a | 58.82 ± 8.96a | 0 (0) | 78 (100) | 78 (100) |
| 18–55b | 149–182b | 35–90b | ||||||
- a Mean ± SD.
- b Min–max.
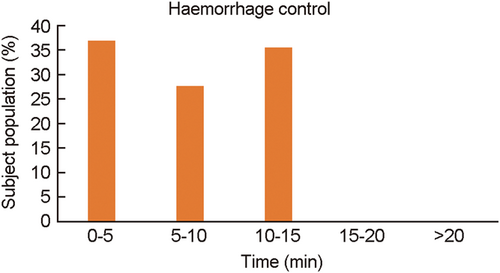
Hemorrhage control, one of the primary outcome measures, was evaluated within 20 min postsurgery. A total of 36.84% of subjects had hemorrhage control within 5 min of surgery, whereas 27.63% of subjects had hemorrhage control within 5–10 min. As shown in Table 3, 35.52% of subjects achieved hemostasis within 10–15 min. The average hemorrhage control time was 7.49 ± 3.90 min (Figure 2).
| Time | Subject population (%) |
|---|---|
| 0–5 min | 36.84 |
| 5–10 min | 27.63 |
| 10–15 min | 35.52 |
| 15–20 min | 0 |
| >20 min | 0 |
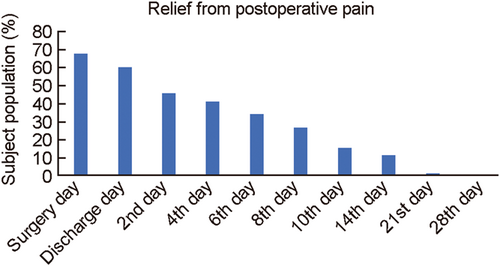
Postoperative pain alleviation was evaluated through pain VAS scale from surgery day to follow-up 9 (Day 28th). A total of 67.10% of subjects reported pain on surgery day which was reduced to 34.24% follow-up 4 and further only 1.35% population had shown pain till follow-up 8 (Table 4). However, by follow-up 9 no patient complained of any pain (Figure 3).
| Follow-up visit no. | Follow-up days | Subject population (%) | |||
|---|---|---|---|---|---|
| Postoperative pain | Moderate pain | Moderate obstruction | Infection | ||
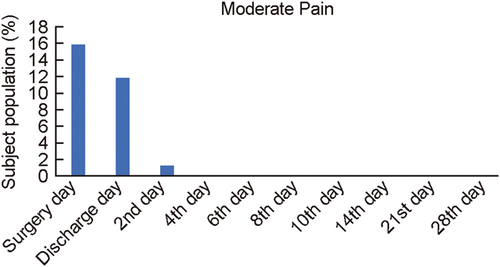
| – | Surgery day | 67.10 | 15.78 | 10.52 (Baseline 42.10) | 0 (Baseline 2.63) |
| 1 | Discharge day | 60.27 | 11.84 | 6.57 | 0 |
| 2 | 2nd day | 45.20 | 1.31 | 1.31 | 0 |
| 3 | 4th day | 41.09 | 0 | 0 | 0 |
| 4 | 6th day | 34.24 | 0 | 0 | 0 |
| 5 | 8th day | 27.02 | 0 | 0 | 0 |
| 6 | 10th day | 15.06 | 0 | 0 | 0 |
| 7 | 14th day | 10.95 | 0 | 0 | 0 |
| 8 | 21st day | 1.35 | 0 | 0 | 0 |
| 9 | 28th day | 0 | 0 | 0 | 0 |
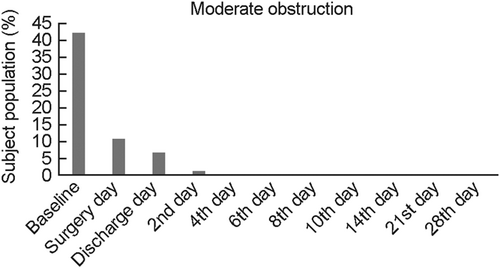
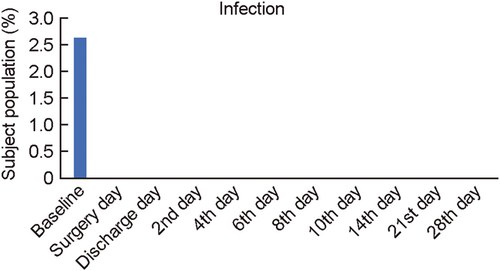
The secondary outcome measures, relief from moderate pain and obstruction were also evaluated through Pain VAS Scale from surgery day to follow-up 9 (Day 28). Moderate pain and obstruction were defined as 5 on the scale (VAS) of 1–10. A total of 15.78% subjects reported moderate pain on surgery day. None of the subjects reported moderate pain from follow-up 3 onwards (Table 4; Figure 4). A total of 42% subject population reported moderate obstruction on the baseline, and 10% on surgery day. None of the subjects reported obstruction from follow-up 3 onwards (Table 4; Figure 5). Infection at the site of VELNEZ application was reported in 2.6% of subject proportion at baseline, with no subject reporting infection at the site on subsequent visit (Table 4; Figure 6).
