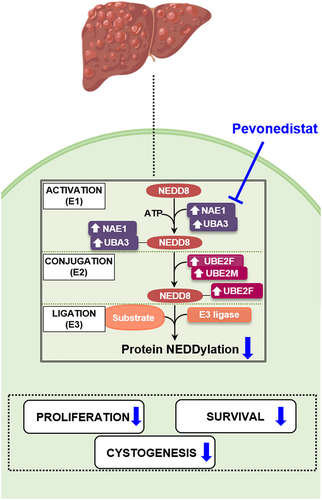
Inhibition of NAE-dependent protein hyper-NEDDylation in cystic cholangiocytes halts cystogenesis in experimental models of polycystic liver disease
Pui Y. Lee-Law and Paula Olaizola contributed equally.
Joost P. H. Drenth and Jesus M. Banales share co-seniorship.
Abstract
Background
Polycystic liver diseases (PLDs) are genetic inherited disorders characterized by the progressive growth of numerous intrahepatic biliary cysts, which are the main cause of morbidity. Previous studies revealed that cystic cholangiocytes are characterized by endoplasmic reticulum stress and aberrant posttranslational modification (PTM) of proteins, in particular hyper-SUMOylation, that promote PLD pathobiology. Protein NEDDylation is a newly characterized PTM that modulates a plethora of biological processes and its dysregulation is associated with the development and progression of several human diseases. However, the role of NEDDylation in PLD remains elusive.
Objective
To explore the role of protein NEDDylation in PLD and its potential therapeutic regulatory value.
Methods
Levels and functional effects of NEDDylation, including response to Pevonedistat (first-in-class selective inhibitor of the NEDDylation E1 enzyme NAE), were assessed in vitro, in vivo, and/or in patients with PLD. NEDDylated protein levels in normal and cystic human cholangiocytes were assessed by immunoprecipitation, and the proteomic profile was further analyzed by mass spectrometry.
Results and Conclusion
The genes involved in the NEDDylation pathway were found overexpressed (mRNA) in polycystic human and rat liver tissue, as well as in cystic cholangiocytes in culture, compared to controls. Elevated levels of NEDDylated proteins were further confirmed in cystic cholangiocytes in vitro, which diminished under Pevonedistat incubation. Pevonedistat promoted apoptotic cell death and reduced proliferation in cystic cholangiocytes in vitro. Comparative proteomic profiling of NEDD8-immunoprecipitated proteins between normal and cystic cholangiocytes in culture reported candidate proteins involved in cystogenesis, mostly associated with protein biogenesis and quality control. All these data indicate that cystic cholangiocytes display increased protein NEDDylation, contributing to cell survival and proliferation, ultimately supporting hepatic cystogenesis. Targeting of protein hyper-NEDDylation in cystic cholangiocytes inhibits cystogenesis in experimental models, representing a novel therapeutic opportunity in PLD.
Graphical Abstract
INTRODUCTION
Key summary
Summarize the established knowledge on this subject
-
Polycystic liver diseases (PLDs) are genetic inherited disorders characterized by progressive growth of intrahepatic biliary cysts.
-
Most PLD-associated genes code for endoplasmic reticulum (ER)-resident proteins.
-
Cystic cholangiocytes are characterized by ER stress, aberrant post-translational modification (PTM) of proteins and abnormal protein homeostasis.
-
Protein hyper-SUMOylation promotes PLD pathobiology.
What are the significant and/or new findings of this study?
-
Protein NEDDylation is dysregulated in PLD, reinforcing the abnormal protein dynamics.
-
Hyper-NEDDylated proteins in PLD are mostly associated with protein biogenesis and quality control.
-
Targeting protein hyper-NEDDylation increases apoptosis and reduces proliferation of cysticcholangiocytes.
