Fluorescence imaging-guided pelvic lymph node localization and resection of bladder cancer after intracutaneous injection of indocyanine green into the lower limbs and perineum
Abstract
Background
Pelvic lymph node dissection (PLND) in radical cystectomy (RC) is of great significance, but the method and scope of PLND remain controversial. Based on the principle of indirect lymphadenography, we designed a method to localize the whole pelvic lymph nodes by intradermal injection of indocyanine green (ICG) through the lower limbs and perineum, and to evaluate the effectiveness of this method.
Methods
In a single center, 54 bladder cancer patients who underwent RC and PLND participated in a prospective clinical trial, which began on February 28, 2022 and ended on December 30, 2022. ICG solution was injected subcutaneously at the medial malleolus of both lower extremities and at both sides of the midline of the perineum. The fluorescent laparoscopy was used to trace, locate, and remove the targeted areas under the image fusion mode. The consistency of lymph node resection was determined by histopathological diagnosis. The impact of ICG guidance on the surgical time of PLND was compared with that of 11 bladder cancer patients who underwent RC and PLND without ICG injection, serving as the control group.
Results
Perineal lower limb combined injection can provide comprehensive visualization of pelvic lymph nodes. This technique reduces PLND surgical time and increases the accuracy of PLND.
Conclusion
Intracutaneous injection of ICG into the lower limbs and perineum can specifically mark pelvic lymph nodes. Intraoperative fluorescence imaging can accurately identify, locate, and resect lymph nodes in the pelvic region, reducing PLND surgical time and increasing the accuracy of PLND.
1 INTRODUCTION
Bladder cancer is one of the ten most common tumors in the world [1]. Radical cystectomy (RC) is the main choice for the treatment of myometrial invasive bladder cancer[2], but the prognosis of patients with RC alone is still poor. The 5-year survival rate is only 58%[3]. The main factor affecting the survival of patients is tumor metastasis. The lymph node metastasis rate of patients with myometrial invasive bladder cancer is as high as 25%[4]. Therefore, more and more evidence shows that simultaneous effective pelvic lymph node dissection (PLND) is of great significance for improving survival rate, accurately evaluating prognosis, and formulating treatment strategies[2, 5-7].
PLND refers to the surgical method of completely removing the lymph node tissue within a certain range of the pelvic cavity. The scope of PLND is mainly formulated by the three-level PLND template system proposed by Leisner et al.[8] in 2004, including four modes: limitation, standard, expansion, and super expansion. Although this surgical method can prolong the survival period of patients, it also has many shortcomings. In particular, extensive resection may cause damage to adjacent organs, large vessels, and nerves, as well as ineffective resection of nonlymphatic tissues, lymphatic leakage and lymph cyst swelling caused by blind closure of lymphatic vessels. Therefore, in the process of lymph node removal, accurate identification and precise removal of lymph nodes among fat, near blood vessels and nerves will significantly shorten the operation time, reduce surgical complications, and lower medical costs.
In recent years, the lymph node tracing technology of near-infrared fluorescence imaging has played an important role in the tracing and positioning of sentinel lymph nodes in breast cancer and penile cancer [9], but the role and significance of indocyanine green (ICG) adjacent to the iliac vessels for tracing sentinel lymph nodes in bladder cancer, prostate, and cervical cancer are still unclear[10-12]. Therefore, on the basis of the successful development of 4 K fluorescent laparoscope and according to the principle of lymphography, the project team has designed for the first time a pioneering research scheme for ICG intradermal injection through the lower limb medial malleolus and perineum, marking pelvic lymph nodes through the principle of lymphatic system reflux, ICG concentration effect, and real-time imaging of intraoperative visible light and fluorescence fusion to accurately locate and identify pelvic lymph nodes in bladder cancer. The aim of this study is to explore the effect and difference of different ICG injection routes to label and locate pelvic lymph nodes and the impact of ICG injection on PLND surgery.