Global assessment for VELNEZ effectiveness by Investigators based on surgeon's questionnaire was done and device performance was rated within the range of 1–3. The surgeons rated the assessment sheet on a scale 1–5, where 1 was easy and 5 was difficult. As shown in Table 5, handling, use and application of VELNEZ was easy. Furthermore, none of the subject reported any adverse event or severe adverse event on using VELNEZ.
| Grading | Ease of handling | Appropriateness of instruction for use | Ease of application | Conformance to tissue surface | Overall performance of device |
|---|---|---|---|---|---|
| 1—Easy | 44 | 47 | 42 | 43 | 40 |
| 2 | 22 | 28 | 18 | 28 | 26 |
| 3 | 09 | 01 | 14 | 05 | 10 |
| 4 | 01 | 0 | 02 | 0 | 0 |
| 5—Difficult | 0 | 0 | 0 | 0 | 0 |
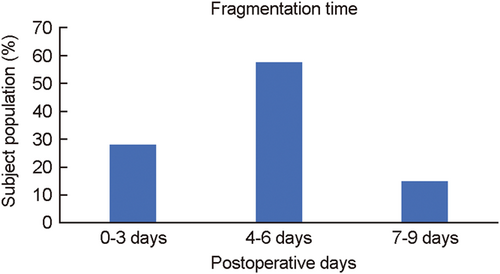
After surgery half of the VELNEZ nasal pack was inserted in each nasal cavity of the patient and fragmentation time was calculated. Its insertion was easy and dissolved with no remnant from surgery day to postoperative 9th day (Table 6; Figure 7). VELNEZ took an average time of 4.7 days to dissolve in the nasal cavity.
| Postoperative days | Subject population (%) |
|---|---|
| 0–3 days | 27.94 |
| 4–6 days | 57.35 |
| 7–9 days | 14.70 |
Subject comfort was evaluated for postoperative pain, pressure effect due to packing, nasal discharge, nasal obstruction, difficulty in swallowing, sleep disturbance, postnasal drip, and general satisfaction. None of the patient reported any problem related to these parameters from follow-up 9 onwards (Table 7).
| Day | Pain | Pressure effect due to Packing | Nasal discharge | Nasal obstruction | Difficulty in swallowing | Sleep disturbance | Postnasal drip | General satisfaction | |
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Baseline | 22.07 | – | 38.96 | 97.4 | 24.67 | 44.15 | – | 67.53 |
| Surgery day | Surgery day | 65.78 | 50 | 47.36 | 47.36 | 50 | 46.05 | 47.36 | 46.05 |
| Follow-up 1 | Discharge day | 60.27 | 46.57 | 45.2 | 45.2 | 43.83 | 45.2 | 45.2 | 45.2 |
| Follow-up 2 | 2nd day | 45.2 | 42.46 | 44.44 | 43.83 | 41.09 | 43.83 | 43.83 | 43.83 |
| Follow-up 3 | 4th day | 41.09 | 35.61 | 42.46 | 46.57 | 34.24 | 42.46 | 42.46 | 41.09 |
| Follow-up 4 | 6th day | 34.24 | 31.5 | 28.76 | 39.72 | 24.65 | 31.5 | 31.94 | 37.5 |
| Follow-up 5 | 8th day | 27.02 | 16.21 | 20.27 | 21.62 | 13.51 | 15.27 | 20.27 | 27.02 |
| Follow-up 6 | 10th day | 15.06 | 0 | 9.58 | 16.43 | 4.1 | 4.1 | 8.21 | 19.17 |
| Follow-up 7 | 14th day | 10.95 | 0 | 1.36 | 2.73 | 0 | 2.73 | 2.73 | 12.32 |
| Follow-up 8 | 21st day | 1.35 | 0 | 0 | 0 | 0 | 0 | 0 | 5.4 |
| Follow-up 9 | 28th day | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5.55 |
DISCUSSION
Nasal packs have marked its place in nasal surgeries since 1847.1 However, it was only in 1951 that it was first reported in the otorhinolaryngologic literature and since then it has been evaluated for the improvement of postoperative wound healing and reducing mucosal bleeding linked to nasal surgery.6 A variety of nasal packing material have evolved over time. But, the type of nasal pack used plays a significant role in the overall wound healing process.
In the present Indian scenario, patients undergoing FESS are conventionally packed with petroleum jelly impregnated gauze for achieving postoperative hemostasis. It is tedious to pack postoperatively if the patient has recovered from anesthesia and is conscious. Furthermore, the patients feel pain, pressure and discomfort while packing and on removing the pack on the first postoperative day. To overcome the disadvantages linked to non-resorbable packs, a variety of biodegradable nasal packs have been developed.7-9 Absorbable packing material are more effective as postsurgery packs as it accelerates the overall mucosal healing process.8, 10 This study was aimed at analyzing the clinical efficacy of a biodegradable and fragmentable nasal pack in subjects undergoing planned nasal surgery.