The great majority of mutations so far described in patients with PLD (i.e., PKD2, PRKCSH, SEC63, GANAB, SEC61B, ALG8, and ALG9) are mapped to genes that code for proteins localized in the endoplasmic reticulum (ER) and thus associated with protein biogenesis and transport, triggering general abnormalities in cell protein homeostasis. Thus, aberrant proteostasis and ER stress are common features of cystic cholangiocytes, contributing to disease pathogenesis. Indeed, strategies aiming at reducing ER stress modify disease burden in experimental models of PLD.2 Furthermore, perturbations in posttranslational modifications (PTMs), particularly in SUMOylation, result in aberrant protein dynamics contributing to the pathology.3 There is elevated protein SUMOylation in cystic cholangiocytes, which drives hepatic cystogenesis in experimental models. Administration of SAMe, an inhibitor of the SUMO-conjugating enzyme UBC9, decreased proliferation and increased apoptotic cell death of cystic cholangiocytes, thus reducing hepatic cystogenesis in vitro and in vivo.3
Protein NEDDylation results from a covalent and reversible attachment of neural precursor cell expressed developmentally down-regulated protein 8 (NEDD8) to a lysine residue in the substrate protein. This type of PTM is catalyzed by a three-step enzymatic cascade involving the heterodimer NEDD8-activating enzyme E1 (NAE1/UBA3), NEDD8-conjugating E2 enzymes (UBE2F and UBE2M), and substrate-specific E3 ligases. Deregulated protein NEDDylation has been found in several human diseases, including different types of cancers, as well as neurodegenerative and cardiac disorders.4 A number of hepatic disorders such as liver fibrosis, hepatocellular carcinoma (HCC), and cholangiocarcinoma (CCA) exhibit aberrant protein NEDDylation.5-8 Evidence for a role of protein NEDDylation remains to be identified for PLDs. Interestingly, Pevonedistat, a potent first-in class selective inhibitor of the sole E1 activating enzyme NAE (a dimeric protein composed of regulatory [NAE1] and catalytic [UBA3] subunits) has emerged as a potential therapeutic agent for disorders presenting aberrant protein hyper-NEDDylation.9 Overall, taking this into account, we hypothesize that protein NEDDylation might be aberrantly increased in PLDs and therefore we aim to investigate the role of NEDDylation in PLD and ascertain the therapeutic value of Pevonedistat.
MATERIALS AND METHODS
Patients and specimens
Biliary cyst tissue specimens were obtained from patients with PLD (n = 16; Radboud University Medical Center, Nijmegen, The Netherlands), while human gallbladder (GB; n = 14) and healthy liver (n = 14) biopsies were collected from human individuals undergoing cholecystectomy due to benign conditions or with colorectal cancer undergoing surgery (Donostia University Hospital, San Sebastian, Spain), respectively. Immunohistochemistry (IHC) was carried out in paraffin-embedded liver tissue from the Radboud University Medical Center. Clinical and biochemical characteristics of the study population are provided in Supplementary Table S1. This study received the approval from the Clinical Research Ethics Committees [MSA-MMR-2017-01 (Donostia) and 2012/317 (Radboudumc)]. All participants provided written consents for the use of their samples and clinical information in biomedical research.
Primary cell cultures
Primary human normal and polycystic cholangiocytes (NHCs and PHCs, respectively), as well as normal and polycystic rat cholangiocytes (NRC and PCK, respectively), were isolated and maintained as previously described.10, 11 Of note, a missense mutation [c.2515C > T,p.(Arg839Trp)] in the GANAB gene is present in PHCs.12 PCK cholangiocytes, isolated from PCK rats, bear a splicing mutation (IVS35-2A > T) in the PKHD1 orthologous gene.13, 14 All cells were cultured in fully supplemented DMEM/F-12 medium as previously described.2, 3, 11, 15, 16
RNA isolation, reverse transcription, and quantitative polymerase chain reaction
RNA was isolated from human cystic wall and rat liver tissues as well as from cell cultures with TRI Reagent (Sigma-Aldrich). RNA was reverse transcribed into cDNA and quantitative real-time polymerase chain reactions (qPCRs) were performed as described in the Supplementary data. Glyceraldehyde-3-phosphate dehydrogenase was utilized as a housekeeping endogenous control. The sequences of the PCR primers (Sigma-Aldrich) are outlined in Supplementary Table S2.
