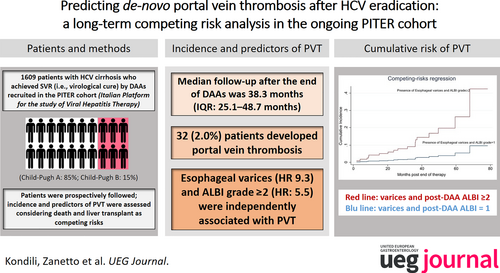
Predicting de-novo portal vein thrombosis after HCV eradication: A long-term competing risk analysis in the ongoing PITER cohort
PITER Collaborating Investigators: Monica Monti (University of Florence); Barbara Coco (University Hospital of Pisa); Roberto Filomia (University Hospital of Messina); Erica Maria Bruno (Villa Sofia-Cervello Hospital, Palermo); Valentina Cossiga (University of Naples Federico II); Simona Amodeo (University of Palermo); Marcello Dallio (University of Campania Luigi Vanvitelli, Naples); Luciano Crapanzano (University of Palermo); Mario Masarone (University of Salerno, Baronissi); Martina De Siena (Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome); Ilaria Serio (Sant'Orsola Malpighi Hospital, Bologna); Martina Loi (University Hospital, Monserrato, Cagliari); Lucia Brescini (Polytechnic University of Marche, Ancona); Antonio Ciaccio (San Gerardo Hospital, Monza); Monica Cucco (Polytechnic University of Marche, Ancona); Xhimi Tata (Istituto Superiore di Sanità, Rome, Italy); Caterina Sagnelli (University of Campania Luigi Vanvitelli, Naples); Costantino Sgamato (University of Naples Federico II); Ernesto Claar (Betania Hospital, Naples), Elena Rosselli Del Turco (University Hospital IRCCS Policlinico Sant'Orsola Malpighi, Bologna), Maria Grazia Rumi (San Giuseppe Hospital, Milan), Giovanni Battista Gaeta (University L. Vanvitelli, Naples), Paolo Simioni (University of Padova).
Loreta A. Kondili and Alberto Zanetto contributed equally and are joint first authors.
Abstract
Background & Aims
Sustained virological response (SVR) by direct-acting antivirals (DAAs) may reverse the hypercoagulable state of HCV cirrhosis and the portal vein thrombosis (PVT) risk. We evaluated the incidence and predictive factors of de novo, non-tumoral PVT in patients with cirrhosis after HCV eradication.
Methods
Patients with HCV-related cirrhosis, consecutively enrolled in the multi-center ongoing PITER cohort, who achieved the SVR using DAAs, were prospectively evaluated. Kaplan-Meier and competing risk regression analyses were performed.
Results
During a median time of 38.3 months (IQR: 25.1–48.7 months) after the end of treatment (EOT), among 1609 SVR patients, 32 (2.0%) developed de novo PVT. A platelet count ≤120,000/μL, albumin levels ≤3.5 mg/dL, bilirubin >1.1 mg/dL, a previous liver decompensation, ALBI, Baveno, FIB-4, and RESIST scores were significantly different (p < 0.001), among patients who developed PVT versus those who did not. Considering death and liver transplantation as competing risk events, esophageal varices (subHR: 10.40; CI 95% 4.33–24.99) and pre-treatment ALBI grade ≥2 (subHR: 4.32; CI 95% 1.36–13.74) were independent predictors of PVT. After HCV eradication, a significant variation in PLT count, albumin, and bilirubin (p < 0.001) versus pre-treatment values was observed in patients who did not develop PVT, whereas no significant differences were observed in those who developed PVT (p > 0.05). After the EOT, esophageal varices and ALBI grade ≥2, remained associated with de novo PVT (subHR: 9.32; CI 95% 3.16–27.53 and subHR: 5.50; CI 95% 1.67–18.13, respectively).
Conclusions
In patients with HCV-related cirrhosis, a more advanced liver disease and significant portal hypertension are independently associated with the de novo PVT risk after SVR.
Graphical Abstract
Key summary
-
Patients with cirrhosis are at risk of portal vein thrombosis (PVT).
-
Achievement of sustained virological response (SVR) in patients with HCV-related cirrhosis may reverse the hyper-coagulable state associated with chronic liver disease.
-
Whether this translates into a reduced risk of PVT is unclear.
-
A better definition of thrombotic risk after SVR may improve individual patient management.
-
This is the largest prospective cohort examining the risk of PVT after SVR in HCV-related cirrhosis.
-
The risk of de novo PVT development was not completely abolished by SVR in patients with HCV-related cirrhosis.
