Seasonal Variation in Trophic Diversity and Relative Importance of Basal Resources Supporting Tropical River Fish Assemblages in Chiapas, Mexico
Abstract
Environmental changes caused by hydrological shifts between wet and dry seasons in the tropics drive variation in resource availability and feeding interactions for riverine fish assemblages, but studies of trophic structure typically take place over short time scales that do not adequately capture this variation. In this study, we used analysis of stable isotopes (δ13C and δ15N) to assess seasonal changes in the diversity and relative importance of production sources supporting fish assemblages in La Venta River, the largest tributary of Netzahualcoyotl Reservoir on the Grijalva River in Chiapas, Mexico. Based on established river ecosystem theories, we expected the relative importance of carbon sources supporting fishes to shift seasonally, with greater use of instream resources during low-water periods and an increasing contribution from terrestrial sources during high-water periods. Abundant, low-trophic-level food resources (i.e., plants and invertebrates) were expected to support most fish species in the wet season, whereas piscivory was predicted to increase as habitat volume declined and fish density increased in the dry season. Somewhat surprisingly, we found that terrestrial energy sources were assimilated more during low-water conditions at some sites, and ranges of δ13C were higher in the dry season. Terrestrial carbon supporting fish production in low-water periods likely came from riparian plant detritus that remained in the river after flows receded and/or leaf litter that accumulated in the dry season. Ranges of δ15N were similar across seasons, with no apparent increase in piscivory during the dry season. Higher trophic redundancy was observed in the wet season, likely due to fish taking advantage of the same readily available food items (e.g., pulses of arthropod prey) during high flows. Documenting seasonal use of trophic resources provides critical information as expanding hydroelectric dam construction threatens the natural variability of energy pathways that support fisheries resources in tropical rivers.
Environmental changes across seasons are known to drive substantial variation in resource availability and trophic interactions in river fish assemblages (Winemiller 1990; Grossman et al. 1998). Most riverine food web studies take place over short time scales, however, resulting in a relative paucity of information on consequences of seasonal variation for the importance of different food sources supporting fish production (Woodward and Hildrew 2002). In the tropics, this variation can be very strong, driven by pronounced hydrological shifts between wet and dry seasons. Seasonal changes in water levels affect trophic structure of tropical fish assemblages through changes in habitat availability and variation in production sources (Junk et al. 1989; Wantzen et al. 2002; Winemiller 2004). For example, in the lower Mekong River basin, Ou and Winemiller (2016) found that fish production was supported largely by benthic algae and seston in the dry season, whereas riparian plants were more important carbon sources for fish in the wet season. Temporal variation in invertebrate prey availability also often leads to differences in trophic structure of fish assemblages across seasons. Wantzen et al. (2002) suggested that fish fed at lower trophic levels during the wet season because the greater availability of invertebrate prey reduced the frequency of piscivory in a Pantanal floodplain lake. To better understand patterns of influential variation, more studies spanning multiple seasons are needed to identify resources supporting fish assemblages in tropical rivers (McMeans et al. 2019), especially as increasing dam construction throughout the tropics threatens the natural flow variability driving temporal shifts (Zarfl et al. 2015).
Some existing theoretical frameworks provide general expectations for spatial and temporal variation in sources supporting fish production in rivers. Humphries et al. (2014) proposed a model to unify river ecosystem theories about the relative importance of different sources of carbon related to variation in flow and river network position. According to their river wave concept, instream and local riparian sources of primary production are expected to largely support fishes and other aquatic consumers during low flows, as suggested by Thorp and Delong’s (1994) riverine productivity model. As water levels rise, terrestrial production sources are expected to become more important as the aquatic–terrestrial interface expands (as described by the flood pulse concept; Junk et al. 1989), terrestrial material is transported from upstream (following the river continuum concept; Vannote et al. 1980), and higher turbidity limits instream production. Expectations are less formalized regarding changes in the diversity of prey types that are expected to directly support fish in rivers in response to seasonal flow variation. Lowe-McConnell (1987) suggested that in tropical rivers, food resources for fish are more limited in the dry season as habitat volume declines. Pool et al. (2017) found that although there was a great deal of niche overlap among species, fishes in Tonle Sap, Cambodia, had broader isotopic niches during the wet season due to an expanded prey resource base and/or access to a broader array of habitats. In terms of the isotopic niche breadth occupied by tropical fish assemblages (sensu Layman et al. 2007), we thus may expect broader ranges of carbon isotope ratios (δ13C) in the wet season, although ranges for nitrogen (δ15N) may be lower if feeding on abundant low-trophic-level resources predominates during high-water periods and piscivory is more common in low-water periods (Winemiller 1989; Wantzen et al. 2002).
The Grijalva–Usumacinta River basin in southern Mexico is the largest hydrological system in Mesoamerica and a center of remarkable fish biodiversity (Hudson et al. 2005; Miller et al. 2009). The diverse fish assemblages support subsistence and artisanal fisheries throughout the basin, including in indigenous communities. Deforestation poses a threat to regional biodiversity (Kolb and Galicia 2012), and a series of dams on the Grijalva River has negatively impacted the native fish fauna (Miller et al. 2009; Gómez-González et al. 2015). These alterations have likely affected the trophic diversity of fish assemblages (Pease et al. 2019), but more information on resource use across seasons is needed. Recent studies in the Grijalva–Usumacinta River basin have provided information on the breadth and relative importance of food resources supporting cichlid species (Pease et al. 2018; Soria-Barreto et al. 2019), but those studies were restricted to the low-water season, when fish sampling can be carried out more efficiently. Stream habitats in this region change markedly in the wet season (Muñoz-Salinas and Castillo 2015), when seasonal rains combined with precipitation related to the tropical storm regime increase discharge and expand habitat volume. Accordingly, there may be substantial seasonal differences in trophic resources supporting fish production that have not yet been documented. Indeed, in wetlands of the Grijalva–Usumacinta River delta, Sepúlveda-Lozado et al. (2017) found that aquatic consumers used a broader array of food resources during the wet season, and they suggested that this was because flooding increased resource availability.
Here, our aim was to examine seasonal changes in the diversity and relative importance of production sources supporting fish assemblages in La Venta River, a large tributary of the Grijalva River in Chiapas, Mexico, using stable isotope analysis. La Venta River is unique compared to many other tropical rivers in which food web studies have been carried out because much of the watershed is dry forest and the climate is relatively dry compared to basins such as those of the Amazon and Mekong rivers. We predicted that in the dry season, ranges of δ13C would be lower, as fish production would be supported mostly by instream sources of carbon. A broader δ13C range, indicating assimilation of a diverse array of basal resources, was expected in the wet season as the terrestrial–aquatic interface expanded. We expected the range of δ15N, which reflects the number of trophic positions (TPs), to be higher during the dry season in accordance with expectations for increased piscivory (and higher TPs) in low-water periods. In the wet season, we expected to see lower δ15N ranges indicative of foraging on lower-trophic-level resources (plants and arthropods) that may become more abundant with rising waters.