Immunohistochemistry
NAE1, UBA3, free NEDD8, and NEDD8-conjugated protein levels were detected by IHC in hepatic paraffin-embedded sections from human patients with ADPKD or PCK rat tissue and compared with respective normal controls as previously described.17 The used antibodies are described in Supplementary Table S3.
Immunoblotting
Whole-cell lysates or NEDD8-immunoprecipitated (IP) cell lysates obtained from cultured PHCs and NHCs were isolated to determine alterations in NEDD8 and NEDD8-conjugated proteins by immunoblotting as described in the Supplementary data.
Three-dimensional cystic organoids
Isolation of intrahepatic biliary cystic organoids from PCK rats was performed and cultured as previously reported.11 In the next day, 0.05 μM Pevonedistat was supplemented in cell culture media on a daily basis. Images of three-dimensional (3D) cystic organoids were taken by light microscopy 24 and 48 h post-incubation. The circumference of cystic organoids was assessed by ImageJ (National Institutes of Health, USA).
Cell proliferation and apoptosis
Cell proliferation and apoptotic cell death were evaluated in NHCs and PHCs under the presence or the absence of different dosages of Pevonedistat using Celltrace CFSE Cell Proliferation Kit (Invitrogen) and FITC Annexin (BioLegend) and TO-PRO-3 iodide (Invitrogen) staining by flow cytometry, respectively, as described in the Supplementary data.
Immunoprecipitation
Incubation with Dynabeads Protein G (Invitrogen) and cross-linking with anti-NEDD8 or immunoglobulin G antibodies (Abcam) was conducted on whole-cell lysates from NHCs and PHCs. Elution of IP proteins from the beads was achieved with 2% sodium dodecyl sulfate as described in the Supplementary data.
Mass spectrometry and proteomic analysis
Comparative shotgun proteomic analyses of NEDD8-IP lysates were performed in a nanoACQUITY UPLC System (Waters), online connected to an LTQ Orbitrap XL mass spectrometer (Thermo Electron). Protein identification was carried out against a database consisting of human entries (Uniprot/Swissprot). Functional analyses of proteins were performed in the STRING database and by gene ontology (GO) enrichment using DAVID Bioinformatics Resources. Details are shown in the Supplementary data.
Statistical analysis
Data are expressed as means ± SEM. Normality was assessed with Shapiro–Wilk normality tests. When comparing two groups, parametric Student’s t-tests or nonparametric Mann–Whitney tests were applied according to the distribution of the population. On the other hand, one-way analysis of variance or nonparametric Kruskal–Wallis tests followed by a posteriori Tukey or Dunns tests, respectively, were used when comparing more than two groups. Results were considered statistically significant when p < 0.05. Statistical analysis was performed with GraphPad Prism software (Version 8.4.3, San Diego, CA, USA).
RESULTS
The NEDDylation system is upregulated in human PLD tissue and cystic cholangiocytes in culture
To explore the global status of the NEDDylation pathway in PLD, the expression (mRNA) levels of NAE1, UBA3, and NEDD8 were assessed in cystic tissue of patients with PLD as well as in healthy human GB and liver tissue. An overexpression of the regulatory subunit of the E1 activating enzyme NAE1 was observed in the cystic tissue of patients with PLD in comparison with both healthy GB and liver tissues (Figure 1a). When evaluating the expression of the catalytic subunit (UBA3) and the ligand itself (NEDD8), a significant upregulation was found in cystic tissue compared to healthy GB tissue, while no changes were found when compared to healthy liver tissue (Figure 1a). In parallel, we also investigated the expression of key NEDDylation pathway components (i.e., NAE1, UBA3, UBE2F, UBE2M, and NEDD8) in primary NHCs and PHCs. An upregulation of NAE1, UBA3, and UBE2F was detected in PHCs, when compared to NHCs, while UBE2M was found downregulated and NEDD8 remained unaltered (Figure 1b). Moreover, immunohistochemical staining in liver tissue of patients with PLD demonstrated a nuclear NEDD8 enrichment (i.e., total sum of NEDD8-conjugated proteins and free NEDD8 ligand) within the biliary cysts compared to the normal bile ducts of healthy controls (Figure 1c). Increased nuclear and cytoplasmic immunoreactivity was also seen in NAE1 and UBA3 (Figure 1c).