2 PATIENTS AND METHODS
2.1 Study population
From February 28, 2022 to December 30, 2022, with the approval of the Medical Ethics Committee, 54 bladder cancer patients undergoing RC were included in the study [13], including 51 men and 3 women. The average age of the combined injection (CI) group was 65.77 ± 6.73 years. The clinical staging was confirmed by computed tomography (CT), magnetic resonance imaging (MRI), and pathology as T2 in 14 cases, T3 in 32 cases, and T4 in 8 cases; one case in the N2 phase, four cases in the Nx phase, five cases in the N1 phase and 44 cases in the N0 phase. All patients were divided into three groups: lower limb medial malleolar injection (LLMMI) group, perineal injection (PI) group, and CI group. See Table 1 for preoperative information of patients.
| Patient information | LLMMI group (n = 6) | PI group (n = 4) | CI group (n = 44) | Control (n = 11) | p-Value (CI group-control) | |
|---|---|---|---|---|---|---|
| Sex, number | 0.36 | |||||
| Male | 6 | 3 | 42 | 9 | ||
| Female | 0 | 1 | 2 | 2 | ||
| Age (years) | 67.83 ± 6.30 | 66.00 ± 4.69 | 65.77 ± 6.73 | 67.18 ± 5.06 | 0.52 | |
| pT stage, number | 0.83 | |||||
| pT2 | 2 | 0 | 12 | 3 | ||
| pT3 | 3 | 4 | 25 | 7 | ||
| pT4 | 1 | 0 | 7 | 1 | ||
| pN stage, number | 0.60 | |||||
| pNx | 1 | 0 | 3 | 0 | ||
| pN0 | 4 | 4 | 36 | 10 | ||
| pN1 | 1 | 0 | 4 | 1 | ||
| pN2 | 0 | 0 | 1 | 0 | ||
- Abbreviations: CI, combined injection; LLMMI, lower limb medial malleolus injection; PI, perineum injection.
To further validate the clinical advantages of ICG-guided PLND, we included 11 bladder cancer patients undergoing RC via 3D laparoscopy during the same period. Among them, there were nine males and two females, with an average age of 67.18 ± 5.06 years. The clinical staging showed T2 in three cases, T3 in seven cases and T4 in one case; N0 stage in 10 cases and N1 stage in one case. ICG was not used intraoperatively in this group, which served as the control group. Preoperative patient information is shown in Table 1.
2.2 Imaging system equipment
The 4 K fluorescent laparoscopic imaging system (Taiyuan Saienshi Technology Development Co. Ltd., China) can display visible light images, fluorescent images, and visible light and fluorescence fusion images in real time. It is equipped with an external (open) imaging lens and an internal imaging laparoscope lens, which can realize various imaging functions and modes of the preoperative external and intraoperative laparoscopes.
2.3 Surgical techniques
2.3.1 Preoparative preparation
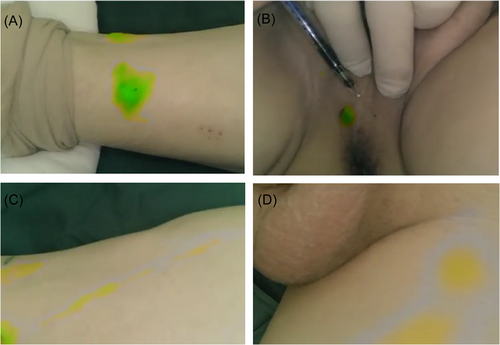
Dilute ICG (25 mg/piece) to 2.5 mg/mL with sterile water for injection about 1–1.5 h before operation. After general anesthesia, take a flat position. Use a 1 mL syringe to inject 0.3–0.5 mL (0.75 mg/piece) ICG solution (Figure 1A,B) intracutaneously at 2 cm above the medial malleolus on both sides and at both sides of the midline of the perineal skin. Fluorescent imaging of the lower limb and perineum uses an external (open) lens configured with a multi-functional fluorescent laparoscope to focus on the injection site, and collect fluorescent images of the lower leg, thigh, and groin respectively along the ICG development sequence (Figure 1C,D). For in vivo fluorescence imaging, the external lens of the same equipment is replaced with a laparoscopic lens.