METHODS
Study area
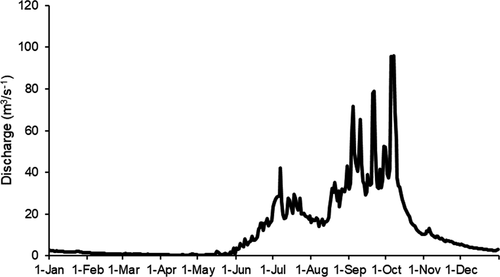
La Venta River flows from its headwaters in the north slope of the Sierra Madre de Chiapas in western Chiapas, Mexico, to its confluence with the Grijalva River at the Netzahualcoyotl Reservoir (Figure 1). The river drains a watershed of 1,304 km2 and is the largest tributary of the middle section of the Grijalva River. The region typically experiences a dry season from December to April (low-flow period) and a rainy season from May through November (high-flow period; Figure 2), with average annual precipitation of approximately 600 mm. The watershed of La Venta River is characterized by karst geology and a mixture of highland and medium-altitude, tropical dry and humid broadleaf forest, with an elevation range from 180 to 1,500 m. Much of the middle and lower watershed is within the Selva El Ocote Biosphere Reserve, a protected area that is considered one of the most important centers of biodiversity in Mexico (CONANP-SEMARNAT 2000). Two small urban areas in La Venta River watershed, Cintalapa and Jiquipilas, have impacted the river through wastewater discharge, riparian deforestation, gravel extraction, and livestock grazing (González-Díaz et al. 2017). Subsistence fishing is common in the lower parts of the river, and the target species for harvest are the Giant Cichlid Petenia splendida and other cichlid species as well as the Macabí Tetra Brycon guatemalensis and Southern Blue Catfish Ictalurus meridionalis (González-Díaz et al. 2017). Nonnative tilapia species and an exotic piscivorous cichlid, the Jaguar Guapote Parachromis managuensis, have been introduced into the Netzahualcoyotl Reservoir, where they are commercially harvested along with the Giant Cichlid. Biogeographically, La Venta River is unique because several fish species in this Gulf of Mexico drainage are otherwise distributed only along the Pacific slope of southern Mexico (González-Díaz et al. 2008; Miller et al. 2009). The occurrence of Pacific drainage species such as the Threespot Cichlid Amphilophus trimaculatus and Blackthroat Cichlid Amphilophus macracanthus in La Venta River supports the theory that there was once a hydrological connection between the Pacific and the Gulf of Mexico in this region (Campbell 1999; Perdices et al. 2002).
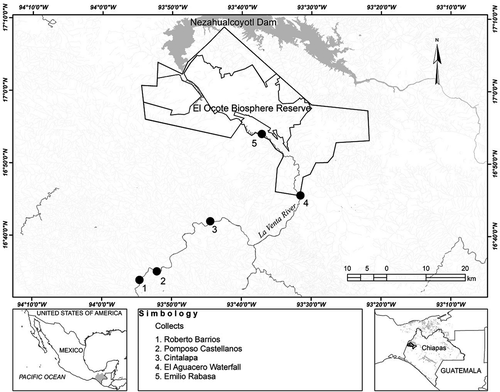
We surveyed fish assemblages and basal food resources at five sites on La Venta River from the headwaters (where it is known as the Cintalapa River) to the confluence of the Negro River (where it is known as the Encajonado River) just above the Netzahualcoyotl Reservoir. The upper La Venta River sites (Roberto Barrios [site 1], Pomposo Castellanos [site 2], and Cintalapa [site 3]) have sandy substrate and are characterized by multi-channel braided geomorphology during the dry season. Even in the rainy season, depths are generally less than 1 m in most mesohabitats at the three upper La Venta River sites. Livestock grazing and farming are common, though not intense, near the river at Roberto Barrios (site 1) and Pomposo Castellanos (site 2). Near the town of Cintalapa (site 3), the river and riparian area are more modified by human uses (deforestation, gravel extraction, modified pool habitats for swimming, and urban wastewater inputs). In the middle section of La Venta River at El Aguacero (site 4), the river enters Selva El Ocote Biosphere Reserve and flows through a steep canyon (average canyon depth = 400 m; CONANP-SEMARNAT 2000). At the El Aguacero site, deeper pool habitats are available and there are large rocks present within the sand-dominated substrate. Below El Aguacero, the river continues through a deep canyon at Emilio Rabasa (site 5), where it has much coarser substrate (85% gravel or larger) and habitat consists of rocky riffles and deep pools just above the transition zone of Netzahualcoyotl Reservoir.
Field surveys and laboratory processing
All sites were surveyed once in April (dry season) and once in July (wet season) of 2015. The timing of fish surveys at least 2 months after the beginning of the dry and wet seasons allowed us to assume that stable isotope ratios in fish muscle tissue represented assimilation of resources from those seasons based on a review of reported turnover times in fish tissues (Boecklen et al. 2011). An intense drought occurred during the dry season of 2015, with unusually low discharge observed in La Venta River (González-Díaz et al. 2017). Sampling of fish and basal resources was carried out within a 150-m study reach at each site. Fish were collected using seine nets, cast nets, and a backpack electrofisher, with the goal of collecting the most common species in local assemblages. At each site, the backpack electrofisher was used for 2 h. The cast net (4-m diameter; 1-cm mesh) was thrown 10 times in pools, and the seine net (10 m long × 2 m wide; 2-cm mesh) was used for 5–10 hauls along sandy shorelines within each study reach. Published diversity surveys of La Venta River (González-Díaz et al. 2008, 2017; Anzueto-Calvo et al. 2016) were consulted to confirm that common species across trophic levels were collected for analyses. We removed muscle tissue from the dorsolateral region of 3–10 individuals of all common fish species at each site. We also collected replicate samples representing available riparian and aquatic carbon sources that supported fish production at each site survey. We gathered samples of the most abundant riparian vegetation and aquatic macrophytes within the 150-m study reach at each site. Because the composition of riparian plant communities was similar across all sites, samples from the same suite of dominant plants (families Salicaceae, Fabaceae, Capparidaceae, Commelinaceae, Onagraceae, and Smilacaceae) were generally collected across sites and seasons. Samples from all available aquatic macrophyte taxa were collected at each site. Riparian and aquatic macrophyte samples were collected from fresh, living plants. Periphyton samples were also scrubbed from submerged rocks where available. Fish, plant, and periphyton samples were preserved in salt in the field following methods recommended by Arrington and Winemiller (2002). Seston samples were collected by filtering 150 mL of water from just below the surface in pre-combusted Whatman GF/F filters (0.7-μm pore size). One to two seston samples were obtained from the thalweg before fish sampling was carried out in each study reach, and filters were frozen prior to laboratory analysis.
