Inflammatory bowel disease cross-sectional imaging: What's new?
Yang-di Wang and Ruo-nan Zhang contributed equally to this work.
Abstract
Cross-sectional imaging—ultrasonography, computed tomography enterography, and magnetic resonance enterography—is a routine and indispensable tool for patients with Crohn's disease (CD) that helps to detect or monitor disease characteristics before, during, and after CD treatment. New emerging radiological technologies may have further clinical applications in the management of CD. In this review article, we focus on the latest developments in cross-sectional imaging in CD research, including its role in intra- and extra-luminal lesion detection, intestinal inflammation and fibrosis grading, therapeutic response assessment and outcome prediction, postoperative recurrence detection and prediction, and the gut-brain axis.
Graphical Abstract
INTRODUCTION
Inflammatory bowel disease (IBD) is a chronic and disabling inflammation of the gastrointestinal tract that includes ulcerative colitis (UC) and Crohn's disease (CD).1 IBD has a serious impact on patients' quality of life, social functioning, and psychological health.1 The confounding pathogenesis and complex clinical features of the natural course of relapsing-remitting IBD result in its inherent complexity and hinder accurate diagnosis and development of precision medicine.2
Compared with UC, CD is a more progressive and destructive disease, with more than half of the patients developing intestinal or extra-luminal complications (such as strictures or penetrating diseases) within 10 years post diagnosis.3 Precise individual stratifying methods hold the promise of improving the management of CD, which may minimise the risk of therapy.4
Cross-sectional imaging, which includes computed tomography enterography (CTE), magnetic resonance enterography (MRE), and ultrasonography, is a key routine examination for patients with CD.5 It can depict a broader perspective of the gastrointestinal tract and detect small bowel inflammation in patients with CD who are identified as normal on endoscopic examination.5 Cross-sectional imaging has played an increasingly important role before, during, and after treatment in CD. A comprehensive and in-depth understanding of recent advances in the field of CD imaging is helpful for improving the quality of diagnosis and therapy for patients with CD. Therefore, the purpose of this review is to provide an update on cross-sectional imaging advances for CD (Figure 1). In addition, future developmental directions of cross-sectional imaging that could improve the understanding of CD pathological processes are discussed.
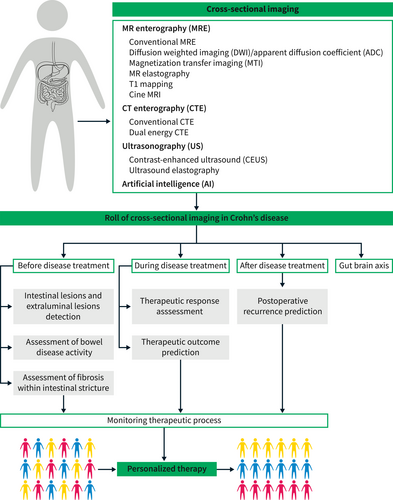
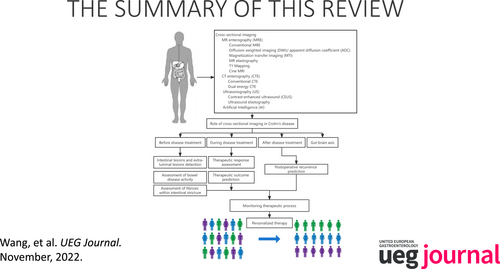
The summary of this review.
THE ROLE OF CROSS-SECTIONAL IMAGING BEFORE CD TREATMENT
Intestinal lesions detection
Although ileocolonoscopy offers a sensitivity of 74%–100% for detecting ileocolic CD6 and capsule endoscopy shows a sensitivity of 90% and specificity of 100% for small bowel CD,7 up to 50% of patients with CD with a normal endoscopic finding illustrates mural abnormalities on cross-sectional imaging,8 since the endoscopy only offers the visualisation of intestinal mucosa. Hence, cross-sectional imaging is an indispensable supplementary tool to endoscopy.
Findings on CTE or MRE of segmental hyperenhancement and wall thickening are highly specific for detection of bowel lesions in patients with suspected CD.9 The accuracy of conventional cross-sectional imaging techniques for the diagnosis of CD is dependent on disease severity. It is inferior to endoscopy for detection of mild lesions.10 In a meta-analysis including 33 studies, the accuracy of ultrasound, MRE and CTE in the diagnosis of IBD were compared with a predefined reference standard.11 For per patient, mean sensitivity (90%, 93% and 84% for ultrasound, MRE and CTE, respectively) and specificity (all over 90%) estimates of the three imaging modalities for diagnosis of CD were high without significantly different among them. For per bowel segment, their mean specificity for diagnosis of CD were high (93%, 94% and 90% for ultrasound, MRE and CTE, respectively), but mean sensitivity was not satisfactory (74%, 70% and 68% in ultrasound, MRE and CTE, respectively).11 In another recent meta-analysis, the sensitivity and specificity of CTE for diagnosis of patients with suspected CD were 87% and 91%, respectively; while the sensitivity and specificity of MRE were 86% and 93%, respectively.12 Performance of cross-sectional imaging in diagnosis of colon and small bowel CD are summarised in Table 1.
