Clinical features and long-term outcomes of patients with type 2 autoimmune pancreatitis
Abstract
Objectives
Type 2 is a rare form of autoimmune pancreatitis (AIP). Despite being considered a benign disease, only few studies with limited sample size and short follow-up have been published on type 2 AIP. The aim of this observational study was to evaluate long-term outcomes, such as the risk of relapse, pancreatic insufficiency and cancer in a large type 2 AIP cohort with long follow-up.
Methods
Patients with definitive or probable diagnosis of type 2 AIP by International Consensus Diagnostic Criteria (ICDC) present in our prospectively maintained database since 1995 at 31.12.2021 were identified. All patients were clinically evaluated during the year 2022. Clinical, radiological, serological, and pathological data were evaluated.
Results
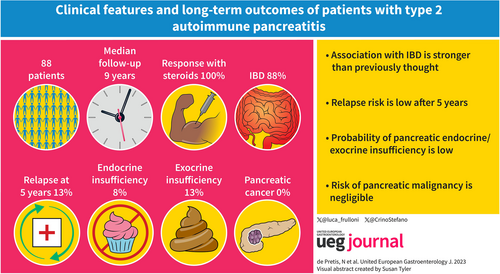
Eighty-eight out of 420 patients present in the database (21%) were diagnosed with type 2 AIP (mean age 33.5 ± 13.5 years). According to the ICDC, 21 patients (23.8%) had a definitive and 67 (76.2%) a probable diagnosis of type 2 AIP. The mean follow-up was 9.2 ± 7.1 years (range 1–27 years). No differences were observed when comparing patients with definitive and probable type 2 AIP diagnosis. Concomitant IBD was reported in 77 patients (87.5%). The probability of disease relapse was lower in patients treated with steroids versus surgery (at 5 years 13% vs. 33%; p = 0.038) but this difference was not statistically significant at multivariable analysis. The risk of endocrine or severe exocrine insufficiency was low (5% and 25%). Four extra-pancreatic malignancies (5%) were diagnosed, none pancreatic. One patient died in a car accident.
Conclusions
Type 2 AIP has benign long-term clinical outcomes. Mortality and cancer rates are low and no specific follow-up is needed after radiological remission.
Graphical Abstract
Key Summary
Summarise the established knowledge on this subject
-
Type 2 is a less frequent form of autoimmune pancreatitis (AIP) compared to type 1
-
Association with inflammatory bowel disease has been described (15%–45%)
-
Type 2 AIP is characterized by dramatic response to steroids and low relapse rate in short term-follow-up
What are the significant and/or new findings of this study?
-
Relapse risk is low even in long-term follow-up and almost negligible after 5 years
-
The risk of extra pancreatic and pancreatic malignancies is very low
-
The association with IBD is stronger (>80%) than previously thought in long-term follow-up
-
Long-term probability of pancreatic endocrine/exocrine insufficiency is low
-
Specific follow-up might not be necessary for patients with a confirmed radiological response to steroids
INTRODUCTION
Autoimmune pancreatitis (AIP) is an inflammatory disease of the pancreas highly responsive to steroids,1, 2 classified according to the International Consensus Diagnostic Criteria (ICDC) into type 1, type 2 and not-otherwise specified (NOS) based on pathological, serological and clinical features.3 Type 1 AIP is considered the pancreatic involvement of a systemic IgG4-related disease and represents more than 60% of AIP patients in Italy.4 Histologically, storiform fibrosis, obliterative phlebitis and dense lymphoplasmacitic infiltrate have been described.5 Patients are generally around the 6th-7th decade of life, with elevated serum IgG4 (>135 mg/dL), potential multiorgan involvement and high risk of recurrence after steroids (>50%).2 Almost the entire literature is focused on this AIP type and there is growing interest in the development of new therapies.