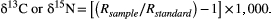
Data analysis
Using the SIAR (Stable Isotope Analysis in R) mixing model (Parnell and Jackson 2008; Parnell et al. 2010) in R (R Core Team 2013), we estimated the relative contribution of production sources assimilated by fish consumers across sites in the dry and wet seasons. The SIAR mixing model uses a Bayesian framework to include natural variation and uncertainties in source and consumer signatures as well as variability in trophic enrichment in iterative model fittings (Parnell et al. 2010). The following sources were included in the models: periphyton, seston, aquatic C3 plants, riparian C3 plants, and riparian C4 plants. These sources were collected and analyzed separately for each site and season. Models were run for entire fish assemblages (all fish species in each site combined) and separately for five common, widespread fish taxa (Astyanax sp., Rhamdia spp., mollies Poecilia spp., Largelip Killifish Profundulus labialis, and Tailbar Cichlid Paraneetroplus hartwegi). Following Post (2002) and Vanderklift and Ponsard (2003), a trophic enrichment factor (TEF) of 0.4 ± 1.3‰ was used for δ13C and a TEF of 2.54 ± 1.27‰ was used for δ15N. As suggested by Phillips et al. (2014), TEF-corrected fish δ13C and δ15N values were plotted to ensure that they were bound within the polygon defined by basal resources prior to running the mixing models. The models were run for 500,000 iterations, with the first 50,000 discarded, and 95% credibility intervals were used to describe the relative importance of each production source.
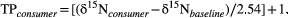
We used assemblage-wide metrics to estimate trophic diversity and redundancy (Layman et al. 2007; Jackson et al. 2011) across sites and seasons based on position and distribution of fishes in niche space as characterized by δ13C–δ15N biplots. We used the SIAR package (Parnell and Jackson 2008; Parnell et al. 2010) in R (R Core Team 2013) to calculate the following metrics described by Layman et al. (2007): δ15N range, δ13C range, total area, centroid distance, mean nearest neighbor distance (MNND), and SD of nearest neighbor distance (SDNND). The range of δ15N describes the distance between fishes with the lowest and highest values of δ15N in local food webs and thus increases with the number of trophic levels. The range of δ13C describes the breadth of food sources supporting fish production. Total area refers to the area of isotopic space occupied by fish assemblages and thus provides a measure of niche breadth. Mean distance to centroid—the average Euclidian distance of each species to the centroid in δ13C–δ15N space—also indicates the breadth of trophic resources used, but it is less affected by outliers than total area (Layman et al. 2007). The MNND and SDNND are measures of trophic redundancy based on species’ positions relative to each other in isotopic niche space (lower MNND suggests high trophic redundancy because species are packed together closely; lower SDNND indicates more evenly distributed trophic niches; Layman et al. 2007). Because the total area metric can be heavily influenced by sample size, we also calculated Bayesian standard ellipse areas corrected for small sample sizes (SEAc) using the SIBER package (Jackson et al. 2011) in R for each fish assemblage. The SEAc provides an estimate of the core amount of trophic niche space occupied and is more robust when used to compare assemblages with differences in sample size (Jackson et al. 2011). Finally, we applied linear mixed-effects (LME) models to test whether the assemblage-wide metrics differed across seasons. For the LME models, we used the lme4 package (Bates et al. 2012) in R (R Core Team 2013) and assigned season as a fixed effect and site as a random factor. We obtained P-values based on likelihood ratio tests of the full model against the model without season as an effect. Dry season samples from site 3 were excluded from production source mixing models and from the assemblage-wide metric calculations described above due to inadequate sampling across trophic levels.
RESULTS
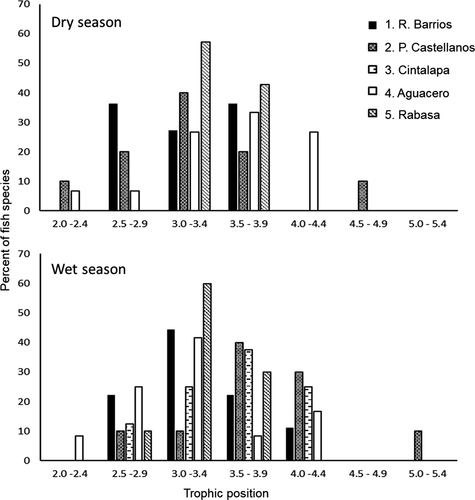
We collected 23 fish species in La Venta River assemblages across the study seasons (Appendix Table A.1). Local richness of common fish species ranged from 7 to 14 at study sites. Assemblage composition was largely consistent across sites and seasons. In general, assemblages had one to three cichlid species, with the Tailbar, Threespot, and Blackthroat cichlids being most commonly collected. All assemblages used in trophic analyses included the characid Astyanax sp., and most assemblages contained the Largelip Killifish, heptapterid catfishes in the genus Rhamdia (Pale Catfish R. guatemalensis and/or Rock Catfish R. laticauda), and poeciliids in the genus Poecilia (Shortfin Molly P. mexicana and/or Mexican Molly P. sphenops). Other frequently encountered species were the Macabí Tetra, Obscure Swamp Eel Ophisternon aenigmaticum, San Jerónimo Livebearer Poeciliopsis fasciata, and Largespot Livebearer Poeciliopsis pleurospilus. Two nonnative cichlid taxa, tilapia Oreochromis spp. and Jaguar Guapote, were collected at some sites. Lowest TPs (mean TP ≤ 2.5) were occupied by the Largespot Livebearer, Brownspotted Killifish Profundulus punctatus, and Oreochromis spp., whereas the Threespot Cichlid, Jaguar Guapote, Giant Cichlid, and Obscure Swamp Eel occupied the highest TPs (mean TP > 4.0). Most fish species were intermediate-level consumers, with TPs between 2.5 and 4.0.
In general, the relative importance of production sources varied across La Venta River sites and seasons (Figure 3). At the upper sites (sites 1 and 2), riparian C3 plants were more important sources in the dry season (along with periphyton at site 1), with a shift toward a greater contribution from aquatic C3 plants in the wet season. Site 4 was unique in that seston was an important source of production in both seasons (with aquatic C3 plants also contributing substantially during the wet season). At site 5, aquatic C3 plants were the primary sources used in the dry season, with a shift toward reliance on riparian C3 plants in the wet season. Relative to other taxa in fish assemblages, Astyanaxsp., Largelip Killifish, and Poecilia spp. were supported more by riparian C3 plants in some sites. Mixing models revealed a shift to greater reliance on aquatic C3 production in the dry season for Largelip Killifish at sites 1, 2, and 4. We documented a shift toward greater use of more riparian C3 production in the wet season for Astyanaxsp. at sites 4 and 5 (Tables A.1, A.2).
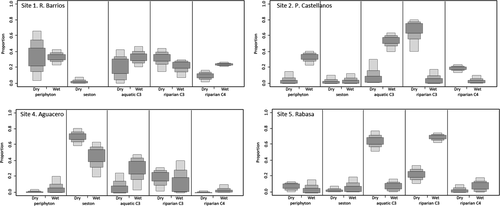
The δ15N values for basal resources and fishes in the upper La Venta River sites (1, 2, and 3) were generally higher than the δ15N values for samples from Selva El Ocote Biosphere Reserve sites (4 and 5; Table A.1). Across sites and seasons, assemblage means for TP ranged from 3.0 to 3.9. Trophic positions of fishes at site 1 were relatively left-skewed (lower TPs) for both seasons (Figure 4). Relatively high TPs were observed for the fish assemblage at site 2 during the wet season. In contrast, the site 4 fish assemblage had higher TPs during the dry season. Site 5 had a very narrow range of TPs around 3.0, especially in the dry season. Sites 2 and 4 had the broadest range of TPs within local assemblages. For the most common fish species collected across sites and seasons, TPs varied spatially and temporally (Tables A.1, A.2). Trophic position ranged from 2.6 to 3.6 for Astyanax sp., from 2.6 to 4.0 for Rhamdia spp., from 3.4 to 4.1 for the Largelip Killifish, from 2.8 to 4.7 for Poecilia spp., and from 2.8 to 4.6 for the Tailbar Cichlid. For Astyanax sp. and Tailbar Cichlid, TPs were lower at site 1 compared to other sites in both seasons. Rhamdia spp. occupied a higher trophic position at site 4 compared to other sites for both seasons. The Mexican Molly occupied a very high TP at site 2 (4.7 in the dry season; 4.2 in the wet season) compared to Poecilia spp. at other sites. Trophic positions of Astyanax sp. and Tailbar Cichlid were very consistent at sites across seasons. Some seasonal variation was observed for Largelip Killifish, with a lower TP in the wet season at site 4.