| Imaging modalities | Sensitivity | Specificity |
|---|---|---|
| Colon | ||
| CTE | 60%–90% | 90%–100% |
| DECT | 74%–92% | 11%–97% |
| MRE | 78%–100% | 46%–100% |
| Ultrasonography | 63%–100% | 77%–100% |
| Small bowel | ||
| CTE | 74%–93% | 64%–100% |
| DECT | NA | NA |
| MRE | 78%–93% | 85%–94% |
| Ultrasonography | 80%–90% | 93%–98% |
- Abbreviations: CTE, computed tomography enterography; DECT, dual-energy computed tomography; MRE, magnetic resonance enterography.
Compared with conventional CTE, dual-energy CTE simultaneously acquires information from high and low levels of energy, which can enhance iodine sensitivity and maximise the image enhancement contrast. Therefore, dual-energy CTE has the advantage of detecting subtle bowel wall thickening or mural enhancement in the early stage of CD using virtual monochromatic imaging or iodine density mapping and can further clearly depict the boundary between the inflamed and normal bowel wall, covering the deficiency of conventional imaging in detecting mild lesions.13 Noteworthily, dual-energy CTE has similar scanning protocol as conventional CTE but produce higher image quality and less image artifacts, without significant increase in radiation dose (Figure 2).14
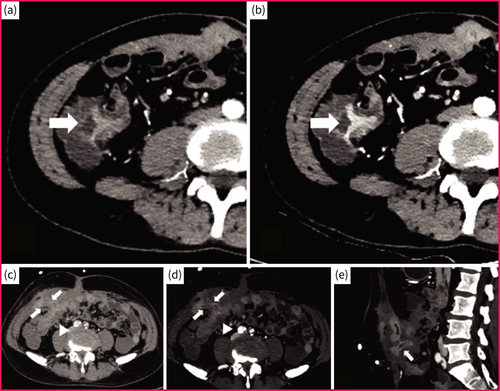
Dual-energy computed tomography enterography images of a 32-year-old (a, b) and a 28-year-old man (c–e) with Crohn's disease. Axial contrast-enhanced image (reconstructed conventional polychromatic image at 120 kVp) shows mild bowel thickening on terminal ileum with iso-enhancement or mild hyper-enhancement (arrow), which mimics the normal bowel and results in a misdiagnosis (a). Under axial mono energetic 45 keV scanning, the bordering of inflamed terminal ileum (arrow) is more clearly depicted due to the improved tissue enhancement from the inflamed to normal bowel, facilitating the improvement of diagnostic accuracy (b). Similarly, axial contrast-enhanced image (reconstructed conventional polychromatic image at 120 kVp) shows active Crohn's disease in terminal ileum (arrowhead) with suspected bowel fistula and abscess (arrows; c), and these penetrating diseases are confirmed on the axial and sagittal mono energetic 45 keV images (d, e) due to the direct imaging evidence of penetrating diseases (i.e. fistula and/or ring-enhancement; arrows) are more clearly illustrated.
However, radiation exposure is still a major limitation of CTE, influencing its extensive use in paediatric patients with CD. MRE and ultrasonography are alternative imaging modalities with the advantages of lacking ionising radiation. In a multi-centre clinical trial, for the detection of inflamed bowels, the sensitivity and specificity of MRE were reported to be 80% and 95%, respectively, and those of ultrasound were range from 74% to 96% and from 80% to 100%, respectively.10 Notably, the accuracy of ultrasonography highly depended on the site of disease, with the highest sensitivity obtained in detecting ileal or left colonic lesions (ileum, 92%; left colon, 87%) but not proximal small bowel and rectal lesions (small bowel, 29%; rectum, 14%).15
30%–40% of CD affected the small bowel, whereas in minority of patients (15%–25%), CD is confined to the colon.16 The role of image techniques in small bowel imaging is well discussed before. However, the detected ability of images in colon disease is needed to be further explored. In some studies, the detection rate in colon was lower than that in small bowel disease (Table 1). The possible reason is that the luminal distension or cleaning enema is not satisfied to diagnosis which producing false negative or false positive results. Compared with using luminal contrast, lower sensitivity was observed in detecting colonic lesions when not using it.17 According to our experience, that patients who suspected to have colonic lesions ingested a polyethylene glycol electrolyte solution for 6–8 h, followed by 1600–2000 ml of 2.5% mannitol solution as an oral contrast agent 1 h before MRE or CTE, can clean and distend colon and obtain satisfied image quality of colon, increasing the diagnostic accuracy rate in MRE or CTE.