2.3.2 Fluorescence image-guided pelvic lymph node extirpation
The Trendelenburg's position was adopted, the operating trocars were placed by conventional drilling, and the fluorescent laparoscope lens was placed. Before the peritoneum the iliac artery was cut, the intraperitoneal observation was conducted: (1) The development of the external iliac artery area (yes/no); (2) Imaging of internal iliac vessels (yes/no); (3) Obturator fossa development (yes/no); (4) Presacral area development (yes/no). Secondly, the peritoneum was cut along the external iliac vessels to expose the external iliac arteries and veins, internal iliac arteries, common iliac vessels and obturator nerves, and then the fluorescent labeled tissues were separated and removed, followed by further completion of PLND within the standard range. After the pelvic tissues were taken out of the body, in vitro fluorescence imaging was performed to preliminarily determine the nature of the fluorescent labeled tissues in combination with vision and touch, and the pathological examinations were numbered. The 3D high-definition laparoscopic group followed the same surgical steps for standard range lymph node dissection.
2.4 Statistical analysis
Statistical analysis was performed using SPSS 25.0. Normally distributed quantitative data were presented as mean ± standard deviation, and paired or unpaired t-tests were utilized for comparison according to the research design. Nonnormally distributed quantitative data were expressed as median (range), and comparisons were made using the Wilcoxon rank-sum test. Qualitative data were described using numbers and percentages, with comparisons conducted using the chi-square test, McNemar's test, or Fisher's exact test. All tests were two-tailed, and a p-value < 0.05 was considered statistically significant.
3 RESULTS
Except for one male patient who received RC and sigmoidectomy, the other patients received RC plus PLND and urinary diversion. RC was performed by standard laparoscopic operation. PLND was guided by fluorescence fusion images, including 48 cases of standard lymph node dissection and 6 cases of enlarged PLND. Urinary diversion included bilateral ureteral extradermal transplantation in 33 cases, Xing type ileal passage in 5 cases, and sigmoid colon orthotopic neobladder in 16 cases. Fluorescence guided PLND with a 4 K ultra-high definition fluorescence laparoscopy imaging system, ICG injection, and labelling methods included six cases in the medial malleolus injection group, four cases in the perineal injection group, and 44 cases in the perineum lower limb combined injection group.
The in vivo and in vitro fluorescence fusion image features of three different injection methods are as follows.
3.1 Perineal injection group
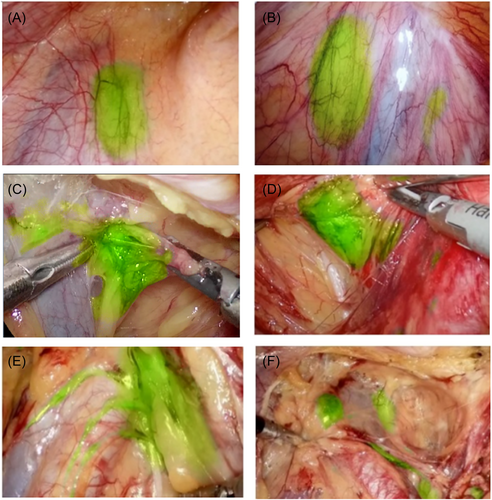
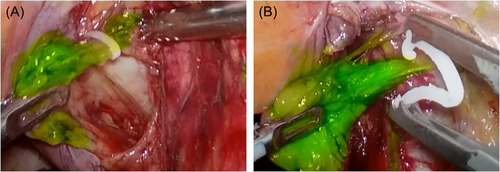
Within 1–4.5 h after ICG injection, only the obturator fossa area showed fluorescent images, while other areas did not. Before the peritoneum was cut, the fluorescence image of obturator fossa area was located at the far end of the fluctuating area of the iliac artery, and the image was green round or oval (Figure 2A). After the peritoneum was cut, the green image gradually increases, and the edge of the green tissue was clear (Figure 2C,D). It ran from the circumflex iliac vein to the obturator nerve along the external iliac vein. When it was close to the white obturator nerve, the fluorescence of tissue disappeared. At the end of the circumflex iliac vein, dense lymphatic vessels converged to the obturator lymph node (Figure 3A).