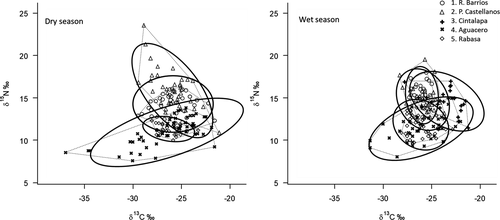
Total convex hull area and SEAc of food resource space used by fish assemblages were greatest for site 4 across seasons, whereas assemblages at site 5 occupied the lowest area of resource space in the dry season and site 1 had the lowest total area and SEAc during the wet season (Table 1; Figure 5). Linear mixed-effects models showed that by some measures, the diversity of trophic resources used by fish assemblages was more constrained during the wet season. The δ13C range and MNND were greater in the dry season than in the wet season (LME for δ13C range: χ2 = 4.92, df = 1, P = 0.03; LME for MNND: χ2 = 6.29, df = 1, P = 0.01). Total area, SEAc, and centroid distance were also greater in the dry season, although marginally nonsignificant (LME for total area: χ2 = 3.20, df = 1, P = 0.07; LME for SEAc: χ2 = 2.83, df = 1, P = 0.09; LME for centroid distance: χ2 = 3.32, df = 1, P = 0.07). No difference between seasons was observed for δ15N range or SDNND (LME for δ15N range: χ2 = 0.74, df = 1, P = 0.39; LME for SDNND: χ2 = 0.50, df = 1, P = 0.48).
| Site number and name | Season | NR | CR | TA | SEAc | CD | MNND | SDNND |
|---|---|---|---|---|---|---|---|---|
| 1. Roberto Barrios | Dry | 3.26 | 4.88 | 9.84 | 7.56 | 1.62 | 0.85 | 0.55 |
| Wet | 4.19 | 1.23 | 2.99 | 2.98 | 1.17 | 0.59 | 0.37 | |
| 2. Pomposo Castellanos | Dry | 5.58 | 3.29 | 9.22 | 9.84 | 1.75 | 1.14 | 0.37 |
| Wet | 5.94 | 2.29 | 7.53 | 5.00 | 1.40 | 0.88 | 0.85 | |
| 3. Cintalapa | Wet | 3.94 | 3.25 | 6.51 | 6.31 | 1.38 | 0.91 | 0.64 |
| 4. El Aguacero | Dry | 5.00 | 10.65 | 19.61 | 12.29 | 2.42 | 0.91 | 1.18 |
| Wet | 4.04 | 5.08 | 13.28 | 8.65 | 1.89 | 0.87 | 0.44 | |
| 5. Emilio Rabasa | Dry | 1.86 | 2.93 | 2.12 | 2.10 | 0.89 | 0.68 | 0.45 |
| Wet | 3.08 | 1.89 | 3.19 | 4.08 | 1.05 | 0.50 | 0.26 |
DISCUSSION
We found that trophic structure and resource use by fish assemblages in La Venta River varied considerably when examined in the wet season versus the dry season. In general, a more diverse array of resources supported fish production in the dry season. Contrary to expectations, ranges of δ13C in fish assemblages were broader in the dry season and δ15N ranges were similar across seasons. Terrestrial energy sources gained more importance in low-water conditions at some sites, with greater assimilation of aquatic macrophyte production in the rainy season at most sites. We noted some exceptions to these broad trends for Largelip Killifish, Astyanax sp., and Poecilia spp., fishes that often relied more on riparian resources compared to assemblage averages. Our results collectively suggest that La Venta River fish production is supported by a combination of riparian and instream sources, with the relative importance of each varying depending on species and on spatial and seasonal habitat differences.
Assemblage-wide metrics of trophic diversity showed that a relatively constrained resource base supported fish during the wet season, and this pattern was largely driven by differences in δ13C range rather than the δ15N range. Generally, the centroids of resource space were positioned very similarly for sites on the δ13C axis across seasons, but the breadth of δ13C resource use was greater in dry season surveys. This suggests that a wider variety of basal resources was assimilated to support fish production in the dry season. Specifically, at sites 1, 2, and 4, we observed reliance mostly on instream production in the wet season (especially aquatic plants, with heavier use of periphyton at site 1). At these sites, the incorporation of more riparian C3 carbon sources broadened the δ13C range in the dry season. At site 5, the opposite pattern occurred: riparian sources predominated during the wet season, and the δ13C range expanded slightly to include more instream production during the dry season (likely driven by the proliferation of aquatic plants observed at this site during the low-water period). This second pattern—greater use of terrestrial resources in the wet season—is what we expected based on the flood pulse concept (Junk et al. 1989) and previous work on the trophic ecology of tropical floodplain river fishes. With rising water levels, the interface with terrestrial resources expands, making off-channel plants and animals more available for aquatic consumers (Junk et al. 1989; Zeug and Winemiller 2008; Humphries et al. 2014). Stable isotope studies of fishes in other tropical rivers have indeed shown that lateral connectivity enhanced by high flows corresponds with greater assimilation of terrestrial food sources (Wantzen et al. 2002; Ou and Winemiller 2016; Correa and Winemiller 2018). At site 5, the relatively high density of riparian trees along the canyon walls probably provided for more local subsidies of terrestrial food resources during high waters.
The pattern of expanded trophic diversity with greater use of terrestrial resources in the dry season countered our expectations based on many other studies of fish resource use in tropical rivers. Rather than incorporating carbon from living inundated terrestrial plants, fish production in the dry season was likely supported by riparian plant detritus that remained in the river after flows receded, leaf litter that accumulated in the dry season, or both. In the river wave concept, Humphries et al. (2014) hypothesized that instream production should be important in low-water periods, but they suggested that inputs from the local riparian zone also contribute to dry season food webs. In the Cinaruco River, Venezuela, Roelke et al. (2006) found that leaves from the riparian forest provided a substantial contribution of carbon available for aquatic consumers during the low-water season. Mesoamerican tropical dry forests tend to drop leaves during the onset of the dry season (Martínez-Yrízar and Sarukhán 1990), so leaf litter may have been more available. In the wet season, the stronger signature of δ13C from aquatic C3 plants may have been driven by connectivity allowing fish to access shallow, off-channel habitats that support high densities of aquatic plants (Welcomme 1985) or by availability of macrophytes that wash into the main channel from these habitats during high-water periods (Winemiller 2004). Patches of the emergent aquatic plant Hymenocallis were observed across sites and seasons in La Venta River, and perhaps they were incorporated into local aquatic food webs at the beginning of the wet season. In a study examining trophic resources supporting piscivorous fishes in a swamp-creek in Venezuela, Winemiller (1989) found that aquatic macrophyte production supporting fish typically peaked in the wet season, with a shift to plant-based detritus in the dry season. A similar pattern was observed in La Venta River: periphyton supported some fish production across seasons, as shown in other studies of tropical rivers (e.g., March and Pringle 2003; Lau et al. 2009), with an increase in importance of aquatic plant production in the wet season at most sites and inclusion of more riparian carbon sources at some sites in the dry season, probably as leaf detritus accumulated and became more available to fish and invertebrate prey.