Type 2 AIP is less common and generally affects young patients (3rd–4th decade) without gender preference and with low recurrence risk after steroid treatment.6 The first reports on a peculiar type of AIP not IgG4-related were published at the beginning of the new century in USA7 and Europe,8 respectively. The typical pathological feature of type 2 AIP is the presence of a fibro-inflammatory process in the pancreatic ducts, with neutrophilic infiltration of medium and small ducts. The most peculiar feature is the presence of granulocytic epithelial lesions in the medium/small-size ducts or in the acini.8 No elevation in serum IgG4 levels and no other organ involvement were detected in these patients. However, a significant proportion of patients with type 2 AIP (15%–45%) have concomitant inflammatory bowel disease (IBD).4, 9-11 According to the ICDC, the diagnosis of type 2 AIP is definitive only if typical histology is available.3 If definitive histology is lacking (not performed or not conclusive), a probable diagnosis of type 2 AIP might be achieved combining the presence of concomitant IBD, steroid responsiveness, and typical or indeterminate pancreatic gland involvement at imaging.3 No significant differences have been described in a retrospective study from US comparing 31 patients with a definitive diagnosis and 12 patients with a probable diagnosis of type 2 AIP.10 Moreover, the literature suggests that definitive diagnosis based on EUS-guided tissue acquisition might be challenging.12, 13 The clinical onset is mainly characterized by acute pancreatitis (AP; >50%) and rarely by jaundice (<25%), which is significantly different compared to type 1 AIP, mostly characterized by jaundice.4, 9-11 Steroid responsiveness is observed in all patients and the risk of recurrence is quite low.6
Very few studies have been published on type 2 AIP with limited sample size and short follow-up. Therefore, despite it being considered to have a benign clinical course, no long-term data are available. In detail, data regarding the long-term risk of relapse, pancreatic insufficiency and pancreatic/extrapancreatic cancer are not currently available.14
Main aims of the present study were to evaluate long-term outcomes in patients with type 2 AIP, particularly risk of recurrence, exocrine/endocrine insufficiency, pancreatic/extra-pancreatic cancer development, and death.
PATIENTS AND METHODS
Patients with definitive or probable diagnosis of type 2 AIP according to the ICDC,3 present in our prospectively maintained database since 1995 and approved by our local ethics committee (protocol number 393CESC), at 31 December 2021 were identified. Patients with an initial diagnosis of AIP type NOS reclassified during follow-up as AIP type 2 were included. All patients classified as AIP type 1 and type NOS were excluded.
All patients were contacted and clinically evaluated during 2022. Uncontactable patients and patients refusing clinical evaluation were considered as lost at follow-up. Clinical, radiological, and pathological data were re-evaluated and analyzed.
Diabetes was defined according to American Diabetes Association criteria.15 Steatorrhea was defined clinically or based on fecal elastase-1 (<100 μg/g of stools) in patients without previous or synchronous diagnosis of IBD. In patients with IBD, steatorrhea was considered only if fecal elastase-1 <100 μg/g of stools.
The diagnosis of IBD was based on histology.
The diagnosis was considered as synchronous if less than 6 months were observed between the diagnosis of type 2 AIP and IBD, and methacronous if more than 6 months were observed.
AP was diagnosed in the presence of two of the three following criteria: typical abdominal pain, serum amylase and/or lipase >3 times the upper limit of normal, and imaging suggestive of pancreatitis.
Response to steroids was evaluated by imaging (CT or MRI) and clinical evaluation at 1–3 months after steroids and was considered effective only in cases of significant improvement of pancreatic abnormalities and complete symptom regression.
Relapse was defined as the reappearance of typical imaging abnormalities with or without clinical symptoms after a confirmed radiological normalization of the pancreas.
Our research follows the Strengthening the Reporting of Observational Studies in Epidemiology statement of observational study. The authors do not have conflicts of interest to disclose.
Statistical analysis
SPSS (version 22, IBM) was used for the statistical analysis. Chi-square test and Fisher's test were used for categorical variables. Kruskal–Wallis test and Mann–Whitney U-test were used for continuous variables. Statistical significance was considered as p value <0.05.
Survival analysis was accomplished by proportional hazards Cox regression model. The proportional-hazard assumption of the Cox model was tested on the basis of Schoenfeld residuals. In addition, the proportionality assumption was checked by graphic methods: it was verified whether -ln[-ln[survival]] curves for each category of risk factors were parallel, when plotted versus ln[analysis time].