Extraluminal lesions detection
CD is a transmural inflammation accompanied by extra-luminal manifestations, such as penetrating diseases (intestinal fistula or abscess) and creeping fat. The sensitivity and specificity of CTE for detecting penetrating diseases that occurring in both small and large bowel ranged from 20%–100% and 91%–100%, respectively.10 Conventional MRI is slightly more accurate than CTE in detecting penetrating complications, with a sensitivity of 40%–100% and a specificity of 93%–100%,18 and its detection can be enhanced after combining it with diffusion-weighted imaging (DWI).19 A similar rate of detection was reported for ultrasonography compared to that with CTE and MRE in identifying penetrating lesions with the sensitivity ranged from 67% to 87% and the specificity ranged from 90% to 100%.10 However, the accuracy of ultrasonography further depends on the physicians' experience of detectors and the location of certain anatomic areas (especially the stomach, deep pelvic part of the sigmoid, and rectum) to be examined.10
Perianal fistulas are a poor prognostic indicator in patients with CD. Approximately 25% of patients develop perianal fistulas during their lifetime. Although the sensitivity of CTE for detecting perianal fistulas was reported to be 70% with a specificity of 97% based on the reference of surgery and endoscopy, CTE is not the best tool for the detailed evaluation of perianal diseases owing to its suboptimal soft tissue resolution.10 Perianal MRI is recognised as the optimal examination method for perianal diseases with a sensitivity and specificity for detection of perianal fistula tracks (100% and 86%, respectively), abscesses (96% and 97%), internal fistula openings (96% and 90%) and horseshoe fistulas (100% and 100%).20 Combining conventional MRI sequences with DWI increases clinicians' confidence in diagnosing perianal fistulae.21 Moreover, the conversion of MRI scans to 3D images could reveal spatial structures on a 1:1 scale with unprecedented precision, allowing a more precise visualisation of perianal fistula before surgery than conventional MRI alone.22 Ultrasound, especially transperineal ultrasound, is also a valuable tool for determining perianal disease in patients with CD, holds a sensitivity of 87% and a specificity of 43%.23 However, it may be limited by unbearable pain if open cutaneous fistulas are present on the perianal skin.
Creeping fat, which is recognised as a hallmark of CD, is associated with transmural fibrosis and stricture formation and regarded as a significant factor in predicting adverse outcomes in patients with CD. Unfortunately, creeping fat can be observed only pathologically or during surgery. Cross-sectional imaging can support the visualisation of 2D or 3D information of mesenteric adipose tissue or creeping fat to some extent. The visceral-to-subcutaneous fat area ratio has been widely reported as a 2D imaging marker to identify patients with aggressive CD.24 This index may partly reflect the degree of hyperplasia of mesenteric adipose tissue; however, it fails to depict the anatomic morphology of the creeping fat. Recently, another imaging marker, the mesenteric creeping fat index, was developed based on the extent of bowel circumference encompassed by the vessels in fat, with a score from 1 to 8.25 It was reported as an accurate parameter to semi-quantitatively characterise the degree of creeping fat wrapping around the inflamed gut in the reference of surgical specimens and enabled the characterisation of fibrotic severity within strictures (area under curve [AUC] of 0.799 in differentiating moderate–severe from mild fibrostenosis; Figure 3).25 It should be noted that these reported imaging indices focused more on quantity than on the quality of adipose tissue. The cause of this knowledge gap may be because of the lack of powerful analytical techniques required to capture these pathophysiological changes within the creeping fat.
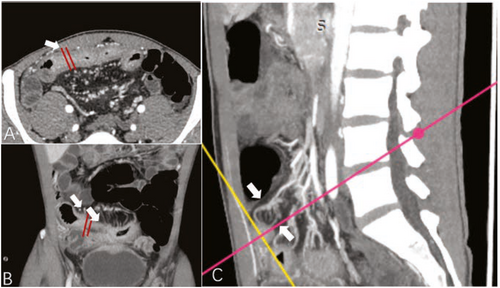
Images from a male patient with Crohn's disease. Transverse (a), coronal (b), and sagittal (c) enhanced CT images showed bowel wall thickening and luminal narrowing of the jejunum (arrow); the MCFI of the designated segment reconstructed from the adjacent mesenteric vessels was scored with 2, because a quarter of the bowel surface was covered by the mesenteric vessels. Note: The MCFI, which reflects the degree of mesenteric fat wrapping around the gut, is scored from 1 to 8 according to the areas of bowel surface covered by the corresponding mesenteric vessels. CT, computed tomography; MCFI, mesenteric creeping fat index.
Assessment of bowel disease activity
Besides confirmed diagnosis of CD, the characterisation of the bowel inflammation activity is crucial for assessment of disease burden. A meta-analysis showed that MRE and CTE are highly accurate for grading CD activity, with the accuracy of 86% and 84% on a per-patient basis, respectively.26 Contrast-enhanced ultrasound can quantitatively illustrate the bowel wall inflammation, because the microbubble contrast agents can perfuse into those areas with active bowel disease, shows increased blood supply. Severity of contrast-enhanced ultrasound in bowel assessment has been demonstrated to be correlated with endoscopic severity (r = 0.90).27 The imaging findings indication of active inflammation are summarised in Table 2. Based on these imaging findings, several imaging scoring systems have been developed to measure and monitor inflammation and to better stratify the risk of patients with CD (Table 3). Previous studies had showed good correlations in grading bowel inflammation between imaging scores and endoscopic scores.28, 29, 34, 35 The Magnetic Resonance Index of Activity (MaRIA) score, one of the most common reported imaging scores, showed high accuracy for detecting active CD (AUC = 0.89).28 Since this index has some limitations such as extra high time-consuming, a simplified version of the MaRIA score (sMaRIA) was developed and validated. It has been reported that the value of sMaRIA≥1 accurately identified active bowel segments with the sensitivity of 90%, specificity of 98%, AUC of 0.94 and correlated well with simpler endoscopy index (r = 0.94 for ileal and r = 0.82 for colon).29 However, the clinical value of these scoring systems still need to be validated in more as well as larger cohorts.