Plant detritus is often a seasonally important carbon source for riverine fish production (e.g., Winemiller 1990; González-Bergonzoni et al. 2019); many tropical fish species consume plant detritus directly and do so more frequently than their counterparts in temperate rivers (Winemiller et al. 2008). Although C3 plant tissue is generally considered refractory for aquatic consumers (Thorp and Delong 2002), high contributions of terrestrial (Forsberg et al. 1993; Davis et al. 2012) and aquatic (Hoeinghaus et al. 2007; Arantes et al. 2019) C3 plant production to fish biomass have been revealed in stable isotope studies of other tropical rivers. Some tropical fish taxa in La Venta River are also known to consume living macrophytes and freshly fallen fruits and seeds, including Astyanax (Burcham 1988; Wootton and Oemke 1992) and Macabí Tetra (Horn 1997). More commonly, however, plant tissue is consumed by tropical fish as coarse or fine detritus (Winemiller 2004). In our system, the low TPs of many fish species in local assemblages support the idea that they may be frequently consuming plant detritus directly, as has been documented for Vieja spp. (Pease et al. 2018), Shortfin Molly (Hinojosa-Garro et al. 2013), and Largespot Livebearer (Pease 2010). Our mixing model analysis suggests that terrestrially derived detritus may have contributed an energy source that expanded the diversity of the resource base in the dry season for some sites.
Lower trophic redundancy in assemblages (as reflected by higher MNND) also suggested broader trophic resource use by La Venta River fish during the dry season. In addition to the expanded carbon base supporting fish in the dry season as described above, this could have been driven by higher trophic niche overlap in the wet season and greater trophic specialization in the dry season. Lowe-McConnell (1987) suggested that in tropical river fish assemblages, reduced habitat space and higher fish densities during the dry season intensify competition for food resources and promote niche segregation. Among neotropical piscivores, Winemiller (1989) found that lower fish density and greater availability of invertebrate prey in the wet season increased trophic redundancy, and high population density with diminished flows was associated with more pronounced trophic segregation. In La Venta River, pulses of invertebrate prey during higher flows may have similarly caused greater overlap in resource use as fish took advantage of the same readily available food items. As flows receded, species may have consumed resources in a more specialized manner as prey availability decreased (Wantzen et al. 2002). Because we did not quantify seasonal differences in food resource availability and fish density, we cannot clearly elucidate the influences of niche overlap and niche partitioning on trophic diversity in this system.
Differences in sampling efficiency in the wet season versus the dry season could have had some influence on the observed seasonal differences in assemblage niche breadth. We did not observe a difference in the size of individuals captured between seasons, but for some sites fewer species were collected in the wet season, likely due to the difficulty of sampling in high-water conditions. For cases in which some higher-trophic-level species were not captured during the wet season, this probably diminished estimates of trophic diversity. For instance, at site 4, two predatory cichlids (Blackthroat and Threespot cichlids) were collected in the dry season only. Similarly, the Obscure Swamp Eel was collected at site 1 in the dry season but not in the wet season survey. Despite these discrepancies, inadequate sampling of a few species at higher trophic levels probably did not have a strong influence on the seasonal trophic diversity patterns we observed, which were largely driven by differences in the breadth of resources supporting fishes at the base of the food web.
Longitudinally, there were some interesting spatial differences in trophic resources supporting La Venta River fish assemblages. At site 1, periphyton was relatively more important as a basal resource. The availability of more coarse substrate and large woody debris compared to other upper La Venta River sites, as well as potential nutrient inputs from agricultural activities, may have facilitated the development of more periphyton at this site across seasons. The higher δ15N found in basal resources and fish from the upper La Venta River sites (1, 2, and 3) compared to the sites in Selva El Ocote Biosphere Reserve (sites 4 and 5) may have been associated with wastewater inputs from the town of Cintalapa and agricultural runoff above the protected area. These watershed influences have been shown to enrich baseline δ15N in other riverine food webs (e.g., Debruyn et al. 2003; Winemiller et al. 2011).
Site 4 was distinctive for several reasons. Unlike sites above and below it, the site 4 fish assemblage was largely supported by seston. The presence of deep pools and a relatively open canopy over much of the channel at site 4 may have supported more phytoplankton (and particulate phytoplankton-based detritus) as a basal resource for stream consumers (Thorp et al. 2006; Atkinson et al. 2009), which has been shown to be important in many other rivers (e.g., Delong and Thorp 2006; Medeiros and Arthington 2011; Hladyz et al. 2012). Incorporation of sestonic resources, potentially including a phytoplankton component, along with carbon from terrestrial and aquatic C3 plants resulted in a much broader carbon range for this assemblage compared to other La Venta River sites. Site 4 also consistently had the highest trophic niche breadth as measured by centroid distance, total area, and SEAc. In previous research comparing different streams across the Grijalva River basin, the fish assemblage in this reach of La Venta River also had relatively high species richness (Pease et al. 2012) and trophic diversity (Pease et al. 2019). At site 4, the river flows through Selva El Ocote Biosphere Reserve, an area with more expansive, intact forest compared to sites upstream of the reserve, which likely boosts the quantity and quality of allochthonous food resources to support trophic diversity. Discharge also increases between sites 3 and 4 due to the confluences of the Catarina River and another small La Venta River tributary. In other rivers, resource subsidies and increased habitat volume below tributary confluences have been associated with greater diversity of food resources used by fish (e.g., Cross et al. 2013; East et al. 2017). The heterogeneity of aquatic habitats was also higher at site 4, with a greater variety of substrate types and a combination of pool habitats (relatively unavailable at sites 1–3) and shallow runs (relatively unavailable at the deep, swift site 5). Spatial heterogeneity in stream habitats is expected to support a broader array of trophic traits in fish assemblages, whereas functional diversity is expected to be more constrained in relatively homogeneous habitats (Townsend and Hildrew 1994). Indeed, we found that centroid distance, total area, and SEAc were lowest at site 5, which was also in the Selva El Ocote Biosphere Reserve but within a reach where fewer different mesohabitat types were available as the river becomes deep and swift above its transition into Netzahualcoyotl Reservoir.