3.2 Inner malleolus injection group
The ICG injection point immediately showed fluorescent images, and gradually moved to the proximal end of the limb, passing through the inner leg, popliteal fossa, and the inner thigh to reach the inguinal lymph nodes. The time to reach the inguinal lymph node was 25–36 min (Figure 1D). Fluorescent images of lymph nodes beside the external iliac artery and vein were first seen in the abdominal cavity (Figure 2E), and no fluorescence was seen in obturator fossa of all cases in this group, which may be related to the lymphatic reflux of lower limbs first reaching the lymph nodes beside the iliac artery.
3.3 Perineal lower limb combined injection group
After ICG injection, the lymph nodes of obturator fossa and iliac vessels region were visualized. (Figure 2B). After the peritoneum was cut along the external iliac artery, the Cloquet lymph node next to the circumflex iliac vein was first seen. There was generally only one lymph node between the genitofemoral nerve and the external iliac artery. The lymph node continued to have lymphatic vessels and ran along the surface of the iliac artery to the proximal end (Figure 2E). The CI of the perineum and lower extremities can make the external iliac, obturator, and internal iliac lymph node be marked (Figure 2F).
After the removal of fluorescent labeled tissues, they should be immediately taken out for fluorescence imaging. The fluorescence fusion image can guide the pathologist to make a preliminary selection of lymph nodes, but it should be noted that formalin cannot be soaked immediately, and the imaging observation time should not exceed 30 min. Because of ICG spillover and cell apoptosis in vitro, it is difficult to distinguish lymph nodes and surrounding fat in vitro. Further confirmation of the accuracy of lymph node tissue clearance was achieved through histopathological examination.
Fifty-four cases of operation confirmed that the pelvic lymph nodes could be visualized 1 h after ICG injection. In 15 patients, the image did not weaken significantly within 4.5 h after injection, and sufficient time was given for surgical operation. In the process of lymph node separation and resection, the operator can accurately identify and finely separate through fluorescence, and a small amount of ICG spillover in the separation did not affect the precise operation (Figure 3A,B). In addition, due to the limited penetration depth of fluorescence, some obese patients need to separate fat to clearly develop lymph nodes and lymphatic vessels. None of the 54 patients in this group suffered from vascular or nerve injuries (Table 2).
| LLMMI group (n = 6) | PI group (n = 4) | CI group (n = 44) | |
|---|---|---|---|
| Labeled lymph node region (<30 min) | External iliac lymph nodes | Obturator lymph nodes | External iliac, internal iliac, and obturator lymph nodes |
| Number of labeled lymph nodes removed | 8.00 ± 1.41 | 4.50 ± 1.29 | 11.52 ± 2.48 |
| The accuracy of PLND (%) | 90.67 | 88.75 | 93.41 |
- Abbreviations: CI, combined injection; LLMMI, lower limb medial malleolus injection; PI, perineum injection; PLND, pelvic lymph node dissection.
We compared the data of PLND between the perineal lower limb CI group and the control group. In the ICG injection group, the average number of lymph nodes removed was 11.52 ± 2.48, with a mean dissection time of 44.07 ± 4.72 min, and an accuracy rate of 93.41%. In the control group, the average number of lymph nodes removed was 10.54 ± 2.38, with a mean dissection time of 50.27 ± 5.18 min, and an accuracy rate of 75.91%. Research on three different injection methods indicates the impact of various injection routes on pelvic lymph node visualization, demonstrating that intraoperative injection of ICG can achieve complete visualization of pelvic lymph nodes. Furthermore, compared to conventional lymphadenectomy procedures, the ICG injection group exhibited significantly reduced operation time and higher accuracy in PLND (Table 3).
| CI group (n = 44) | Control (n = 11) | p-Value | |
|---|---|---|---|
| Number of lymph nodes removed | 11.52 ± 2.48 | 10.54 ± 2.38 | 0.25 |
| The accuracy of PLND (%) | 93.41 | 75.91 | 0.01 |
| Lymph node dissection time (min) | 44.07 ± 4.72 | 50.27 ± 5.18 | 0.01 |
- Abbreviations: CI, combined injection; PLND, pelvic lymph node dissection.