-
The presence of esophageal varices and ALBI grade ≥2 (both pre-treatment and prospectively evaluated within 1 year after the end of treatment (EOT)) were independent predictors of PVT.
-
Patients who did not achieve a significant improvement in liver function after antiviral therapy remained at risk of PVT.
INTRODUCTION
Portal vein thrombosis (PVT) is the most common thrombotic complication in patients with cirrhosis. Its prevalence ranges between 1% and 25%, and it is significantly higher in decompensated patients (10%–25%) than in compensated ones (1%–5%).1 Such differences are related to multiple factors, such as a progressive decrease in the velocity of portal vein blood flow,2 the development of local alterations in the antithrombotic properties of portal endothelium,3 and probably a more pronounced hypercoagulable state in decompensated patients.4
The effect of PVT on the natural history of cirrhosis remains unresolved due to conflicting data from studies that included patients with different severity of liver disease, different degrees of PVT, and the use of different therapeutic strategies.5, 6
However, PVT is associated with an increased risk of failure to control bleeding after variceal hemorrhage and higher mortality after liver transplantation (LT) or exclusion from the listing.7-11 Thus, by preventing the development of PVT, patient outcomes might be improved.
Sustained virological response (SVR) by direct-acting antivirals (DAAs) in patients with chronic hepatitis C virus (HCV)-related cirrhosis is associated with a decrease in the risk of hepatic decompensation and mortality.12 However, whether eradicating HCV by DAAs leads to a decrease in the risk of PVT remains unclear.
Studies conducted by our group and others showed that improving liver synthetic functions, driven by virological cure, is associated with a reversal of plasmatic hypercoagulability,13, 14 which might decrease the risk of PVT.15
The improvement in coagulation after treatment with DAAs is inversely correlated with the severity of cirrhosis. A study by Mandorfer et al. showed that the risk of PVT persisted although an SVR was elicited, and PVT was independently associated with the baseline severity of chronic liver disease.16
A better definition of the thrombotic risk after SVR in patients with cirrhosis might improve risk stratification and hemostatic management.
In this study, we aimed to [a] evaluate the incidence of de novo PVT in a large, real-world cohort of patients with HCV cirrhosis who achieved SVR after DAA treatment and [b] identify pre-treatment and post-treatment variables associated with the development of PVT.
PATIENTS AND METHODS
Study population
The study population consisted of patients with chronic HCV infection consecutively enrolled in the ongoing prospective PITER cohort (the Italian Platform for the study of Viral Hepatitis Therapy) from 40 centers specialized in liver and infectious diseases from teaching hospitals and general hospitals throughout Italy.17 The data were accurately and prospectively collected in dedicated electronic case report forms at pre-defined time points, that is, before DAA therapy and during the follow-up, following the clinical practice of each center. The Child-Pugh-Turcotte (CPT) stage, albumin-bilirubin (ALBI) score,18 Baveno expanded,19 Fibrosis-4 (FIB-4) index,20 and RESIST score21 were calculated using well-established formulas. Following the PITER protocol, decompensated cirrhosis was diagnosed based on the presence or appearance of ascites and/or gastrointestinal bleeding due to portal hypertension and/or hepatic encephalopathy and/or jaundice and/or spontaneous bacterial peritonitis.8 The presence of hepatic steatosis by abdominal ultrasound (abdominal-US) examinations associated with hypertension, cardiovascular disease, type 2 diabetes, and body mass index (BMI) >25 kg/m2 were used as surrogate markers of metabolic syndrome in patients who did not report alcohol consumption.
Exclusion and inclusion criteria and duration of follow-up
We evaluated patients with pre-treatment liver cirrhosis included in the PITER cohort between June 2014 and March 2020 for inclusion in this study. Cirrhosis was defined by liver biopsy (Metavir ≥4 or Ishak score ≥6) or Transient Elastometry (liver stiffness measurement [LSM] >12.5 kPa) or biochemical and/or imaging indications of clinically significant portal hypertension (i.e., presence of esophageal and/or gastric varices and/or platelet [PLT] count ≤150,000/μL with spleen enlargement). A PLT count of ≤120,000/μL and an albumin level of ≤3.5 g/dL were chosen as the cut-off for determining the risk of liver disease progression due to their better performance in predicting severe liver cirrhosis in our study population, as also reported in previous studies.22, 23
As per the initial Italian regulations on treating patients with HCV-related liver cirrhosis without an indication for LT, only those belonging to CPT stages A and B at pre-treatment evaluation were eligible for antiviral treatment.24 Thus, no CPT stage C patient at the time of the treatment start was available for this analysis. Patients with a pre-treatment compensated CPT-A or B class or with a previous decompensation history but under a stable clinical condition and having a compensated liver disease within the last 12 months before DAA treatment were included.