Our results also revealed some notable distinctions in feeding ecology at the species level within La Venta River fish assemblages. For example, within local food webs, Astyanax sp., Largelip Killifish, and Poecilia spp. were generally supported more by riparian production sources compared to the assemblage average. Astyanax sp. and Largelip Killifish likely assimilated riparian carbon through consumption of terrestrial arthropods, as has been shown in previous studies (Miller et al. 2009; Pease 2010; Small et al. 2011), whereas Poecilia spp. likely consumed plant detritus of riparian origin (Hinojosa-Garro et al. 2013). Notably, we documented relatively high TPs for Poecilia spp. (2.8–4.7) in this study, suggesting that they were feeding at a higher trophic level in La Venta River compared to what has been reported in studies of their diets in other systems (e.g., Scharnweber et al. 2011; Hinojosa-Garro et al. 2013). These unusually high δ15N values could also be caused by nutritional stress (starvation), as reported by Bowes et al. (2014) for the Guppy Poecilia reticulata, but we cannot evaluate that potential influence without measurements of body condition. Nonetheless, across sites and seasons many La Venta River fishes fed at relatively low TPs (<3.0), a common pattern for tropical fish assemblages (e.g., Winemiller 1990; Wootton and Oemke 1992; Pease et al. 2019). Winemiller (1991) suggested that fish in tropical streams generally feed at lower trophic levels compared to fish in temperate systems, where most of the direct consumption of primary producers is carried out by invertebrates.
La Venta River is biogeographically unique compared to the relatively limited spectrum of tropical systems for which seasonal changes in the trophic structure of fish assemblages have been investigated. Compared to more studied systems like the Mekong and Amazon rivers, fish assemblages in rivers of southern Mexico are composed of mostly secondary freshwater fish lineages (especially cichlids and poeciliids). Additionally, much of the basin of La Venta River is characterized by dry broadleaf forest, whereas most stream food web studies in the Neotropics have been carried out in watersheds draining moist forests. La Venta River basin also receives less precipitation compared to many other tropical river ecosystems that have been well studied. Documentation of seasonal shifts in La Venta River thus broadens the body of knowledge on temporal changes in resources supporting fish biomass across diverse systems. Our work confirmed some patterns documented elsewhere, such as higher assimilation of carbon from aquatic plants during the high-water season, but some other expectations were not confirmed (e.g., the range of δ13C was greater in the dry season and the range of δ15N was relatively stable across seasons). Our results also confirm studies in other tropical rivers, which have revealed the importance of riparian forest resources in supporting fish assemblages, either through direct consumption of terrestrial plant tissue and riparian arthropods (e.g., Wootton and Oemke 1992; Correa and Winemiller 2018) or through detrital energy pathways (e.g., Hoeinghaus et al. 2007; González-Bergonzoni et al. 2019). This study provides information that may be valuable for further refinement of expectations regarding the consequences of anthropogenic change, such as deforestation or hydropower expansion, on fisheries resources in tropical rivers.
ACKNOWLEDGMENTS
We thank Fideicomiso El Colegio de la Frontera Sur (784-1004) and Consejo Nacional de Ciencia y Tecnología (PDCPN-2013/214650). The Comisión Nacional de Áreas Naturales Protegidas staff provided facilities to carry out fieldwork in the Selva El Ocote Biosphere Reserve, especially Carlos Morales, Karla Leal, and Roberto Escalante. Miguel Ángel Trujillo, Anuar Caballero, Abraham Aragón, Yuriria Olvera, Carlos Garita, and Beatriz Naranjo helped with field data collection. Dario Navarrete provided the map used in Figure 1. A permit for fish collection was provided by Secretaria de Agricultura, Ganaderia, Desarrollo Rural, Pesca y Alimentacion and Comisión Nacional de Acuacultura y Pesca (PPF/DGOPA249/14). There is no conflict of interest declared in this article.
Appendix: Appendix: Isotope Signatures and Mixing Model Results
| Site number and name | Season | Species | n | δ13C (‰) | δ15N (‰) | TP |
|---|---|---|---|---|---|---|
| 1. Roberto Barrios | Dry | Astyanax sp. | 4 | −24.3 ± 1.1 | 12.6 ± 0.6 | 2.6 |
| Pale Catfish Rhamdia guatemalensis | 5 | −23.5 ± 1.7 | 14.2 ± 1.2 | 3.2 | ||