| Imaging findings | Description/definition | Detection in techniques |
|---|---|---|
| Segmental mural hyperenhancement | Increased attenuation/signal intensity from contrast-enhanced scan on bowel segment in comparison with nearby normal bowel segments | CT, MRI, US |
| Wall thickening | Bowel wall thickening >3–5 mm, 3 mm, 3–4 mm from CT, MRI, US, respectively | CT, MRI, US |
| Intramural edema | The grey attenuation layer of the water halo sign in CT represents edema in submucosa; hyperintense signal from fat-saturated T2-weighted scan in MRI | CT, MRI |
| Stricture | Luminal narrowing (at least 50% reduction of luminal diameter in CT or MRI, <10 mm in CT or US) in area of Crohn's disease with unequivocal upstream dilation (luminal diameter is >3 and 2.5 cm in CT and US; 1.5-fold greater than normal loop in MRI) | CT, MRI, US |
| Ulcerations | Appear as small focal breaks in the intraluminal surface of the bowel wall with focal extension of air or enteric enhancement from the inflamed bowel wall | CT, MRI, US |
| Fistulas | Appears as an extra-enteric tract, with or without internal air or fluid. Including simple/complex fistulas, sinus tract and perianal fistulas | CT, MRI, US |
| Inflammatory mass | Ill-defined mass-like process of mixed fat and/or soft tissue attenuation/signal intensity (non-water attenuation/signal intensity) usually associated with penetrating disease, such as complex fistulas | CT, MRI, US |
| Abscess | Mesenteric/peritoneal/perianal fluid collection with rim enhancement and/or internal air | CT, MRI, US |
| Perienteric edema/inflammation | Increased attenuation (CT) or high fat-saturated T2-weighted signal (MR) in mesenteric fat adjacent to abnormal bowel loops | CT, MRI |
| Comb sign | Engorged vessels that supply an inflamed bowel loop | CT, MRI, US |
| Fibrofatty proliferation | Increased fat adjacent to abnormal bowel, displacing bowel loops; usually along mesenteric border, but can be circumferential | CT, MRI, US |
| Adenopathy | Lymph node >1.5 cm (in short axis) | CT, MRI |
- Abbreviations: CT, computed tomography; MRI, magnetic resonance imaging; US, ultrasonography.
| Features | Calculation formula | Inflammatory activity grading | Strengths | Limitation | Reference | |
|---|---|---|---|---|---|---|
| MRI | ||||||
| MaRIA score |
|
1.5 × wall thickness (mm) + 0.02 × relative contrast enhancement + 5 × edema + 10 × ulceration |
|
|
|
Rimola et al28 |
| sMaRIA |
|
(1 × thickness >3 mm) + (1 × edema) + (1 × fat stranding) + (2 × ulcers) |
|
|
Incapable of entire small bowel valuation | Roseira et al29 |
| Clermont score |
|
−1.321 × ADC (mm2/s) + 1.646 × wall thickening + 8.306 × ulcers + 5.613 × edema + 5.039 |
|
|
|
Buisson et al30 |
| London score |
|
1.79 + 1.34 × mural thickness + 0.94 × mural T2 score | Score ≥4.1: active CD |
|
Does not evaluate the entire small bowel | Steward et al31 |
| MEGS |
|
Score per segment × multiplication score per segment (jejunum, ileum, terminal ileum, cecum, ascending, transverse, descending, sigmoid and rectum based) + additional score per patient (lymph node + comb sign + abscess + fistula) | MEGS >10: active disease |
|
|
Makanyanga et al32 |
| US | ||||||
| Semiquantitative bowel ultrasound scoring system |
|
(2.5 × US pattern) + (1.5 × bowel thickness) + (3 × presence of fistulas/abscesses) + (1.5 × presence of stenosis) | Score >3: Predicted the high risk of surgery | Non-radiation | Unknown reproducibility | Rigazio et al33 |
- Abbreviations: ADC, apparent diffusion coefficient; CD, Crohn's disease; MaRIA, magnetic resonance index of activity; MEGS, magnetic resonance enterography global score; MRI, magnetic resonance imaging; sMaRIA, simplified MaRIA; US, ultrasonography.