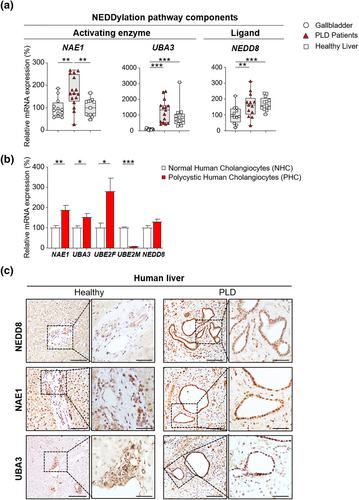
Aberrant expression of NEDDylation pathway components in cystic human tissue and cholangiocytes. (a) mRNA levels of NEDDylation core components in healthy human tissue [gallbladder (n = 12) and liver (n = 13–16)] and cystic tissue of patients with polycystic liver disease (PLD) (n = 13–16). (b) mRNA levels of NEDDylation core components in normal (n = 6) and cystic (n = 6) human cholangiocytes. (c) Representative images of immunostaining of NEDD8, NAE1 and UBA3 in healthy liver tissue and cystic tissue of patients with PLD. Scale bar: 100 μm, 50 μm (cropped). *p < 0.05; **p < 0.01; ***p < 0.001. (One-Way analysis of variance or Kruskal–Wallis tests)
The NEDDylation machinery is also upregulated in rat PLD tissue and cystic cholangiocytes in culture
Similarly, an equivalent array of genes was evaluated to establish the global NEDDylation status in cystic liver tissue of PCK rats as well as healthy liver tissue of wild-type rats. Nae1 was upregulated in cystic rat tissue, while Uba3 and Nedd8 showed no significant changes when compared to normal rat tissue (Figure 2a). Furthermore, overexpression of nearly every key NEDDylation pathway components (i.e., Nae1, Uba3, Ube2m, and Nedd8) was observed in the PCK cholangiocytes compared to normal controls (NRC), with the only exception of Ube2f that was found downregulated (Figure 2b). The overexpression of NEDD8 in vitro was further supported by an NEDD8 enrichment in the nuclei of cyst-lining cholangiocytes in liver tissue of PCK rats compared to bile duct epithelium of wild-type rats (Figure 2c). Additionally, increased immunoreactivity in NAE1 and UBA3 was detected in PCK rats compared to wild-type animals (Figure 2c). Taking into account these data and the results obtained in patients, hyper-NEDDylation seems to be a common event in PLD, independently of the underlying mutations.
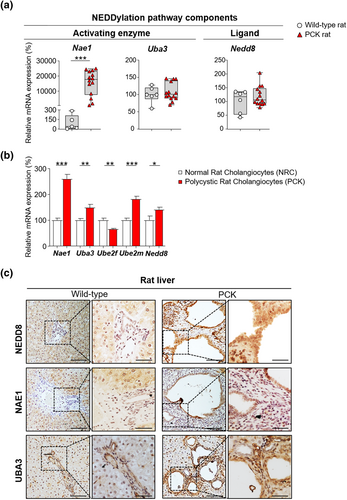
Dysregulated expression of NEDDylation pathway components in cystic rat tissue and cholangiocytes. (a) mRNA levels of NEDDylation core components in wild-type rat tissue (n = 5–6) and cystic tissue of PCK rats (n = 14). (b) mRNA levels of NEDDylation core components in normal (n = 10–11) and cystic (n = 11) rat cholangiocytes. (c) Representative images of immunostaining of NEDD8, NAE1 and UBA3 in wild-type rat tissue and cystic tissue of PCK rats. Scale bar: 100 μm, 50 μm (cropped). *p < 0.05; **p < 0.01; ***p < 0.001. (One-way analysis of variance or Kruskal–Wallis tests)
Pevonedistat inhibits protein NEDDylation in cystic human cholangiocytes
In agreement with the above-mentioned upregulation of the NEDDylation system in cystic cholangiocytes, immunoblotting revealed increased levels of NEDD8 and NEDD8-conjugated proteins, under baseline conditions, in PHCs when compared to NHCs in culture (Figure 3). Consequently, we evaluated the effect of Pevonedistat, a selective inhibitor of NAE, on the protein NEDDylation levels. Of note, the levels of NEDD8-conjugated proteins were dramatically diminished upon incubation of cells with Pevonedistat, whereas no significant changes on NEDD8 protein levels were observed.