| Obscure Swamp Eel Ophisternon aenigmaticum | 1 | −25.0 | 15.1 | 3.5 | ||
| Shortfin Molly Poecilia mexicana | 5 | −26.8 ± 1.3 | 14.9 ± 0.3 | 3.5 | ||
| Mexican Molly Poecilia sphenops | 4 | −25.4 ± 1.0 | 15.9 ± 0.6 | 3.9 | ||
| San Jerónimo Livebearer Poeciliopsis fasciata | 4 | −27.4 ± 1.1 | 14.2 ± 0.3 | 3.2 | ||
| Largespot Livebearer Poeciliopsis pleurospilus | 4 | −28.4 ± 1.3 | 12.8 ± 0.2 | 2.6 | ||
| Largelip Killifish Profundulus labialis | 5 | −26.5 ± 1.5 | 15.5 ± 0.3 | 3.7 | ||
| Threespot Cichlid Amphilophus trimaculatus | 3 | −26.1 ± 1.1 | 15.2 ± 0.7 | 3.6 | ||
| Blackthroat Cichlid Amphilophus macracanthus | 1 | −25.5 | 13.2 | 2.8 | ||
| Tailbar Cichlid Paraneetroplus hartwegi | 5 | −25.5 ± 0.5 | 13.1 ± 1.4 | 2.8 | ||
| Periphyton | 2 | −25.6 ± 1.6 | 10.5 ± 0.7 | |||
| Seston | 2 | −24.3 ± 2.0 | 6.6 ± 2.3 | |||
| Aquatic C3 plants | 6 | −28.4 ± 1.2 | 9.4 ± 0.8 | |||
| Riparian C3 plants | 20 | −28.9 ± 1.0 | 10.3 ± 4.0 | |||
| Riparian C4 plants | 5 | −14.1 ± 0.3 | 13.5 ± 2.0 | |||
| Wet | Astyanax sp. | 5 | −25.7 ± 0.4 | 13.8 ± 0.4 | 2.6 | |
| Pale Catfish | 3 | −25.3 ± 0.3 | 15.4 ± 0.3 | 3.3 | ||
| Rock Catfish Rhamdia laticauda | 2 | −25.3 ± 0.9 | 13.8 ± 1.5 | 2.6 | ||
| Mexican Molly | 5 | −26.5 ± 0.5 | 15.6 ± 0.5 | 3.4 | ||
| Largelip Killifish | 5 | −26.4 ± 1.0 | 16.3 ± 0.8 | 3.6 | ||
| Threespot Cichlid | 4 | −25.7 ± 0.5 | 16.6 ± 0.7 | 3.7 | ||
| Blackthroat Cichlid | 1 | −25.9 | 14.9 | 3.1 | ||
| Giant Cichlid Petenia splendida | 1 | −25.5 | 18.0 | 4.3 | ||
| Tailbar Cichlid | 5 | −26.0 ± 0.7 | 14.8 ± 1.1 | 3.0 | ||
| Periphyton | 1 | −30.1 | 7.7 | |||
| Aquatic C3 plants | 3 | −30.1 ± 0.5 | 11.3 ± 0.2 | |||
| Riparian C3 plants | 18 | −29.6 ± 1.6 | 11.1 ± 5.3 | |||
| Riparian C4 plants | 9 | −13.7 ± 0.7 | 14.5 ± 2.4 | |||
| 2. Pomposo Castellanos | Dry | Astyanax sp. | 5 | −24.9 ± 1.2 | 15.3 ± 1.2 | 3.2 |
| Pale Catfish | 5 | −24.6 ± 1.9 | 14.2 ± 1.8 | 2.7 | ||
| Rock Catfish | 4 | −24.3 ± 0.7 | 15.1 ± 1.5 | 3.1 | ||
| Mexican Molly | 5 | −27.6 ± 1.3 | 19.2 ± 3.4 | 4.7 | ||
| San Jerónimo Livebearer | 4 | −25.4 ± 1.8 | 16.4 ± 0.7 | 3.6 | ||
| Largespot Livebearer | 5 | −27.2 ± 1.0 | 17.3 ± 1.6 | 3.9 | ||
| Largelip Killifish | 5 | −27.4 ± 1.3 | 16.0 ± 1.0 | 3.4 | ||
| Threespot Cichlid | 1 | −26.1 | 15.5 | 3.2 | ||
| Blackthroat Cichlid | 5 | −25.6 ± 0.8 | 13.8 ± 1.2 | 2.6 | ||
| Tilapia Oreochromis sp. (juv) | 1 | −26.6 | 13.6 | 2.5 | ||
| Periphyton | 2 | −28.6 ± 0.8 | 9.4 ± 1.6 | |||
| Seston | 1 | −22.5 | 6.2 | |||
| Aquatic C3 plants | 6 | −31.0 ± 0.5 | 11.8 ± 1.2 | |||
| Riparian C3 plants | 9 | −29.6 ± 0.6 | 13.0 ± 2.6 | |||
| Riparian C4 plants | 6 | −14.0 ± 1.0 | 7.2 ± 3.0 | |||
| Wet | Astyanax sp. | 5 | −25.4 ± 1.3 | 13.5 ± 0.6 | 3.3 | |
| Pale Catfish | 5 | −25.2 ± 0.7 | 14.4 ± 0.9 | 3.6 | ||
| Obscure Swamp Eel | 1 | −24.8 | 15.5 | 4.1 | ||
| Mexican Molly | 5 | −27.1 ± 0.9 | 15.7 ± 1.2 | 4.2 | ||
| Largespot Livebearer | 5 | −26.0 ± 0.9 | 14.4 ± 0.3 | 3.6 | ||
| Threespot Cichlid | 2 | −25.1 ± 0.8 | 18.6 ± 1.1 | 5.3 | ||
| Blackthroat Cichlid | 2 | −26.2 ± 0.6 | 12.7 ± 0.0 | 3.0 | ||
| Oreochromis sp. (juv) | 1 | −25.6 | 14.2 | 3.6 | ||
| Tailbar Cichlid | 1 | −25.4 | 14.1 | 3.5 | ||
| Periphyton | 1 | −19.8 | 12.8 | |||
| Seston | 1 | −24.0 | 6.7 | |||
| Aquatic C3 plants | 6 | −30.8 ± 0.4 | 11.4 ± 0.7 | |||
| Riparian C3 plants | 21 | −30.4 ± 0.5 | 7.4 ± 4.0 | |||
| Riparian C4 plants | 9 | −14.2 ± 2.1 | 6.4 ± 3.4 | |||
| 3. Cintalapa | Dry | Threespot Cichlid | 2 | −22.8 ± 0.6 | 16.0 ± 0.0 | 4.4 |
| Oreochromis sp. (juv) | 1 | −20.4 | 14.7 | 3.9 | ||
| Seston | 2 | −21.5 ± 0.1 | 8.0 ± 0.5 | |||
| Riparian C3 plants | 6 | −30.5 ± 0.6 | 3.2 ± 1.0 | |||
| Wet | Astyanax sp. | 3 | −23.3 ± 0.3 | 15.1 ± 0.7 | 3.6 | |
| Pale Catfish | 1 | −21.7 | 14.3 | 3.3 | ||
| Rock Catfish | 4 | −21.9 ± 0.5 | 12.5 ± 0.8 | 2.6 | ||
| Mexican Molly | 4 | −23.6 ± 2.8 | 15.7 ± 1.5 | 3.8 | ||
| San Jerónimo Livebearer | 4 | −24.0 ± 1.1 | 15.1 ± 0.3 | 3.6 | ||
| Largespot Livebearer | 4 | −24.9 ± 0.8 | 14.1 ± 0.3 | 3.2 | ||
| Largelip Killifish | 1 | −23.0 | 16.5 | 4.1 | ||
| Tailbar Cichlid | 1 | −23.1 | 16.3 | 4.1 | ||
| Seston | 1 | −24.7 | 5.2 | |||
| Aquatic C3 plants | 6 | −29.1 ± 0.6 | 12.3 ± 2.0 | |||
| Riparian C3 plants | 12 | −30.3 ± 0.5 | 6.1 ± 5.0 | |||
| Riparian C4 plants | 6 | −14.0 ± 1.1 | 12.0 ± 0.5 | |||
| 4. El Aguacero | Dry | Macabí Tetra Brycon guatemalensis (juv) | 3 | −22.9 ± 1.1 | 10.3 ± 0.9 | 3.2 |
| Astyanax sp. | 5 | −24.6 ± 1.5 | 10.8 ± 0.7 | 3.4 | ||
| Obscure Swamp Eel | 2 | −26.1 ± 4.9 | 11.8 ± 1.5 | 3.8 | ||
| Pale Catfish | 2 | −23.8 ± 0.1 | 12.4 ± 0.9 | 4.0 | ||
| Rock Catfish | 2 | −23.5 ± 1.3 | 12.2 ± 0.9 | 3.9 | ||