4 DISCUSSION
RC + PLND is the standard treatment for bladder cancer, but the optimal range of PLND is still a hot issue in debate. How to locate or characterize metastatic lymph nodes during surgery has always been a clinical problem faced by urologists around the world[14, 15]. In recent decades, the concept, location, and resection of sentinel lymph nodes have received extensive attention. Sentinel lymph node biopsy has been proven to be feasible and safe for patients with breast cancer, penile cancer and melanoma, and is helpful for intraoperative lymph node dissection. However, the evidence on whether sentinel lymph node biopsy is valuable for RC + PLND is insufficient, and there are few clinical studies.
As early as 1996, Scelsi et al.[16] carried out a study on the intravesical injection of ICG to track bladder sentinel lymph nodes, and found that the pressure change in the bladder directly affected the lymphatic return of the bladder and the accurate labeling of ICG. Knapp et al.[17] believed that effective control of bladder pressure was the key parameter to obtain the best imaging effect. Schaafsma et al.[18] confirmed that keeping the bladder full of saline for 15 min can promote ICG: HSA to return lymph nodes. Since then, Nynke and Liedberg et al.[19] had found that the false-negative rate of this technology can reach 19%. Other studies also confirmed that intravesical injection of ICG for sentinel lymph node imaging and bladder tumor tracking cannot replace routine lymph node dissection. In addition, the location, method, dose, and injection depth of ICG injected into the bladder are also difficult to be accurate, and ICG leakage and fluorescence pollution are easy to occur. The stability of ICG mediated pelvic lymph node imaging technology will be affected by factors such as bladder wall thickness, bladder internal pressure, bladder filling degree, and so on[20]. It is generally believed that the intraoperative lymph node real-time location and recognition technology based on ICG labeling and near-infrared fluorescence imaging had certain clinical application value, but the false negative rate was high and the operation was not standardized, which needed further improvement.
In the early stage of this study, the lymphatic drainage system of normal lower limbs, perineum, pelvis, bladder[21], prostate[22], and uterus[23, 24] were analyzed. It was believed that normal lymphatic drainage of pelvic organs and lymph nodes of tumor metastasis were both inherent lymph nodes of normal pelvic anatomy, and the heterogeneity of organs or tumor sites led to differences in lymph node metastasis sites. Lymphatic return and tumor metastasis of the lower limbs, perineum, and pelvic organs involves the same intra-iliac, extra-iliac, obturator, and presacral lymph nodes. Early iodine lymphography fully confirmed that tumor lymph node metastasis can be diagnosed by X-ray through the injection of a contrast agent into the lymphatic vessels of the lower limbs, and also directly proved the feasibility of the lower limb ICG injection to mark pelvic lymph nodes[25, 26].
Therefore, we combined lymphography and fluorescence imaging technology to explore and verify the way to locate and identify pelvic lymph nodes through the combination of medial malleolus and perineum injection of ICG. The clinical practice of 54 cases of RC + PLND patients with intraoperative fluorescent image-guided lymph node tracing and recognition had proved that this technology can completely and clearly display pelvic lymph nodes, and had far-reaching significance in standardizing all surgical methods requiring PLND.
In this group of four patients with simple perineal injection, only obturator lymph nodes were found on intraoperative fluorescence imaging. At the same time, lymphatic vessels returned from obturator lymph node to the proximal end of external iliac artery. In six patients with simple medial malleolus injection, ICG could be observed flowing back to the proximal end, reaching the superficial inguinal lymph node through the inner leg, popliteal fossa, and the inner thigh, and then entering the deep inguinal lymph node, extra-iliac lymph node, and common iliac lymph node, without obturator lymph node development. However, it was found that after the lymph returned from the lower limb entered the pelvic cavity, it first entered the Cloquet lymph node near the circumflex iliac vein. After the Cloquet lymph node, there were branches that entered the iliac paravascular lymph node and obturator lymph node respectively. There was lymphatic communication between the obturator lymph node and the extrailiac paravascular lymph node. Many previous studies had believed that the first station of metastasis of bladder cancer, prostate cancer, and cervical cancer was the obturator lymph node, while the obturator, internal iliac, and external iliac lymph nodes had an intersecting lymphatic network, and external iliac lymph nodes may be the second station of metastasis. Therefore, the use of fluorescence imaging technology for in-depth study of lymph node metastasis has far-reaching significance.