Patients previously diagnosed with PVT or with a diagnosis of malignant PVT and those who either underwent or were awaiting LT were excluded. A total of 6 patients with malignant PVT were excluded from the present analysis (4 with pre-therapy hepatocellular carcinoma (HCC) and 2 with post-therapy HCC). Patients with less than 12 weeks of follow-up after the EOT (i.e., in whom SVR could not be evaluated) were excluded.
For each patient, the duration of follow-up was calculated between the EOT and the last follow-up.
Screening for portal vein thrombosis
The results of abdominal ultrasound examinations, routinely performed for HCC screening every 6 months for patients with cirrhosis, were prospectively collected. The PVT was defined as the presence of hyper-echogenic material within the portal vein main trunk and/or intrahepatic branches, determined by ultrasound examinations or through the visualization of non-contrasted material at the venous phase of the CT scan or T2 sequence at MRI. PVT was classified as complete when blood flow in the portal vein was absent and as partial when the lumen was only partially occluded, and the flow was still present at Doppler evaluation. Only PVT occurring after the EOT was considered to be de novo PVT.
As per the current guidelines, all patients with a new diagnosis of PVT based on ultrasound examinations underwent second-line imaging (either CT scan or MRI).5, 6 In patients with a history of HCC, the malignant nature of thrombosis was excluded according to the following criteria: the presence of thrombus enhancement, PVT adjacent to HCC, and an increase in the diameter of the portal vein.25
Data collection
The case report forms recorded detailed demographic, clinical, and laboratory characteristics. The outcomes collected during the follow-up after viral eradication included de novo PVT, development of hepatic decompensation, de novo HCC, LT, and patient survival. In patients who experienced PVT, data on the extension of thrombosis were also collected.
Statistical analysis
The main characteristics of the patients are reported as the median and interquartile range (IQR) or as proportions (N and %) for continuous and categorical variables, respectively. Albumin, alanine aminotransferase (ALT), bilirubin, creatinine, International Normalized Ratio (INR), PLT, and LSM were evaluated using clinically relevant threshold cut-offs below or above which the risk could be assumed to be homogeneously distributed.
The Mann-Whitney U test was used for analyzing continuous variables to assess differences between distributions, and the Chi-squared test was used for comparing proportions. The Wilcoxon matched-pairs signed-rank test was used to assess the differences between matched pairs of observations.
To evaluate the effect of demographic data and clinical variables on the risk of development of PVT, a Fine-Gray competing-risks regression model was applied, considering death or LT as competing events (i.e., events that could prevent the event of interest). Potential multicollinearity was tested among all the variables included in the final models. The possible presence of multicollinearity was checked by estat vce, corr STATA command. A correlation coefficient <0.5 indicated predictor variables in the same model are not correlated, and they can independently predict the value of the dependent variable.
Univariate and multivariate analyses were performed. In the multivariable analysis, a forward stepwise selection method was used to obtain robust results. The results are presented as a sub-hazard ratio (subHR) and the corresponding 95% Confidence Interval (95% CI).
For all analyses, differences were considered to be statistically significant at p < 0.05. All statistical analyses were performed on STATA version 16.1 (Stata Corp, College Station, TX, USA).
RESULTS
Baseline characteristics of the study population based on the occurrence of PVT
The baseline characteristics of 1609 patients who achieved the SVR and were followed up for a median time of 38.3 months (IQR: 25.1–48.7 months) after EOT are shown in Table 1. Of these, 32 patients (2.0%) experienced PVT; the median time of PVT development was 36.1 months after EOT; IQR: 19.1–46.2 months). PVT was complete in 9 patients and partial in 23 patients. Of these patients, 21 (65%) were treated with anticoagulation. The median duration of anticoagulant therapy was 7 months (IQR: 3.0–24.0). Of the treated patients, 3 (14%) achieved complete resolution of PVT and 5 (24%) achieved a partial response (i.e., any improvement in PVT extension without complete resolution). In 13 (62%) patients, PVT either remained stable or progressed despite anticoagulant treatment.