VELNEZ nasal pack, a patented product, manufactured and marketed by Datt Mediproducts Pvt. Ltd. is a biodegradable composite that fragments within a few days of its application as a nasal dressing. At present, VELNEZ is one of its kind biodegradable nasal packs which can fragment on aspiration with saline. This biodegradable composite has an average fragmentation time of 4.7 days in the nasal cavity. Therefore, on using VELNEZ patients do not have to go through the bleeding and pain associated with the pack removal.
As postoperative bleeding and pain are the two major concerns during nasal surgery,7, 11 a suitable nasal pack should be able to control both these aspects. In this study, hemorrhage control time, was evaluated within 20 min postsurgery. As it was the first in-human study of its kind, this wide time window was chosen. A total of 36.84% population achieved hemostasis with in the first 5 min of surgery. Interestingly all subject population achieved hemostasis within 5–15 min of VELNEZ application (Table 3; Figure 2) with an average hemorrhage control time of 7.49 ± 3.90 min. This study showed that VELNEZ was efficient in attaining hemostasis in a short span of time.
In a nasal surgery patient experiences pain postoperation, during nasal packing and throughout the duration of its application and during the removal of the pack. This study assessed the postoperative and the moderate pain through a VAS on surgery day, discharge day and 28 days postoperation. A total of 67.10% of subjects complained of pain on surgery day which was reduced to 41.09% on 4th day (follow-up 3), and by 21st day only 1.35% patient complained of pain (Table 4; Figure 4). In a similar 3-week analysis of pain post-septoplasty, Tutar et al.12 reported there was a significant decline in the pain scores of third week when compared to first week. In addition, in the present study no patient complained of moderate pain after second day of surgery. Pain alleviation is an important factor deciding the efficacy of the nasal pack. The present study results highlighted that VELNEZ was successful in ameliorating both postoperative and moderate pain.
Subject comfort is another critical parameter to evaluate the clinical efficacy of the pack. In situ nasal packing by VELNEZ caused minimal discomfort in patients, with none of the patient complaining of any nasal obstruction from Day 4 onwards (Figures 4 and 5). Subjects did not report of any infection at the site of VELNEZ application during postsurgery visits (Figure 6). Parameters including pressure effect due to packing, nasal discharge, difficulty in swallowing, and sleep disturbance were also assessed during this study (Table 7). This came out as a safe nasal pack, as no subject reported any adverse or severe adverse events on its use In addition, VELNEZ effectiveness was rated within the range of 1–3 by investigators based on the surgeon's questionnaire (Table 5) due to its ease of handling, application, overall performance, and so forth. Conclusively, VELNEZ, can be considered as a safe, efficient and tolerable postoperative nasal pack which protects patients from all postoperative adverse events associated with nasal surgeries.
CONCLUSION
VELNEZ was shown to be safe, effective and tolerant as a nasal pack postseptoplasty. This novel fragmentable composite easily dissolved in the nasal cavity, thereby avoiding the painful nasal pack removal linked to conventional nasal packs. VELNEZ, as a nasal pack, exhibited favorable hemostatic property along with reduced postoperative and moderate pain. Subject comfort and the ease of handling this pack were the added advantages of this biodegradable composite. Most importantly, no adverse events or severe adverse events were reported in any patients, on using VELNEZ. To summarize, in this prospective interventional, single arm, open label study VELNEZ, a fragmentable composite, was considered suitable as a nasal pack.
AUTHOR CONTRIBUTION
Shama Bellad: Study design; acquisition of data; statistical review; manuscript preparation; and final review. Nagula Parusharam: Study design; acquisition of data; statistical review; manuscript preparation; and final review. Vineeta Dhyani: Study design; acquisition of data; statistical review; manuscript preparation; and final review. Pankaj Bablani: Study design; acquisition of data; statistical review; manuscript preparation; and final review. Siddharth Pandey: Study design; acquisition of data; statistical review; manuscript preparation; and final review.
ACKNOWLEDGMENTS
The project is funded by Datt Mediproducts Pvt. Ltd.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
This study was conducted at Mahatma Gandhi Memorial Hospital, Warangal, India and KLE's Dr. Prabhakar Kore Hospital & Medical Research Centre, Belagavi, Karnataka, India, following approval by Internal institutional Ethics Committee (CTRI/2018/12/016535). It was performed in accordance with ICH E6(R2) (Step 5) Guideline for good Clinical Practice, New Drugs and Clinical Trials Rules 2019 (G.S.R.227(E)), ICMR guidelines (2017).
Open Research
DATA AVAILABILITY STATEMENT
All the data has been originally developed by authors and raw data files is available with Datt Mediproducts Pvt. Ltd.