Assessment of fibrosis within intestinal stricture
Intestinal strictures are the main morbidity factor in patients with CD. More than 30% of patients with CD develop fibrotic stricture within 10 years from diagnosis.36 The core objective of imaging diagnosis in strictures is to differentiate fibrostenosis from the inflammatory component, because patients who developed fibrotic strictures are recommended endoscopic or surgical intervention, whereas inflammatory strictures may be relieved by anti-inflammatory therapy. To date, the differentiation of fibrosis from inflammation remains challenging because non-invasive assessment of bowel fibrosis is difficult. To solve this problem, several novel imaging techniques have been explored and adopted to detect bowel fibrosis.
Magnetisation transfer magnetic resonance imaging
Magnetisation transfer imaging (MTI) is a method with the mechanism of determining of the fraction of large macromolecules or immobilised phospholipid cell membranes in tissue, such as collagens.37 The MT ratio (MTR) is a quantitative parameter index used to characterise the magnetisation transfer effect. MTI is reported to show a satisfactory ability to grade bowel fibrosis.38-40 The normalised MTR (i.e. MTR of inflamed gut divided by MTR of muscle) increases with the fibrotic severity of bowel wall in patient with CD. Significant difference was found among nonfibrotic (0.51 ± 0.07), mildly (0.67 ± 0.13), moderately (0.77 ± 0.11), and severely (0.88 ± 0.14) bowel wall (p = 0.000).40 Using a cut-off value of 0.71, normalised MTR can differentiate moderate-severe fibrotic from non-mild fibrotic segment with a sensitivity of 84.42% and a specificity of 90%.40 Noteworthily, the diagnostic performance of MTI was not affected by the severity of coexisting inflammation. Therefore, it will offer a stable and reliable MTR in assessing fibrosis. Hence, MTI can potentially help clinicians formulate individualised treatment strategies.
MR elastography
Magnetic resonance (MR) elastography is a technique used for the quantitative assessment of tissue stiffness.41 It uses additional hardware to generate propagating shear waves and induces micromovements inside the tissue.41 A stiffness map can be generated and used for quantitative measurements by analysing the local wavelength, which is depicted with a phase-contrast sequence. One prospective CD study demonstrated the role of MR elastography in the grading of intestinal fibrosis.42 In this study, a stiffness of over 3.57 kPa was shown to be a reliable predictor of a worse disease outcome with the AUC of 0.82. Compared with ultrasound elastography, MR elastography is more likely to reveal the entire spectrum of the small bowel. Studies using MR elastography for intestinal diseases are scarce. Further exploration of the role of MR technology in CD is necessary.
T1 mapping
T1 mapping can depict the smallest variations in T1 values within tissues, which are based on a tissue's intrinsic longitudinal magnetisation relaxation time and rises with increasing extracellular water.43 It has been demonstrated to have good performance in the evaluation of fibrosis in other organs, such as the myocardium and liver.44 A preclinical CD study model demonstrated the potential value of T1 mapping in grading the severity of bowel fibrosis, in which the T1 value of none-to-mild fibrotic bowel walls was lower than that of moderate-to-severe ones.45 Although the feasibility of T1 mapping for characterising bowel lesions of patients has been confirmed in a patient-based study (inflammatory activity was measured using T1 mapping), its ability to assess intestinal fibrosis in patients with CD requires further research (Figure 4).46
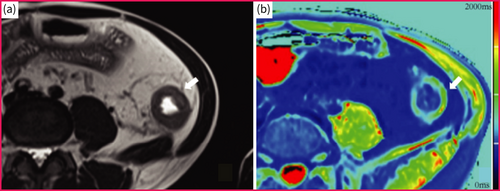
T1 mapping of a f Crohn's disease suffered female patient with moderate-to-severe fibrosis in descending colon. (a) Axial T2-weigheted image showed bowel wall thickening of the descending colon. (b) The T1 mapping shows that the T1 value of affected descending colon was 1430 ms.
‘CINE’ MRI
Owing to the revolution of image diagnosis methods from barium small bowel follow-through to CTE and MRE, the degree of physiological information (e.g. degree of change in bowel movement) was lost. Motility or ‘CINE’ MRI allows acquisition of a series of images at the same anatomical location for a period of time (Video S1 showing normal bowel motility of a healthy volunteer). The dynamic loop movement in the bowel segment provides additional information about equivocal or poorly distended loops, as seen from conventional T2-weighted or T1-weighted imaging in patient with CD.47 It was reported that the decreased motility may be associated with the fixed small bowel stricture (Video S2 showing decreased sigmoid motility of a patient with sigmoid CD).47 Bowel motility can be affected by several mechanisms, including restriction of the mesentery, fat wrapping, and fibrosis involving the bowel wall or serosa, which may lead to a sophisticated motility pattern within or beyond bowel lesions.48 Motility MRI enables real-time observation of anatomical changes in the inflammatory bowel and can be used for therapeutic management.