| No PVT (N = 1577a) | PVT occurrence (N = 32a) | TOTAL (N = 1609a) | ||
|---|---|---|---|---|
| Epidemiological features | Median (IQR) | Median (IQR) | p b | Median (IQR) |
| Age (years) | 65 (55–72) | 67 (58–71) | 0.671 | 65 (56–72) |
| N. | % | N. | % | p c | N. | % | |
|---|---|---|---|---|---|---|---|
| Sex | |||||||
| Male | 875 | 55.5 | 14 | 43.8 | 0.186 | 889 | 55.3 |
| Female | 702 | 44.5 | 18 | 56.3 | 720 | 44.7 | |
| BMI | |||||||
| Underweight-normal | 683 | 43.3 | 13 | 40.6 | 0.761 | 696 | 43.3 |
| Overweight-obese | 894 | 56.7 | 19 | 59.4 | 913 | 56.7 | |
| Alcohol use | |||||||
| Never | 1059 | 68.0 | 25 | 78.1 | 0.230 | 1084 | 68.2 |
| Current | 157 | 10.1 | 4 | 12.5 | 161 | 10.1 | |
| Past | 342 | 22.0 | 3 | 9.4 | 345 | 21.7 | |
| HCV- genotype | |||||||
| 1 | 1116 | 70.8 | 21 | 65.6 | 0.328 | 1137 | 70.7 |
| 2 | 232 | 14.7 | 8 | 25.0 | 240 | 14.9 | |
| 3 | 135 | 8.6 | 1 | 3.1 | 136 | 8.5 | |
| Other | 94 | 6.0 | 2 | 6.3 | 96 | 6.0 | |
| Clinical features | |||||||
| Platelets count | |||||||
| ≤120,000/μL | 821 | 52.5 | 28 | 87.5 | <0.001 | 849 | 53.2 |
| >120,000/μL | 742 | 47.5 | 4 | 12.5 | 746 | 46.8 | |
| Albumin (g/dL) | |||||||
| ≤3.5 | 347 | 23.3 | 19 | 59.4 | <0.001 | 366 | 24.1 |
| >3.5 | 1140 | 76.7 | 13 | 40.6 | 1153 | 75.9 | |
| ALT (IU/L) | |||||||
| ≥35 | 1356 | 86.5 | 29 | 90.6 | 0.501 | 1385 | 86.6 |
| <35 | 211 | 13.5 | 3 | 9.4 | 214 | 13.4 | |
| Bilirubin (mg/dL) | |||||||
| ≥1.1 | 466 | 30.5 | 20 | 62.5 | <0.001 | 486 | 31.2 |
| <1.1 | 1061 | 69.5 | 12 | 37.5 | 1073 | 68.8 | |
| Creatinine (mg/dL) | |||||||
| ≥1.2 | 91 | 6.0 | 2 | 6.3 | 0.954 | 93 | 6.0 |
| <1.2 | 1425 | 94.0 | 30 | 93.8 | 1455 | 94.0 | |
| INR | |||||||
| ≥1.1 | 745 | 50.1 | 22 | 68.8 | 0.037 | 767 | 50.5 |
| <1.1 | 742 | 49.9 | 10 | 31.3 | 752 | 49.5 | |
| Liver stiffness | |||||||
| ≥20 | 606 | 48.6 | 11 | 64.7 | 0.186 | 617 | 48.8 |
| Measurement (kPa) | |||||||
| <20 | 642 | 51.4 | 6 | 35.3 | 648 | 51.2 | |
| History of previous HCC | 121 | 7.7 | 3 | 9.4 | 0.721 | 124 | 7.7 |
| Steatosis | 416 | 26.4 | 4 | 12.5 | 0.077 | 420 | 26.1 |
| History of ascitesd | 115 | 7.3 | 8 | 25.0 | <0.001 | 123 | 7.6 |
| Esophageal varices | 345 | 21.9 | 26 | 81.3 | <0.001 | 371 | 23.1 |
| Esophageal | |||||||
| F1 | 225 | 70.1 | 10 | 45.5 | 0.044 | 235 | 68.5 |
| Varices Grade | |||||||
| F2 | 85 | 26.5 | 10 | 45.5 | 95 | 27.7 | |
| F3 | 11 | 3.4 | 2 | 9.1 | 13 | 3.8 | |
| History of bleedingd | 35 | 2.2 | 5 | 15.6 | <0.001 | 40 | 2.5 |
| History of encephalopathyd | 42 | 2.7 | 2 | 6.3 | 0.218 | 44 | 2.7 |
| Previous decompensationd | 166 | 10.5 | 13 | 40.6 | <0.001 | 179 | 11.1 |
| Child-Pugh class | |||||||
| A | 1353 | 85.9 | 19 | 59.4 | <0.001 | 1372 | 85.3 |
| B | 223 | 14.1 | 13 | 40.6 | 236 | 14.7 | |
| Potential metabolic syndrome | 218 | 13.8 | 2 | 6.3 | 0.217 | 220 | 13.7 |
| Non-selective beta blockers use | 101 | 6.4 | 10 | 31.3 | <0.001 | 111 | 6.9 |
| Score | |||||||
| ALBI | |||||||
| Grade 1 | 643 | 44.0 | 3 | 9.4 | <0.001 | 646 | 43.3 |
| Grade 2 | 794 | 54.4 | 28 | 87.5 | 822 | 55.1 | |
| Grade 3 | 23 | 1.6 | 1 | 3.1 | 24 | 1.6 | |
| BAVENO | |||||||
| OUT | 593 | 53.4 | 15 | 93.8 | 0.001 | 608 | 53.9 |
| Expanded | |||||||