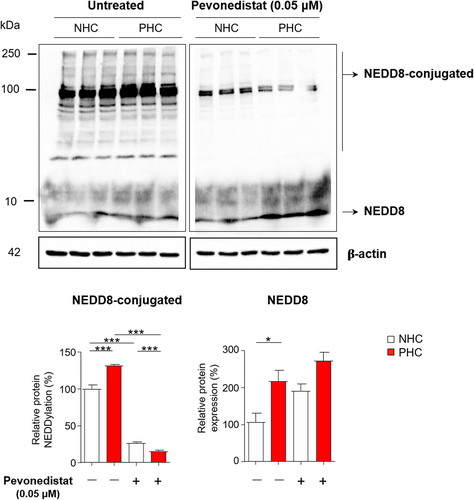
Pevonedistat blocks protein NEDDylation in cystic cholangiocytes in vitro. Representative immunoblotting images and quantification of NEDD8 and NEDD8-conjugated proteins in normal and cystic human cholangiocytes (NHC and PHC, respectively) cultured in the presence or in the absence of Pevonedistat. *p < 0.05; ***p < 0.001. (One-way analysis of variance tests)
Pevonedistat impacts on cystic growth by reducing cell proliferation and survival
The effect of Pevonedistat on cysts growth was investigated by analyzing its impact on cell proliferation and survival. Initially, isolated biliary cysts from PCK rats were cultured on a 3D collagen type I matrix to monitor their growth for 3 days. Remarkably, Pevonedistat completely abrogated the 3D growth of cystic organoids (Figure 4a). Furthermore, exposure to several dosages of Pevonedistat had a substantial dose-dependent antiproliferative effect in both NHCs and PHCs (Figure 4b). The half maximal inhibitory concentration (IC50) for Pevonedistat in both cell lines was established at 0.05 μM. Interestingly, Pevonedistat (0.05 μM) inhibited proliferation but did not induce apoptosis in NHCs or PHCs (Figure 4c). However, higher concentrations significantly triggered apoptotic cell death in both normal and cystic cholangiocytes (Figure 4c). Finally, Pevonedistat (0.05 μM) increased the expression of several biliary epithelia (CK7 and SOX17) and cell polarity (ZO-1) markers in those viable PHCs, while no alterations were observed in NHCs (Figure 4d).