Ultrasound elastography
Ultrasound elastography, including strain elastography and shear wave elastography (SWE), has demonstrated with ability to assess bowel fibrosis by measuring tissue stiffness. The former method measures the degree of tissue deformation with external compression and transfers the value into a real-time colour spectrum, instead of direct pressure; SWE applies energy to the tissue through a pulse wave that is propagated throughout the tissue according to its stiffness.49 Series of studies on ultrasound elastography showed significantly different values between normal and strictured segments (14.4 ± 2.1 kPa vs. 23.0 ± 6.3 kPa, p = 0.008), and correlated well with the severity of pathological bowel fibrosis (r = 0.536, p < 0.001) (Figure 5).50 Compared with B-mode ultrasonography alone, combination of strain elastography with B-mode ultrasonography may increase the accuracy of the visual differentiation of fibrotic bowel segments from inflammatory segments (accuracy, 25% vs. 75%).51 However, colour scale interpretation of ultrasound elastography can be affected by peristaltic movement, which needs to be considered when interpreting it in practical applications.
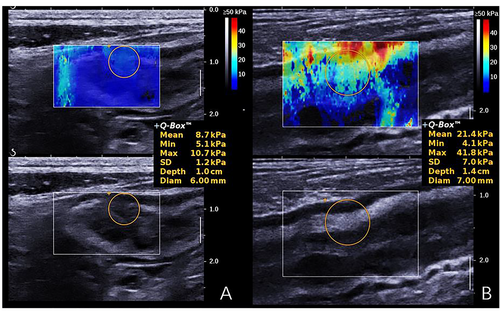
Examples of shear wave elastography for diagnosis of different degrees of bowel fibrosis in a 32-year-old (a) and a 47-year-old male patient (b) with Crohn's disease. The mean shear wave elastography value of mildly fibrotic bowel wall (a) was significantly lower than that of severely fibrotic bowel wall (b) (8.7 vs. 21.4 kPa).
AI in stricture
The advent of artificial intelligence (AI), particularly machine learning, has opened an avenue for the efficient integration and interpretation of big datasets in discovering clinical imaging information. Radiomics, a machine learning technique, involves the computer-based extraction of large amounts of high-dimensional features from images and uncovers disease characteristics that are invisible to clinicians.52 In a multi-centre study reported by Li et al., a CTE-based radiomic model was developed with four selected features using logistic regression.53 This radiomic model enabled the accurate differentiation of moderate-severe from non-mild intestinal fibrosis in CD with an AUC of 0.888 in the training cohort and 0.832 in the test cohort, showing remarkable robustness in different inflammatory severities, CD locations, and CT scanners.53 However, this CTE-based radiomic model was a subjective model because the radiomic feature extraction was handcrafted and was ectra time-consuming for manual segmentation of the bowel. Deep learning is a series of machine learning algorithms that automatically extract features with an outstanding ability to decode the contents of images. Hence, Li et al. further developed a deep learning model using a 3D deep convolutional neural network based on the same patient cohorts, showed an AUC of 0.828 in the training cohort and 0.811 in the test cohort for fibrosis diagnosis.54 The diagnostic performance of the deep learning model was not inferior to that of the prior radiomic model, but it was more time-saving than the radiomic model (48.4 vs. 599.8 s).54 Notably, both radiomic and deep learning models significantly outperformed the radiologist's interpretation of fibrosis grading.53 Hence, the application of AI has the potential to help radiologists diagnose fibrosis more accurately and quickly. The role of cross-sectional imaging based AI in CD has been listed in Table 4.
| Type of subjects | Type of study design | Sample size | Study results | Reference | AUC in the training cohort | AUC in the test cohort |
|---|---|---|---|---|---|---|
| Adult patients with CD | Retrospective multicenter study | 212 bowel lesions of 167 CD patients | This radiomic model enabled the accurate differentiation of moderate-severe from non-mild intestinal fibrosis in CD, showing remarkable robustness in different inflammatory severities, CD locations, and CT scanners | Li et al53 | 0.888 | 0.832 |
| Adult patients with CD | Retrospective multicenter study | 312 bowel segments of 235 CD patients | Based on the same patient cohorts mentioned above (reference52), the diagnostic performance of the deep learning model developed by using a 3D deep convolutional neural network was not inferior to that of the radiomic model, but it was more time-saving than the radiomic model (48.4 vs. 599.8 s) | Meng et al54 | 0.828 | 0.811 |
| Paediatric patients with CD | Retrospective single institution study | 64 bowel segments of 25 patients | Texture analysis of enteric phase T1-weighted fat suppressed postcontrast MRI images can distinguish fibrotic from nonfibrotic strictures, providing a noninvasive biomarker of stricture composition that can guide therapy arrangement | Tabari et al55 | NA | 0.995 |
| Rat model of colitis | Retrospective animal study | 45 rat model of inflammation (10 control and 35 irradiated with visible lesions) | This approach offers practitioners a valuable tool to evaluate antifibrotic treatments under development and to extrapolate such noninvasive MRI scores model for patients with the aim of identifying early stages of fibrosis improving disease management | Morilla et al56 | NA | 0.875 |
- Abbreviations: AUC, area under the curve; CD, Crohn's disease; IBD, inflammatory bowel disease; MRI, magnetic resonance imaging.