| IN | 518 | 46.6 | 1 | 6.3 | 519 | 46.1 | |
| FIB4 | |||||||
| >3.25 | 1060 | 68.3 | 29 | 90.6 | 0.007 | 1089 | 68.8 |
| ≤3.25 | 492 | 31.7 | 3 | 9.4 | 495 | 31.3 | |
| RESIST | |||||||
| OUT | 915 | 61.9 | 32 | 100.0 | <0.001 | 947 | 62.7 |
| IN | 564 | 38.1 | 0 | 0.0 | 564 | 37.3 | |
- Abbreviations: IQR, interquartile range; PVT, portal vein thrombosis.
- a For some variables inconsistencies are due to missing values.
- b p value Mann–Whitney rank-sum test.
- c p value Chi-square test.
- d All patients did not have signs of liver decompensation at treatment start.
Age, sex, BMI distribution, alcohol use, and presence of surrogate markers of metabolic syndrome were similar among patients who achieved the SVR and developed PVT and those who did not develop PVT. In contrast, a PLT count of ≤120,000/μL, albumin levels ≤3.5 mg/dL, bilirubin >1.1 mg/dL, a history of liver decompensation, and ALBI, Baveno, FIB-4, and RESIST scores were all significantly different among patients who developed PVT versus those who did not (indicating greater severity of advanced liver disease in PVT patients vs. non-PVT patients). The use of non-selective beta-blockers (NSBBs) was more frequent in patients who developed PVT versus those who did not. The proportion of patients who were administered anticoagulants was similar between the two groups (p = 0.262, data not shown). A transjugular intrahepatic portosystemic shunt was reported in four patients who did not develop PVT and in none of the patients who developed PVT (data not shown). The overall comorbidity pattern and those that might be associated with liver disease progression were not different among patients who developed PVT and those who did not (Figure S1).
Pre-treatment predictive factors for the occurrence of PVT
Considering death or LT as competing risk events, the pre-treatment variables independently associated with the development of PVT in patients who achieved the SVR were evaluated (Table 2). No multicollinearity in the parameters chosen to be evaluated as predictive factors of PVT (all correlation coefficients were <0.5) was found. The presence of esophageal varices and an ALBI grade ≥2 were significantly associated with the risk of PVT development. Similar results were obtained after excluding the data on patients with a history of previous decompensation from the analysis (Table S1).
| Pre-treatment factors | Univariable | Multivariablea | ||||
|---|---|---|---|---|---|---|
| SubHR | 95% CI | p | SubHR | 95% CI | p | |
| Sex (ref. male) | 1.43 | 0.71–2.88 | 0.312 | |||
| Age (increasing years) | 1.01 | 0.98–1.05 | 0.406 | |||
| Previous decompensation | 4.80 | 2.38–9.68 | <0.001 | |||
| Esophageal varices | 13.21 | 5.37–32.54 | <0.001 | 10.40 | 4.33–24.99 | <0.001 |
| Platelets (ref. >120,000/μL) | 6.00 | 2.11–17.07 | 0.001 | |||
| ALBI (ref. Grade 1) | 7.15 | 2.18–23.41 | 0.001 | 4.32 | 1.36–13.74 | 0.013 |
| INR (ref. <1.1) | 2.13 | 1.01–4.51 | 0.048 | |||
- Note: Competing Risk model results. PVT events N = 32.
- Abbreviations: CI, Confidence Interval; SubHR, SubHazard Ratio.
- a Stepwise Forward selection including variables with a p value <0.10 at univariable analysis.