Within 1–4.5 h after the CI of ICG into the perineum and lower extremities, bilateral Cloquet lymph nodes, internal iliac, external iliac, obturator, iliac, and presacral lymph nodes were effectively labeled. Fluorescent labeling and fluorescence images of lymph nodes were stable, and no fluorescence contamination or ineffective diffusion were found. We believe that this stable fluorescent labeling is related to the stability and continuous return of lymph after ICG is injected into the surrounding tissues. Due to the characteristics of ICG's molecular structure and molecular weight, ICG only flows in the lymphatic vessels and does not spill into the blood vessels, and finally flows into the right lymphatic vessels and thoracic ducts to enter blood metabolism. This is also an important theoretical basis for ICG lymphofluorescence imaging to track lymph nodes and lymphatic vessels.
In this study, by comparing the data of PLND between the group receiving intraoperative injection of ICG and the control group, we found that intraoperative ICG guidance significantly shortened PLND operation time and markedly improved the accuracy of PLND. There was no significant difference in the number of lymph nodes removed between the two groups. Numerous studies have shown[27] that patients can achieve better oncological outcomes when more than 10 lymph nodes are removed. The American Joint Committee on Cancer recommends the removal of 12 lymph nodes during RC to stage regional lymph nodes[28]. Improving the accuracy of lymph node removal can help reduce the unnecessary removal of fibrofatty tissue during PLND, effectively reducing operation time. The accuracy rate of lymph node removal in the group receiving lower limb and perineal injection of ICG was 93.00%, possibly due to some lymphatic vessels being contained within pathologically confirmed fibrofatty tissue.
5 LIMITATION AND FURTHER STUDY
The shortcomings of this study are: (1) The specificity of ICG is not high. Although ICG can clearly show the situation of lymph nodes and lymphatic vessels, it cannot specifically show positive lymph nodes. The number of lymph nodes cleared is large and the scope is wide, resulting in a lower positive detection rate than nonfluorescent laparoscopy. Therefore, the research and development of fluorescent probes with different tumor specificity is a hot spot at home and abroad. (2) The follow-up time is short, and the long-term control effect of RC + PLND in this way on tumor metastasis needs further research.
6 CONCLUSIONS
ICG can be injected intradermally through lower limbs and perineum under 4 K fluorescent laparoscopy to realize the identification, localization, and resection of pelvic proper lymph nodes. This approach effectively enhances the accuracy of lymph node dissection and reduces the operation time of PLND. This technique has far-reaching significance in standardizing PLND and studying the lymphatic metastasis pathway of tumor.
AUTHOR CONTRIBUTIONS
Yangbing Wei and Xiaofeng Yang had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. The study concept was designed by Xiaofeng Yang. Surgical operations and acquisition of data were performed by Chen Liu, Haifeng Hao, and Libing Sun. Bo Wu and Fan Liu were responsible for analysis and interpretation of data. Hua Yang and Yangbing Wei drafted the manuscript. Critical revision of the manuscript for important intellectual content was made by Nianzeng Xing. Liang Wei and Qiang Jing were in charge of statistical analysis. Chao Liu and Xiaofeng Yang were responsible for obtaining funding. Xiaoming Cao was in charge of administrative, technical, and material support.
CONFLICT OF INTEREST STATEMENT
The authors declare that there is no conflict of interest except for Nianzeng Xing (who is Editor-in-Chief of UroPrecision). He was excluded from the peer-review process and all editorial decisions related to the acceptance and publication of this article. Peer review was handled independently by the other editors to minimize bias.
ETHICS STATEMENT
This study received ethical approval from First Hospital of Shanxi Medical University. Patient identifiers have been anonymized or removed to protect their identity and privacy.
Open Research
DATA AVAILABILITY STATEMENT
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.