THE ROLE OF CROSS-SECTIONAL IMAGING DURING CD TREATMENT
Therapeutic response assessment
Cross-sectional imaging, especially MRE, can provide disease information regarding improvement or deterioration by revealing anatomical changes after treatment. In the therapeutic response assessment of CD, mucosal and transmural healing are the two novel concerns in clinical management.
Mucosal healing is associated with significant improvements in health-related quality of life and lower risk of disease progression.57 Endoscopy is regarded as the gold standard for determining mucosal healing; however, its invasiveness limits its repeated use during disease monitoring. In this context, some MRE scoring systems have been used as alternative tools for assessing mucosal healing.58 MaRIA was reported as a promising index for assessing therapeutic response in patients with CD with an accuracy of 90% in determining ulcer healing and 83% in assessing endoscopic remission, and MaRIA <7 is identified as an effective indicator of mucosal healing with a sensitivity of 85% and specificity of 78%.59 Moreover, deep MR remission (no segmental MaRIA >7/no segmental Clermont >8.4) could be used to predict mucosal healing with a specificity >85%.60 However, these MRI scoring systems used for the assessment of mucosal healing should be carefully validated in a larger patient cohort.
CD is characterised by transmural inflammation, that is, active intramural inflammation can occur concurrently with endoscopically demonstrated mucosal healing.61 Currently, transmural healing has been proposed as a potential new treatment endpoint for patients with CD. Preliminary studies have shown that transmural healing is associated with lower rates of drug escalation, CD-related hospitalisation, and surgery.62 A recent intestinal ultrasound study defined transmural healing as bowel wall thickness ≤3 mm without hyperaemia on colour Doppler, inflammatory fat, or disrupted bowel wall stratification, which showed that sonographic healing is associated with improved clinical outcomes, such as reduced risk of medication escalation (p = 0.0018), corticosteroid use (p = 0.0247), hospitalisation (p = 0.0102), and surgery (p = 0.083), therefore along with clinical remissions in patients with CD.63 Generally, the most important and reliable parameter for predicting transmural healing is the normalisation of bowel wall thickness (>3 mm being the most common threshold for pathology) with normalisation of stratification, no hypervascularisation, or resolution of mesenteric inflammatory fat on cross-sectional images.64 However, a recognised imaging definition of transmural healing has not yet been established. Further studies are needed to determine whether transmural healing, assessed using cross-sectional images, is a treatment target.
Therapeutic outcome prediction
Over the past decades, with the revolution of biological agents, the management of CD has significantly changed. Unfortunately, up to one-third of patients are primarily non-responsive to biologic agents, and some patients who show an initial response can also lose response over time. Additionally, the high cost and adverse events of biological agents also indicate the need to evaluate the risk of a nonresponse as a component of ‘precision medicine’.
Cross-sectional imaging can provide information about the inflammatory bowel, which correlates with therapeutic response. In a prospective CD study, lower apparent diffusion coefficient (a quantitative index of DWI that characterising the diffusion of water molecules in biologic tissue) values were correlated with clinical and biochemical remission under anti-tumour necrosis factor (TNF) therapy (1.89 ± 0.25 mm2/s vs. 2.05 ± 0.22 mm2/s, remission vs. no remission at week 12).65 Ultrasonography strain elastography also enabled to predict the therapeutic response to anti-TNF therapy in patients with CD. Orlando et al. reported that the surgery-free survival was significantly reduced in patients with strain ratio (ratio between strain of the bowel wall and the mesenteric tissue) ≥2 as compared with those with strain ratio <2 after anti-TNF therapy treatment.66 Additionally, perfusion index delivering from the contrast-enhanced ultrasound is another promising index to predict the therapeutic response. It was reported that the contrast enhanced ultrasound parameter of increased wash-out rate showed an odds ratio of 0.76 in predicting treatment response to anti-TNFα.67 However, theoretically, the prediction of therapeutic outcome is complex and difficult to achieve using limited routine parameters. Cross-sectional image-based AI approaches may pave the way to address this issue. Recently, Chen et al. reported that a radiomic model with 2D texture analysis of the intestinal segment on CTE images could predict secondary loss of response to infliximab in patients with CD.68 This promising result demonstrates the feasibility of applying AI to clinical decision support systems.
THE ROLE OF CROSS-SECTIONAL IMAGING IN THE POST-OPERATIVE SETTING
Surgical resection is an established treatment for CD, especially in patients who are refractory to aggressive medical therapy or develop fibrotic strictures. Postsurgical recurrence is very common and is observed endoscopically in almost 73% of patients within 1 year and in almost 90% of patients within 3 years after surgery, even with the absence of symptoms.69 Prediction of postoperative recurrence is crucial because risk stratification can help identify patients who would most benefit from intensive monitoring and potentially prevent unnecessary treatment in patients with a low recurrence risk.