RESULTS
General features
Among 420 patients with AIP included in the database, 88 were classified as type 2 AIP (21%) and were included in the study (55 male, 33 females, mean age at clinical onset 33.5 ± 13.5 years). Twenty-six patients were alcohol drinkers (30%), with a mean alcohol intake of 22.5 ± 20.9 g/day, and 26 were smokers (30%), with a mean cigarette use of 14.5 ± 7.3/day.
Forty-five patients had a diffuse (51%) and 43 had focal involvement (49%) of the pancreatic gland. Among focal forms, the inflammatory process involved the pancreatic head in 27 patients (60%) and body-tail in the remaining 16 patients (40%).
Only 6 patients were clinically asymptomatic at the time of diagnosis (7%), all suffering from IBD. Five of them had asymptomatic elevation of pancreatic enzymes and the last one underwent abdominal ultra-sound for IBD follow-up. Among the 82 symptomatic patients at the time of diagnosis (93%), AP and weight-loss were the most reported symptoms (47% and 49%, respectively). Symptoms at clinical onset were reported in Table 1.
| Symptoms | Nr. of patients (%) |
|---|---|
| Acute pancreatitis | 41 (47) |
| Weight lossa | 43 (49) |
| Abdominal painb | 26 (29.5) |
| Jaundice | 18 (20.5) |
| Diabetesc | 2 (2.3) |
| Steatorrhea | 2 (2.3) |
| Asymptomatic | 6 (6.8) |
- Abbreviation: AIP, autoimmune pancreatitis.
- a Involuntary loss of >5% of total body weight in the previous 3 months.
- b Not attributable to acute pancreatitis or ulcerative colitis.
- c New-onset diabetes (diagnosis in the 6 months before diagnosis of AIP).
IBD was diagnosed in 77 patients (87.5%), all ulcerative colitis (UC). In 26 patients, the diagnosis of type 2 AIP and UC was synchronous (33.7%) and in 51 the diagnosis was methacronous (66.3%). The diagnosis of UC anticipated the diagnosis of type 2 AIP in 26 patients (33.7%), with an average time interval of 4.8 ± 5.5 years (range 1–19 years). The treatment for UC was based exclusively on oral or topical mesalazine. Finally, the diagnosis of UC followed the diagnosis of AIP in 25 patients (32.5%), with an average time interval of 3.2 ± 3.5 years (range 1–18 years). Among these 25 patients, 6 had a previous definitive histological diagnosis of type 2 AIP, while 19 had a previous diagnosis of NOS AIP and were reclassified as type 2 AIP during follow-up because of the development of UC.
A definitive diagnosis of type 2 AIP according to the ICDC was achieved in 21 patients (23.8%), 10 on surgical specimens after resective pancreatic surgery (47.6%) for the suspicion of cancer (9 Whipple resections and 1 distal resection), and 11 on EUS-guided fine needle biopsies (52.4%). EUS-guided tissue acquisition was attempted in other 24 type 2 AIP patients (11 FNB and 13 FNA) without achieving definitive histological diagnosis, with an overall diagnostic accuracy of EUS-guided tissue acquisition of 31.5%.
The diagnosis of type 2 AIP was not based on histology and, therefore, considered as probable according to the ICDC in 67 patients (76.2%).
The mean age at clinical onset of 10 operated patients was significantly higher compared with 78 non-operated patients (respectively 42.2 ± 18.3 vs. 32.4 ± 12.5 years) (p = 0.03). Among the 78 patients who did not undergo pancreatic surgery, 72 were treated with steroids (92.3%) with complete clinical and radiological response. The oral prednisolone dose was 0.8–1.0 mg/kg/day, which was administered for 4 weeks and then gradually tapered by 5 mg/week.
Six patients did not require steroids (8.3%) because they already presented a spontaneous normalization of the pancreas on imaging at the time of our observation after a first evaluation in another institution.