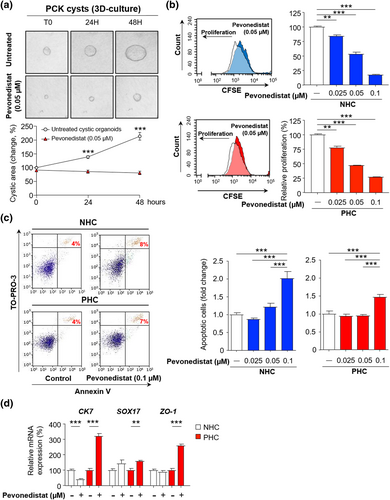
Pevonedistat inhibits cholangiocyte proliferation and survival in vitro. (a) Representative images and quantification of three-dimensional-cultured PCK cysts in the absence or in the presence of Pevonedistat (n = 12). (b) Cell proliferation (n = 3) and (c) apoptosis (n = 5) in the presence or in the absence of Pevonedistat in normal and cystic human cholangiocytes (NHC and PHC, respectively). (d) mRNA levels of epithelial and polarity markers in the presence or in the absence of Pevonedistat. **p < 0.01; ***p < 0.001. (Two-tailed t tests or one-way analysis of variance tests)
NEDDylated proteins in PHCs are mostly associated with the regulation of protein homeostasis
To identify the proteins that are being NEDDylated in PLD, these NEDDylated proteins were immunoprecipitated with an NEDD8-specific antibody and subjected to proteomic analysis by mass spectrometry. The isolation of NEDD8-IP proteins was verified by showing enrichment of these proteins by immunoblotting, when comparing to the inputs of their respective cell controls (Figure 5a). Afterward, shotgun proteomic analyses identified 140 NEDDylated proteins in both cell lines. Of those 140 proteins, 27 proteins were differentially identified between NHCs and PHCs. In PHCs, the levels of two proteins were found downregulated (7%; in blue), whereas the levels of the other 25 proteins were upregulated (93%; in red) in comparison with NHCs (Figure 5b,c, Supplementary Table S4). According to GO analysis, the upregulated NEDDylated proteins in PHCs are engaged in several biological processes, mostly related to response to unfolded protein (i.e., HSPD1 and HSP90AB1) and cell-cell adhesion (i.e., RPL23A and PRDX1) (Figure 5d). Furthermore, protein–protein interaction analysis revealed various associations linked to cytoskeleton organization, cellular protein localization, E3 ubiquitin protein ligase complex and yet again to, response to unfolded protein and cell-cell adhesion (Figure 5e). Therefore, the analyses revealed a connection of protein hyper-NEDDylation with protein biogenesis and quality control in PLD.
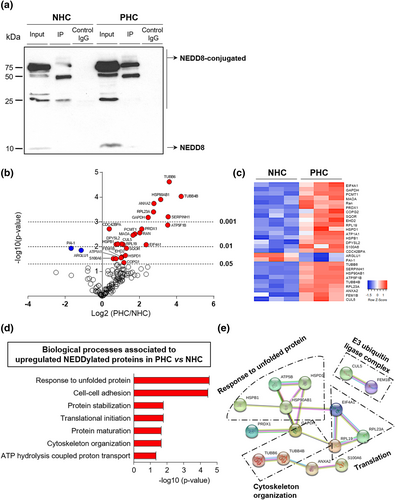
Increased NEDDylated proteins in cystic cholangiocytes, mostly involved in the translational machinery. (a) Representative immunoblotting of NEDD8-IP proteins in normal and cystic human cholangiocytes (NHC and PHC, respectively). (b) Volcano plot of all identified NEDD8-IP proteins (n = 140) by mass spectrometry comparing fold enrichment in PHCs to NHCs. Proteomic analyses of significant identified proteins (n = 27) in PHCs and NHCs by (c) heatmap representation, (d) gene ontology analysis, and (e) protein–protein interaction (PPI) network. Line color in PPI network indicates type of interaction evidence
DISCUSSION
This study reveals that abnormal protein NEDDylation has a significant role in the pathogenesis of PLD. Our key findings demonstrate that (i) expression of enzymes involved in the NEDDylation pathway is increased in liver tissue from patients and rats with PLD, as well as in cystic cholangiocytes in vitro, compared to respective controls; (ii) protein NEDDylation is upregulated in cystic cholangiocytes in vitro; (iii) Pevonedistat causes depletion of NEDD8-conjugated proteins in cystic cholangiocytes in culture; (iv) cyst growth, cell proliferation, and survival are reduced by Pevonedistat in vitro; and lastly (v) proteomic analyses of NEDD8-IP proteins reveal that the majority of the upregulated NEDDylated proteins are involved in protein synthesis and quality control. These data suggest that increased NEDDylation of certain proteins promotes cell proteostasis, survival, and ultimately cystogenesis. Hence, targeting protein NEDDylation (such as Pevonedistat) might serve as a potential therapeutic tool to halt disease progression of PLD.