Prospective evaluation of the predictors of PVT measured after viral eradication
The changes in the biochemical markers of chronic liver disease severity (PLT, albumin, and bilirubin measurement) before antiviral treatment (baseline) and after a median time of 6 months after EOT (IQR: 4.7–7.9 months, with no difference among patients who developed PVT and those who did not: p = 0.977) according to the PVT development are shown in Figure 1. For these three parameters, there were significant variations (all p < 0.001) in the values evaluated after viral eradication versus pre-treatment values in patients who did not develop PVT, whereas in those who developed PVT, PLT count, albumin, and bilirubin levels remained unchanged (p = 0.759, p = 0.054, and p = 1.000, respectively).
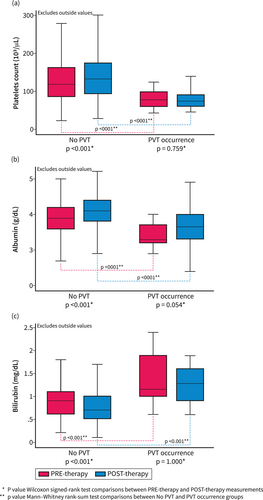
Pre-treatment and post-treatment evaluation of factors associated with the severity of liver disease in patients with SVR. Changes in the markers of chronic liver disease severity were evaluated in patients with data available before antiviral treatment (baseline) and within the first year after EOT (follow-up) based on the development of PVT. Pre-treatment and post-treatment platelet counts (a), albumin (b), and bilirubin levels (c) in patients with and without PVT. The values were reported as the median and interquartile range. PVT, portal vein thrombosis.
The biochemical characteristics evaluated post-EOT (independently and combined with the ALBI or RESIST scores) have shown that despite viral eradication, patients who developed PVT remained with parameters of more severe liver disease compared with those who did not (Table 3).
| No PVT | PVT occurrence | p a | |||
|---|---|---|---|---|---|
| N. | % | N. | % | ||
| Platelets count (103/μL) | N = 817 | N = 18 | |||
| ≤120,000/μL | 355 | 43.5 | 15 | 83.3 | 0.001 |
| >120,000/μL | 462 | 56.5 | 3 | 16.7 | |
| Albumin (g/dL) | N = 741 | N = 18 | |||
| ≤3.5 | 99 | 13.4 | 6 | 33.3 | 0.015 |
| >3.5 | 642 | 86.6 | 12 | 66.7 | |
| Bilirubin (mg/dL) | N = 775 | N = 18 | |||
| ≥1.1 | 164 | 21.2 | 11 | 61.1 | <0.001 |
| <1.1 | 611 | 78.8 | 7 | 38.9 | |
| ALBI | N = 710 | N = 18 | |||
| Grade 1 | 464 | 65.4 | 3 | 16.7 | <0.001 |
| Grade 2–3 | 246 | 34.6 | 15 | 83.3 | |
| RESIST | N = 736 | N = 18 | |||
| OUT | 368 | 50.0 | 16 | 88.9 | 0.001 |
| IN | 368 | 50.0 | 2 | 11.1 | |
- a p value Chi-square test.
Post-treatment predictive factors related to the occurrence of PVT
Post-treatment evaluated variables associated with the development of PVT in patients who achieved the SVR are shown in Table 4. The correlation coefficients suggested weak correlations (all coefficients were <0.5), indicating no multicollinearity in the parameters chosen for the regression model used. The presence of esophageal varices and post-treatment ALBI grade ≥2 were significantly associated with the risk of PVT. For patients with esophageal varices, the adjusted (independent) risk of developing PVT was 9.32 times higher than the risk of developing PVT in patients with no esophageal varices. For patients with ALBI grade ≥2, the adjusted (independent) risk of developing thrombosis was 5.50 times higher than the risk of patients with ALBI grade 1; similar results were obtained after excluding patients with previous decompensation from the analysis (Table S2).
| Variables | Univariable | Multivariablea | ||||
|---|---|---|---|---|---|---|
| SubHR | 95% CI | p | SubHR | 95% CI | p | |
| Sex (ref. male) | 1.09 | 0.44–2.73 | 0.847 | |||
| Age (increasing years) | 1.03 | 0.98–1.10 | 0.293 | |||
| Previous decompensation | 2.61 | 0.87–7.82 | 0.085 | |||
| Esophageal varices | 14.16 | 4.58–43.79 | <0.001 | 9.32 | 3.16–27.53 | <0.001 |
| Post-treatment factors | ||||||
| Platelets (ref. >120,000/μL) | 5.86 | 1.69–20.31 | 0.005 | |||
| ALBI (ref. grade 1) | 9.56 | 2.75–33.14 | <0.001 | 5.50 | 1.67–18.13 | 0.005 |
- Note: Competing Risk model results. PVT events N = 18.