Long term outcomes
Nine patients (10.2%) were uncontactable or refused clinical evaluation and, therefore, were considered as lost at follow-up. The mean follow-up time was 9.2 ± 7.1 years (range 1–27 years).
The overall recurrence rate was 13% at 5 years and 13% at 10 years of follow-up (Figure 1). The recurrence rate was 33% at 5 and 10 years among 10 patients who underwent resective surgery, and 10% at 5 and 10 years among the 78 patients who did not undergo surgery. This difference was statistically significant (p = 0.038) and Figure 2 shows the probability of remission in these two groups. No other factors were associated with increased risk of relapse in unavariable analysis. Moreover, multivariable analysis with the Cox regression model showed similar results to the unadjusted model, except for pancreatic surgery, which did not reach statistical significance as a risk factor for disease relapse. The variable “type of disease (focal vs. diffuse)” was excluded from the multivariable analysis since it exhibited strong collinearity with pancreatic surgery (Table 2).
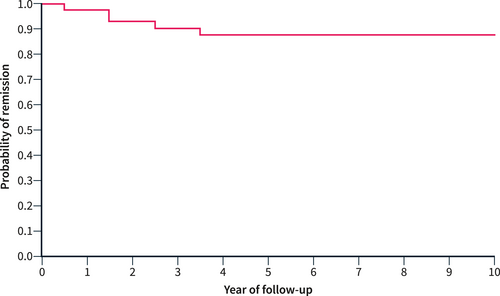
The probability of relapse-free survival in 88 type 2 autoimmune pancreatitis patients.
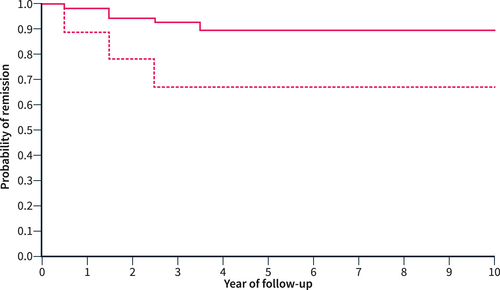
The probability of relapse-free survival in 78 non-surgically resected patients (solid line) and in 10 surgically resected patients (dashed line). The difference was statistically significant (p = 0.038) at univariable analysis.
| Characteristics | Unadjusted haz. ratio [95% conf. interval] | Unadjusted p-value | Adjusted haz. ratio [95% conf. interval] | Adjusted p-value |
|---|---|---|---|---|
| Gender (male vs. female) | 2.68 (0.57–12.62) | 0.213 | 2.51 (0.52–12.01) | 0.251 |
| Acute pancreatitis (yes vs. no) | 1.74 (0.49–6.16) | 0.392 | 3.32 (0.73–15.12) | 0.121 |
| IBD (yes vs. no) | 0.26 (0.68–1.03) | 0.055 | 0.25 (0.05–1.32) | 0.104 |
| Type of disease (focal vs. diffuse) | 2.37 (0.61–9.15) | 0.212 | / | / |
| Pancreatic surgery (yes vs. no) | 3.95 (1.02–15.31) | 0.038 | 2.82 (0.60–13.36) | 0.191 |
- Note: Hazard ratios and p value were obtained by univariable and multivariable Cox regression model.
Among the 10 patients with disease relapse, 3 were asymptomatic and 7 reported symptoms such as pancreatitis or abdominal pain. All relapses were detected within 5 years from the diagnosis of AIP.
Two patients had diabetes at clinical onset and 5 more patient developed diabetes during follow-up. Among the 10 surgically resected patients, 3 had diabetes (30%) compared to 4 out of 78 not resected patients (5.1%) (p = 0.030).
Steatorrhea was diagnosed in 5 out of 10 resected patients (50%) and in 6 out of 78 not resected patients (7.7%) (p = 0.002). Fecal elastase was available in 28 out of 78 non-operated patients (35.9%) at the time of diagnosis. Seven (25%) had severe exocrine insufficiency, 5 (18%) had mild exocrine insufficiency, and 16 (57%) had normal exocrine function. Fecal elastase was available during follow-up (after radiological remission at imaging) in 46 patients (59%). Eleven (23.9%) had severe exocrine insufficiency, 4 (8.7%) had mild exocrine insufficiency, and 31 (67.4%) had normal exocrine function.