Previous studies have also reported overactivation of the NEDDylation pathway in patients with liver fibrosis5 and HCC.6, 7 NEDDylation inhibition with Pevonedistat has been shown to halt tumor growth6, 18 as well as to reduce hepatic stellate cell activation and decrease liver fibrosis, ultimately diminishing liver injury.5 Thus, Pevonedistat administration might not only halt hepatic cystogenesis but also attenuate liver fibrosis in PLD. Besides, studies in CCA have shown increased protein levels of NEDD8, NAE1, UBA3, and UBE2M.8 Interestingly, increased NAE1 levels were shown to correlate with worse prognosis (i.e., overall and recurrence-free survival) in patients with HCC and CCA, as well as in other types of cancers such as lung and colorectal.6, 8, 19, 20 Consequently, the NEDDylation pathway is commonly deregulated in different liver diseases, including PLD, thus being amenable for therapeutic targeting.
The mechanism of action of Pevonedistat in PLD was evaluated by immunoblotting and conducting several functional assays. At baseline conditions, PHCs showed increased levels of NEDDylated proteins, which drastically decreased after the administration of Pevonedistat. Furthermore, Pevonedistat considerably restricted the growth of 3D-cultured cysts and reduced the proliferation and survival of cystic cholangiocytes in culture. Multiple mechanisms to explain the antiproliferative properties of Pevonedistat have been proposed. For instance, it is thought that chromatin licensing and DNA replication factor 1, a DNA replication-related protein accumulates after Pevonedistat administration, consequently leading to the activation of DNA damage checkpoints, cell cycle arrest, and ultimately apoptosis or senescence.21
Pevonedistat is a small first-in-class inhibitor of NAE, which processes NEDD8 before its conjugation to target substrates. Noteworthy, the therapeutic efficacy of Pevonedistat is currently being investigated in several clinical trials for hematological malignancies and advanced solid tumors, presenting good tolerability and clinical response in already published phase I studies.22-24 Hepatotoxicity and sepsis syndromes have been reported as side effects of high doses (beyond 100 mg/m2) of Pevonedistat used for hematological malignancies.22
Most mutated genes in PLD encode for ER-resident proteins that are mainly required for proper protein folding, transport, and maturation. Experimental studies highlighted polycystin-1 (PC1) as the main key determinant in PLD pathogenesis since most of the mutations in PLD result in decreased functional PC1 levels and therefore in cyst formation.25 These findings emphasize the importance of the ER in the pathobiology of PLD. In this regard, we have previously reported abnormal protein homeostasis (i.e., proteostasis) and ER stress in PLD, together with a significant upregulation of the unfolded protein response pathway.2 Importantly, treatment with the chemical chaperone, 4-phenylbutyric acid, normalized the ER stress and aberrant proteostasis and inhibited hepatic cystogenesis in vivo. In this regard, most of the NEDDylated candidates identified upregulated in PHCs after high throughput proteomic analysis on NEDD8-immunoprecipitated proteins corresponded to proteins involved in response to unfolded protein (i.e., HSPD1 and HSP90AB1). Notably, the HSP90 family (i.e., HSP90AB1) includes important chaperone proteins that mediate correct protein folding and maturation of hundreds of target proteins involved in cell proliferation, apoptosis, and survival.26 These data indicate that NEDDylation of HSPD1 and HSP90AB1 are pro-survival mechanisms compensatory to the aberrant proteostasis and ER stress present in cystic cholangiocytes.