- Abbreviations: CI, Confidence Interval; SubHR, SubHazard Ratio.
- a Stepwise Forward selection including variables with a p value <0.10 at univariable analysis.
The competing risk regression curves showed that for all patients in whom the presence of esophageal varices was reported, the cumulative PVT incidence rates in patients with post-treatment ALBI grade ≥2 versus those with post-treatment ALBI grade 1 were 27% versus 6% at 60 months and 43% versus 10% at the end of the observation period (80 months) (Figure 2).
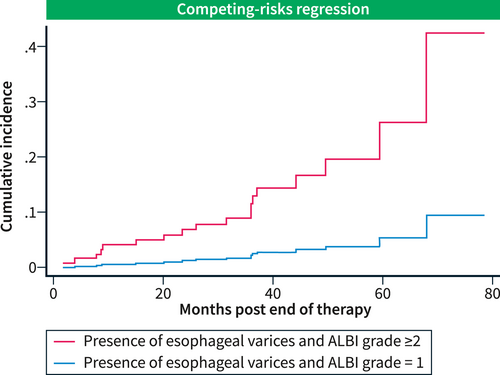
Competing risk regression curves. The cumulative incidence rates of PVT in patients with esophageal varices and ALBI grade ≥2 versus those with ALBI grade 1.
The outcomes of patients according to the development of PVT after the SVR are shown in Table S3. Patients who experienced PVT had higher rates of liver decompensation and mortality.
DISCUSSION
The effect of etiological therapy on the risk of PVT in patients with HCV-related cirrhosis is still debated.26 In this study, we addressed this question by investigating prospectively the multicenter PITER cohort, which represents the current, real-life state of HCV clinical care in Italy. Patients with HCV-related chronic liver disease have been enrolled in the PITER cohort since 2014 and followed for a prospective assessment of the clinical effect of DAAs on the natural history of HCV-related chronic liver disease.17
The positive effectiveness of SVR on PVT development is inversely correlated with the severity of cirrhosis.12 Indeed, we observed that the risk of PVT was not completely abolished by SVR. We found that patients who developed PVT had a more advanced liver disease severity. Similar to the findings of another study,16 we showed that the severity of baseline liver dysfunction (i.e., pre-treatment ALBI grade ≥2) and severe portal hypertension (i.e., esophageal varices) were independently associated with the PVT risk. Despite the use of NSBBs in our cohort being significantly higher in patients who developed PVT, we did not consider NSBBs in the multivariable model because our data are not sufficient to perform a time-dependent analysis, and thereby take into consideration the real dynamic changes in PVT after their use.2 Furthermore, the presence of esophageal varices is a more specific marker for clinically significant portal hypertension and is a well-known risk factor for PVT.1
The findings of several studies suggested that the estimation of thrombo-hemorrhagic risk in cirrhosis should reflect the dynamic changes in coagulation and liver function observed in these patients.27-30 Therefore, we assessed whether the evolution of the chronic liver disease and portal hypertension after the virological cure were associated with the risk of PVT. A low platelet count (PLT ≤120,000/μL), the presence of esophageal varices, history of previous decompensation and ALBI grade ≥2 were associated with the PVT risk on the univariable analysis. Using a multivariable model, the ALBI score evaluated after viral eradication and the presence of esophageal varices were independently associated with the PVT risk. The dynamics of platelet count after SVR do not mirror those of the hepatic vein pressure gradient. Our data further confirm that despite a PLT count of ≤120,000/μL did not remain an independent factor, significant portal hypertension as defined by the presence of esophageal varices was independently associated with the PVT risk.31
Some studies have shown that most patients who achieve the SVR by DAA treatment experienced a significant decrease in the portal pressure32, 33 and an increase in the portal blood flow velocity.34, 35 Since slow portal blood flow is a major risk factor for the development of PVT,2 this might partly explain the low incidence of PVT in our cohort. Virological cure might lead to an improvement in HCV-associated hyper-coagulability13, 14, 36 and a decrease in the thrombotic tendency associated with cirrhosis.37 Patients with more advanced liver disease in whom SVR seems not to be associated with a significant reversal of coagulopathy13 would remain at risk of thrombosis. However, whether coagulopathy in patients with cirrhosis is implicated in the pathophysiology of PVT independently of the reduced portal vein blood flow is still under investigation,38, 39 and further studies are required to elucidate the effect of SVR on Virchow's triad in HCV cirrhosis.4, 40, 41
Our study had several limitations. First, the number of patients with Child-Pugh stage B cirrhosis was small, and no Child-Pugh C patient was included. Therefore, the effect of SVR on the thrombotic risk associated with decompensated cirrhosis and its complications (i.e., the most at risk) could not be thoroughly assessed.42 Second PITER is a multicenter real-life prospective cohort, neither designed to specifically assess the incidence of PVT (which might be underestimated) nor to evaluate the specific effect of PVT and its extension on the outcomes of patients. However, the accuracy of the prospectively collected data included in PITER is constantly checked by a dedicated team as per the study protocol. Thirdly, given the low number of events, we could not perform a separate analysis for complete and partial PVT. Dedicated studies that evaluate portal vein blood flow velocity or specific coagulation markers could improve the identification of patients who remain at risk of PVT after SVR and the specific effect of PVT on the patient outcome. In this study, we censored the patients at the last clinical visit and not at the time of PVT diagnosis. However, patients with cirrhosis included in PITER are followed up at least every 6 months, and the imaging and biochemical tests are performed a few days before the clinical visit or during the visit. Thus, the risk of bias regarding the duration of follow-up was minimal.