Four patients developed extra-pancreatic cancer (1 kidney, 1 rectum, 1 prostate and 1 breast), and none developed pancreatic cancer during the follow-up. Only 1 patient died during follow-up of a car accident. Three patients among the 77 with UC underwent total colectomy (3.9%).
All the principal clinical features of the study population have been reported in Table 3.
| Number of patients | 88 (100) |
| Males nr. (%) | 55 (62.5) |
| Years of follow-up (SD) | 9.2 (7.1) |
| Age at clinical onset in years (SD) | 33.5 (13.5) |
| Diffuse disease nr. (%) | 45 (51) |
| Definitive diagnosis nr. (%) | 21 (23.8) |
| IBD nr. (%) | 77 (87.5) |
| IBD before AIP nr. (%) | 26 (33.7) |
| AIP before IBD nr. (%) | 25 (32.5) |
| Synchronous AIP and IBD nr. (%) | 26 (33.7) |
| Symptoms at clinical onset nr. (%) | 82 (93.2) |
| Relapse at 5 years nr. (%) | 10 (13) |
| Endocrine insufficiency in follow-up nr. (%) | 7 (8) |
| Exocrine insufficiency in follow-up nr. (%) | 11 (12.5) |
| Pancreatic cancer nr. (%) | 0 (0) |
| Extra-pancreatic cancer nr. (%) | 4 (4.5) |
- Abbreviations: AIP, autoimmune pancreatitis; IBD, inflammatory bowel disease; Nr., number; SD, standard deviation.
DISCUSSION
Type 2 AIP is a distinct form of AIP that is less known and investigated compared to type 1 AIP. Despite it being considered to have a benign clinical course, very few studies have been published, with very limited follow-up. Therefore, little is known about the long-term outcomes of type 2 AIP.14
The largest published single-center experience on type 2 AIP included 43 patients from Mayo Clinic with a median follow-up of 2.9 years10 and the largest study from Eastern countries, including 27 patients, had a median follow-up of 32.3 months (2.7 years).9 Moreover, Lorenzo et al. published a multicenter study from France and Belgium focused on patients with the association IBD and AIP, reporting globally 91 patients with a mean follow-up of 5.7 years.11 In the present study, the mean follow-up time was significantly longer (9.1 years), with a limited number of drop-outs.
Different from type 1 AIP, a significant gender prevalence in type 2 AIP has never been clearly described. However, in our study 62.5% of patients were males, similar to the study of Oh et al. (70.4% of males),9 while in the study from Hart et al.10 and Lorenzo et al.,11 the proportion of males was slightly lower (53.5% and 48%). Despite these conflicting data, a significant proportion of males was observed even in type 2 AIP.
Similar to previous published studies,4, 9-11 our data confirm that pancreatitis is the most frequent symptom in type 2 AIP (47%), while jaundice is relatively rare (20%). Signs of pancreatic insufficiency were rare, whereas we found a significant number of patients with a specific symptoms, such as weight-loss and abdominal pain. Only 7% of patients were completely asymptomatic at the time of diagnosis, all suffering from IBD. This proportion is higher compared to previous published studies (0%–2%),9-11 but still low. Therefore, an active radiological evaluation of the pancreas (MRI scan) might be considered only in patients with IBD and specific pancreatic symptoms.
Young age at clinical onset (33.5 years) and low incidence of jaundice explain the relatively small risk of pancreatic resection for the suspicion of pancreatic cancer reported in our study (11%). The significantly higher age at clinical onset of type 2 AIP in operated patients compared with non-operated patients seems to confirm this hypothesis.