On the other hand, the most widely characterized substrates of NEDDylation are the cullins. The cullins are the substrates of Cullin-RING Ligases (CRL), functioning as scaffold proteins and providing support for E3 ubiquitin ligases. CRL are the largest known family of E3 ubiquitin ligases, controlling the degradation of approximately 20% of proteasome-regulated proteins.27, 28 The activation of CRL by NEDDylation is required to facilitate degradation via the ubiquitin-proteasome system (UPS).27 Another potential candidate protein that was also found upregulated in NEDD8-immunoprecipitated proteins from cystic cholangiocytes is CUL5. This protein is a component of an E3 ubiquitin protein ligase complex that was also previously related with HSP90 proteins. A previous study showed CUL5-mediated degradation via UPS of several HSP90 client proteins (i.e., ErbB2 and Hif1-α) through interaction with the HSP90 chaperone complex and respective HSP90 client protein.29 The regulation of HSP90 client proteins is controlled by UPS and many of them are aberrantly overexpressed in diseases.26 As such, CUL5 hyper-NEDDylation could lead to the activation of CRLs and degradation of HSP90 client proteins, maintaining protein homeostasis. Pevonedistat was shown to effectively block NEDDylation of the cullins, inactivating CRLs.9 Therefore, Pevonedistat might result in the accumulation of multiple CRL substrates, and thus, increasing cellular stress and, ultimately, inducing apoptosis of cystic cholangiocytes.9
In summary, our study provides new molecular insights on the role of NEDDylation in the pathobiology of hepatic cystogenesis and reveals the therapeutic value of Pevonedistat in PLD. These data support the evaluation of Pevonedistat in vivo, although its economic high cost is a current limitation. Furthermore, studying the potential crosstalk between NEDDylation and SUMOylation, two PTMs known to be deregulated in PLD would be of great interest. Overall, new effective therapies might emerge to treat patients with PLD and to increase their outcome and welfare.
ACKNOWLEDGMENTS
Jesus M. Banales received funding from Spanish Carlos III Health Institute (ISCIII; FIS PI12/00380, PI15/01132, PI18/01075 and Miguel Servet Program CON14/00129 and CPII19/00008); “Diputación Foral Gipuzkoa” (DFG15/010 and DFG16/004); Department of Health of the Basque Country (2017111010); “Euskadi RIS3” (2016222001, 2017222014, 2018222029, 2019222054, and 2020333010); Department of Industry of the Basque Country (Elkartek: KK-2020/00008). Maria J. Perugorria received funding from ISCIII (FIS PI14/00399, PI17/00022, and PI20/00186); Department of Health of the Basque Country (2019111024 and 2015111100); Spanish Ministry of Economy and Competitiveness (MINECO: “Ramón y Cajal” Program RYC-2015-17755). Pedro M. Rodrigues was funded by ISCIII (Sara Borrell CD19/00254). Maria L. Martinez-Chantar received funding from Ministerio de Ciencia, Innovación y Universidades (MICINN; Maria L. Martinez-Chantar: SAF2017-87301-R). Patricia Aspichueta was funded by “Ayudas para apoyar grupos de investigación del Sistema Universitario Vasco” (IT971-16). Marco Marzioni got financial support from Università Politecnica delle Marche PSA2017_UNIVPM grant. Pui Y. Lee-Law received a grant from the European Association for the Study of the Liver (EASL; Sheila Sherlock Award 2017), Francisco J. Caballero-Camino from the Spanish Ministry of Science and Innovation (BES-2014-069148), and Paula Olaizola from the Basque Government (PRE_2016_1_0269). Jesus M. Banales and Maria L. Martinez-Chantar were also supported by BIOEF (Basque Foundation for Innovation and Health Research: EiTB Maratoia BIO15/CA/016/BD), “Fundación Científica de la Asociación Española Contra el Cáncer” (AECC Scientific Foundation) and La Caixa Scientific Foundation (HR17-00601). Jesus M. Banales, Maria J. Perugorria, Maria L. Martinez-Chantar, and Luis Bujanda received funding from CIBERehd (ISCIII). ISCIII was cofinanced by “Fondo Europeo de Desarrollo Regional” (FEDER). The authors are thankful to MINECO for the Severo Ochoa Excellence Accreditation to CIC bioGUNE (SEV-2016-0644). The funding sources had no involvement in study design, data collection and analysis, decision to publish, or preparation of the article.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
Proteomics data has been deposited in PRIDE repository with the dataset identifier PXD022571. The remaining research data is confidential.