In conclusion, patients with more advanced chronic liver diseases before antiviral therapy and those with severe portal hypertension whose liver function did not significantly improve, despite the achievement of SVR by a DAA treatment, remained at a higher risk of de novo PVT. Further studies are required to determine whether and how the development of de novo PVT in this population affects the risk of hepatic decompensation after SVR.
AUTHOR CONTRIBUTIONS
Loreta A. Kondili: Performance of the research; interpretation of the data, writing of the manuscript, revision of the manuscript and final approval. Alberto Zanetto: Research idea and performance of the research; interpretation of the data, writing of the manuscript, the revision of the manuscript. Maria Giovanna Quaranta: Performance of the research, writing of the manuscript, the revision of the manuscript. Luigina Ferrigno: Statistical analysis. Valentina Panetta: Statistical analysis. Vincenza Calvaruso: Revision of the manuscript. Anna Linda Zignego, Maurizia R. Brunetto, Giovanni Raimondo, Elisa Biliotti, Donatella Ieluzzi, Andrea Iannone, Salvatore Madonia, Liliana Chemello, Luisa Cavalletto, Carmine Coppola, Filomena Morisco, Francesco Barbaro, Anna Licata, Alessandro Federico, Federica Cerini, Marcello Persico, Maurizio Pompili, Alessia Ciancio, Fabio Piscaglia, Luchino Chessa, Andrea Giacometti, Pietro Invernizzi, Giuseppina Brancaccio, Antonio Benedetti, Leonardo Baiocchi, Ivan Gentile, Nicola Coppola, Gerardo Nardone, Antonio Craxì: Patients' recruitment and clinical assessments. Francesco Paolo Russo: Research idea and performance of the research; interpretation of the data, writing of the manuscript, revision of the manuscript and final approval.
ACKNOWLEDGMENTS
The authors wish to thank all PITER Collaborating Group and all participating centers, investigators and research staff (available in www progettopiter.it) who are involved in the study on a voluntary basis for their time and effort. We also thank Giampaolo La Terza (Medisoft Informatic Services) for Database maintenance and implementation. We additionally acknowledge Federica Magnani, Rosangela Duranti, Erika Olivieri, and Alessandra Mattei for secretarial and administrative assistance. This study was supported by the Italian Ministry of Health (Grant number RF-2016-02364053 to LAK).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Ethics approval
The study was conducted following the guidelines of the Declaration of Helsinki and the principles of Good Clinical Practice. The study protocol was approved by the Ethics Committee of the Istituto Superiore di Sanità (Italian National Institute of Public Health) and by the local ethics committees of each clinical center. The data on the patients were evaluated through pseudonymous analysis using codes generated by the electronic case report forms. Written informed consent was obtained from all patients to participate in the PITER study.
Open Research
DATA AVAILABILITY STATEMENT
This study was approved by the Ethical Committee of Istituto Superiore di Sanità and the local Ethical Committees of the participating centers. By the protocol, the property of the data is of participating clinical centers, while Istituto Superiore di Sanità acts as a coordinating center for data management and analysis. Cumulative data are reported within the paper, whereas each patient's data are not fully available and without restrictions for ethical reasons. Dr. LA Kondili ([email protected]) is in charge of data management, and the readers may contact her for specific data requests. She will provide the necessary ethical clearances for access to data.