Interestingly, we found an association with IBD in 87.5% of patients, which is significantly higher compared to other published studies (44%–45%). This is probably related to the long follow-up period, considering that only 52 patients (59%) had a previous or contemporary diagnosis of IBD. In 25 patients (28%), 6 of whom had a previous histological diagnosis of type 2 AIP, the diagnosis of IBD was achieved during the follow-up with a mean interval time >3 years. These data confirm the relationship between type 2 AIP and IBD, suggesting that it might be even stronger than previously thought. Moreover, it confirms that a significant proportion of patients classified as type NOS AIP-based on the ICDC might be reclassified during follow-up as type 2 because of IBD diagnosis, suggesting that a part of patients with type NOS AIP is at risk of developing UC, as we reported in a previously published paper.16 Finally, the presence of IBD is extremely important to achieve the diagnosis of type 2 AIP if histology is lacking or inconclusive. EUS-guided FNA or FNB appear to have limitations in achieving a definitive diagnosis in these patients. Based on the limited published reports, a definitive histological diagnosis is achieved after EUS guided tissue acquisition with FNA or FNB in only 40% of patients.12, 17
Interestingly, 6 patients (7%) showed a spontaneous normalization of the pancreas without steroids, suggesting that the disease might be self-limiting in some patients. This percentage is quite low compared to the data of Lorenzo et al. reporting 42 patients (46%) with type 2 AIP presenting spontaneous clinical resolution with conservative care without steroids.11 However, the Authors evaluated remission mainly based on the persistence or resolution of symptoms, and not on imaging. In our study, all patients underwent imaging before starting steroids (CT/MRI scan) and spontaneous remission was considered only in case of complete normalization of both imaging and symptoms. Finally, all 72 treated patients responded to steroids. However, we cannot exclude the possibility that some patients might not respond to steroids since the response to steroids is a criterion for the diagnosis in non-histologically proven type 2 AIP.
The overall recurrence rate was 13% at 5 years and 13% at 10 years of follow-up, which is slightly higher compared to previous published studies with shorter follow-up (8%–10%).9-11 Therefore, the recurrence of the disease is expected within 5 years, and after 5 years, the probability of recurrence is negligible.
Different from the study from Hart et al.10 we reported a significant decrease in the recurrence rate at 5 and 10 years in patients treated with steroids compared with those treated with resective surgery at univariable analysis (10% vs. 33%). However, this difference was not statistically significant at multivariable analysis, which might be related to the small simple size of patients who experienced disease relapse (10 patients). Nevertheless, surgery should be avoided in patients with type 2 AIP not only for the operation-related risk but also for possible better long-term outcomes in terms of remission.
The risk of endocrine and exocrine insufficiency was extremely low in non-operated patients, suggesting that the pancreatic function is preserved over time in type 2 AIP patients. Similar data have been published by Eder and colleagues, reporting 20% exocrine insufficiency and 11% endocrine insufficiency in 96 patients with concomitant IBD and AIP (including type 1, 2 and NOS).18
Finally, the risk of pancreatic cancer (0%) or extra-pancreatic cancer (4.5%) development is negligible, differently from type 1 AIP where a higher risk of cancer has been observed, even though it is still debated.19, 20 The low risk of cancer development and death (1.1%) is probably related to the young age at clinical onset in type 2 AIP patients. These data suggest that patients with preserved endocrine/exocrine pancreatic function do not need specific pancreatic follow-up after steroid treatment considering the benign course of the disease.
The strength of the present study is the good simple size, considering the rarity of type 2 AIP, the long follow-up (>9 years), and the limited number of patients lost at follow-up (10%). Moreover, all patients were contacted and evaluated during the year 2022.
Limitations to our study are the retrospective design partially compensated by the prospective maintained data collection.
In conclusion, this is the largest single-center study of patients with type 2 AIP with a very long follow-up. We confirmed that AIP type 2 patients are young and AP is the most frequent symptom at clinical onset. The relationship with IBD appears more frequent than previously published short follow-up studies. We confirmed that patients with type 2 AIP have a low risk of relapse, as well as a low risk of exocrine/endocrine pancreatic insufficiency (if not surgically resected), pancreatic/extra-pancreatic cancer, and death. Therefore, patients with type 2 AIP without endocrine/exocrine insufficiency do not need specific clinical or radiological follow-up.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare.
Open Research
DATA AVAILABILITY STATEMENT
Data available on request from the authors.