MRI has been reported to be valuable for predicting the risk of clinical recurrence in patients with postoperative CD.70 An MRI index developed using seven items (bowel wall thickness, contrast enhancement, T2WI hyperintensity, DWI hyperintensity, mural oedema, ulcers, and the length of diseased segment) was significantly associated with Rutgeerts score and was reported as an easy-to-apply tool that can be used in clinical practice to predict the postoperative recurrence with a AUC of 0.85 in an independent test cohort.71 Additionally, Maconi et al. demonstrated a significant correlation between the length of bowel wall thickening and surgical recurrence in a prospective study based on ultrasonography image features (28.4 ± 10.7 vs. 21.0 ± 11.2 cm, p = 0.04).72
ROLE OF BRAIN MR IMAGING IN GUT-BRAIN AXIS RESEARCH IN CD
Recent studies have pointed to gut-brain axis dysfunction as a key player in the occurrence and development of CD. Disclosing the neurological characteristics of patients with CD may help shed light on its pathogenesis and provide potential therapeutic targets. Neuroimaging and bowel MRE of the gut-brain axis are vital for disclosing specific effects of gut microbiota on brain structure and function, as well as for revealing the correlation between the gut, brain, and microbiome.
Prior CD studies based on functional MRI have demonstrated abnormal neural activity and functional connectivity, regional homogeneity, and amplitude of low-frequency fluctuations in various regions primarily associated with emotion, pain, and cognitive-related functions, which would provide information to further understand the neural mechanisms of CD.73 Moreover, multimodal MRI that combines functional and structural MRI can provide additional information.74 Prior multimodal studies of voxel-based morphometry and functional connectivity indicated that differences were observed in the insula, paracentral lobule, cingulate gyrus, and medial frontal gyrus, which are related to the mental state, pain, and quality of life of patients with CD. These neural correlations potentially serve as useful biomarkers for evaluating the treatment efficacy and differential points for diagnosing diseases.
Notably, the anterior cingulate cortex has been widely studied in all brain structures in CD.75 Recent MR spectroscopy study showed that patients with CD experiencing abdominal pain have an imbalance in glutamate and γ-aminobutyric acid levels in the anterior cingulate cortex, whereas another CD study showed that lower grey matter volumes in the anterior cingulate cortex correlated strongly with higher pain scores.76 In terms of function, connectivity within the default mode network was increased in the anterior cingulate cortex and decreased between the anterior cingulate cortex and amygdala, which may be attributed to negative emotions and changes in gut microbiota.77 Collectively, multidimensional brain MRI data suggest that the anterior cingulate cortex is associated with pain and depression in patients with CD, indicating that the anterior cingulate cortex might be a potential neural alternative for managing the progression of CD.
Moreover, prior diffusion tensor imaging study proved that alterations (significantly decreased fractional anisotropy values on regions of the bilateral cingulum and significantly increased mean diffusivity values on regions of the left cingulate gyrus, left inferior fronto-occipital fasciculus and bilateral superior longitudinal fasciculus) in white matter of brain was responsible for psychological related perceptions and decreased language function.78 Results from another verbal fluency task-based brain activation fMRI study in remission CD showed a higher bi-hemispheric activation compared to controls.79 Besides of focussing on the emotional and pain aspects of patients with CD, changes in language and other networks are also worthy of our attention. Further research with larger cohort is needed to verify this link and to explore more connection between gut and brain function.
FUTURE DIRECTION AND CONCLUSION
Precision medicine holds great promise for improving the landscape of the CD course of care for individual patients. AI, which has a powerful ability in information processing, has a promising future in CD diagnosis. Standardisation of data and large-scale validation of cohorts from different international centres are needed to improve the performance of AI in clinical management.
Molecular imaging of CD may play an essential role in improving the understanding of its pathophysiology by targeting molecular and cellular events.80 Future studies should focus on the development of different types of cross-sectional molecular imaging, such as hyperpolarised 13C spectroscopic MRI or 68Ga-FAPI PET/CT, to investigate the pathophysiology of CD by targeting cellular receptors or labelling therapeutic agents.
The gut-brain-microbiome axis is an emerging research field in CD. Cross-sectional imaging, especially functional and structural brain MRI, has been increasingly applied to study neurological characteristics in patients with CD. Future studies should also aim to integrate multiple ‘omics’ techniques, such as radiomicrobiomics, and explore the causality between the gut and brain.
A new era in the management of CD is emerging. The role of cross-sectional imaging in CD is indispensable but still need deep exploitation and investigation. Along with the illuminating of pathogenetic mechanisms in CD, new options in cross-sectional imaging would be appeared in future and therefore feedback to the disease management.30-33, 55, 56
ACKNOWLEDGEMENTS
The authors thank Zhoulei Li (Department of radiology, The First Affiliated hospital of Sun Yat-Sen University, China) for commenting on this revised manuscript and editing the language carefully. The authors also thank Yujun Chen (Department of Ultrasound, The First Affiliated hospital of Sun Yat-Sen University, China) for providing ultrasound images for this manuscript. This study was supported by National Natural Science Foundation of China (82070680, 82072002).
CONFLICTS OF INTEREST
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. Xue-hua Li takes responsibility for the integrity of the work as a whole, from inception to published article. All authors drafted and edited the manuscript, and approved the final version of the article, including the authorship list.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable—no new data generated, or the article describes entirely theoretical research.