| Shortfin Molly | 5 | −28.6 ± 1.3 | 10.3 ± 0.4 | 3.2 | ||
| San Jerónimo Livebearer | 6 | −28.7 ± 1.3 | 9.5 ± 1.1 | 2.9 | ||
| Largespot Livebearer | 3 | −27.5 ± 1.8 | 9.9 ± 3.0 | 3.0 | ||
| Brownspotted Killifish Profundulus punctatus | 5 | −33.5 ± 2.8 | 8.5 ± 0.3 | 2.5 | ||
| Largelip Killifish | 5 | −25.7 ± 0.6 | 12.7 ± 0.9 | 4.1 | ||
| Threespot Cichlid | 3 | −25.5 ± 0.9 | 12.8 ± 1.0 | 4.2 | ||
| Blackthroat Cichlid | 5 | −25.8 ± 1.1 | 11.7 ± 0.4 | 3.7 | ||
| Jaguar Guapote Parachromis managuensis | 1 | −24.6 | 13.5 | 4.5 | ||
| Tailbar Cichlid | 5 | −24.5 ± 1.8 | 11.3 ± 0.5 | 3.6 | ||
| Periphyton | 2 | −15.0 ± 0.8 | 5.9 ± 1.6 | |||
| Seston | 2 | −24.1 ± 0.7 | 8.3 ± 1.3 | |||
| Aquatic C3 plants | 3 | −29.0 ± 0.3 | 4.4 ± 0.3 | |||
| Riparian C3 plants | 35 | −30.7 ± 1.8 | 4.1 ± 0.9 | |||
| Riparian C4 plants | 4 | −14.1 ± 1.2 | 4.6 ± 1.4 | |||
| Wet | Macabí Tetra (juv) | 3 | −24.5 ± 0.6 | 10.2 ± 0.3 | 3.0 | |
| Astyanax sp. | 5 | −25.1 ± 1.0 | 11.0 ± 0.9 | 3.3 | ||
| Obscure Swamp Eel | 4 | −24.8 ± 0.6 | 12.6 ± 0.8 | 3.9 | ||
| Pale Catfish | 6 | −24.2 ± 2.0 | 12.9 ± 1.4 | 4.0 | ||
| Rock Catfish | 4 | −26.4 ± 1.5 | 12.9 ± 0.8 | 4.0 | ||
| Shortfin Molly | 1 | −27.5 | 9.9 | 2.8 | ||
| Mexican Molly | 3 | −29.3 ± 2.0 | 10.2 ± 0.9 | 3.0 | ||
| Largespot Livebearer | 2 | −27.7 ± 1.1 | 8.9 ± 1.0 | 2.4 | ||
| Brownspotted Killifish | 2 | −27.5 ± 1.1 | 10.5 ± 0.9 | 3.1 | ||
| Largelip Killifish | 5 | −28.6 ± 2.0 | 11.4 ± 1.0 | 3.5 | ||
| Tailbar Cichlid | 4 | −25.1 ± 2.0 | 11.2 ± 0.5 | 3.4 | ||
| Periphyton | 1 | −20.5 | 5.7 | |||
| Seston | 1 | −23.2 | 6.5 | |||
| Aquatic C3 plants | 3 | −30.8 ± 0.4 | 4.8 ± 0.2 | |||
| Riparian C3 plants | 21 | −30.2 ± 1.8 | 4.6 ± 2.4 | |||
| Riparian C4 plants | 6 | −14.4 ± 0.4 | 5.6 ± 2.7 | |||
| 5. Emilio Rabasa | Dry | Macabí Tetra (juv) | 5 | −25.7 ± 0.4 | 10.5 ± 0.3 | 3.1 |
| Astyanax sp. | 5 | −24.7 ± 1.0 | 11.3 ± 0.4 | 3.4 | ||
| Pale Catfish | 3 | −26.4 ± 1.0 | 11.7 ± 0.5 | 3.5 | ||
| Southern Blue Catfish Ictalurus meridionalis (juv) | 5 | −26.7 ± 0.4 | 11.3 ± 0.3 | 3.4 | ||
| Largelip Killifish | 5 | −27.2 ± 0.6 | 11.8 ± 0.4 | 3.6 | ||
| Almoloya Cichlid Paraneetroplus regani (juv) | 2 | −27.6 ± 0.1 | 12.4 ± 0.4 | 3.8 | ||
| Tailbar Cichlid | 5 | −26.8 ± 0.8 | 11.2 ± 0.5 | 3.3 | ||
| Periphyton | 7 | −13.2 ± 1.6 | 6.6 ± 0.5 | |||
| Seston | 2 | −13.5 ± 0.1 | 3.9 ± 1.0 | |||
| Aquatic C3 plants | 6 | −27.9 ± 1.5 | 7.7 ± 2.2 | |||
| Riparian C3 plants | 27 | −30.7 ± 1.1 | 5.2 ± 2.2 | |||
| Riparian C4 plants | 12 | −14.1 ± 1.0 | 5.1 ± 0.6 | |||
| Wet | Macabí Tetra (juv) | 5 | −25.3 ± 0.1 | 11.3 ± 0.9 | 3.2 | |
| Astyanax sp. | 5 | −25.1 ± 1.3 | 11.9 ± 0.9 | 3.4 | ||
| Southern Blue Catfish | 1 | −25.8 | 12.5 | 3.6 | ||
| Shortfin Molly | 5 | −26.3 ± 1.5 | 11.1 ± 0.5 | 3.1 | ||
| San Jerónimo Livebearer | 5 | −26.5 ± 0.7 | 11.6 ± 0.8 | 3.3 | ||
| Largespot Livebearer | 5 | −26.7 ± 1.2 | 10.1 ± 0.2 | 2.7 | ||
| Largelip Killifish | 5 | −26.8 ± 0.4 | 12.9 ± 0.3 | 3.8 | ||
| Almoloya Cichlid | 5 | −27.0 ± 0.4 | 13.2 ± 0.7 | 3.9 | ||
| Oreochromis sp. (juv) | 1 | −25.6 | 11.1 | 3.1 | ||
| Tailbar Cichlid | 5 | −25.1 ± 1.6 | 12.1 ± 0.9 | 3.5 | ||
| Periphyton | 1 | −15.0 | 5.8 | |||
| Seston | 1 | −16.7 | 5.8 | |||
| Aquatic C3 plants | 5 | −24.4 ± 6.3 | 6.5 ± 0.9 | |||
| Riparian C3 plants | 32 | −30.5 ± 0.7 | 6.6 ± 2.9 | |||
| Riparian C4 plants | 6 | −14.0 ± 0.5 | 6.3 ± 4.7 |
| Site number and name | Season | Periphyton | Seston | Aquatic plants | Riparian C3 plants | Riparian C4 plants |
|---|---|---|---|---|---|---|
| Astyanax sp. | ||||||
| 1. Roberto Barrios | Dry | 0.21 (0.00–0.38) | 0.25 (0.02–0.41) | 0.23 (0.02–0.39) | 0.23 (0.02–0.37) | 0.18 (0.05–0.28) |
| Wet | 0.34 (0.13–0.55) | n/a | 0.28 (0.04–0.46) | 0.17 (0.00–0.35) | 0.24 (0.17–0.29) | |
| 2. Pomposo Castellanos | Dry | 0.13 (0.00–0.35) | 0.08 (0.00–0.31) | 0.23 (0.00–0.40) | 0.29 (0.04–0.52) | 0.24 (0.08–0.33) |
| Wet | 0.25 (0.07–0.40) | 0.20 (0.00–0.35) | 0.33 (0.14–0.53) | 0.15 (0.00–0.33) | 0.05 (0.00–0.19) | |
| 3. Cintalapa | Wet | n/a | 0.09 (0.00–0.37) | 0.37 (0.08–0.58) | 0.12 (0.00–0.38) | 0.33 (0.21–0.44) |
| 4. El Aguacero | Dry | 0.07 (0.00–0.25) | 0.46 (0.13–0.75) | 0.15 (0.00–0.38) | 0.20 (0.00–0.38) | 0.05 (0.00–0.21) |
| Wet | 0.17 (0.00–0.36) | 0.24 (0.00–0.44) | 0.26 (0.02–0.43) | 0.25 (0.00–0.42) | 0.07 (0.00–0.24) | |
| 5. Emilio Rabasa | Dry | 0.07 (0.00–0.24) | 0.03 (0.00–0.18) | 0.44 (0.18–0.73) | 0.30 (0.04–0.51) | 0.04 (0.00–0.22) |
| Wet | 0.06 (0.00–0.24) | 0.07 (0.00–0.27) | 0.15 (0.00–0.41) | 0.57 (0.28–0.70) | 0.06 (0.00–0.25) | |
| Catfishes Rhamdia spp. | ||||||
| 1. Roberto Barrios | Dry | 0.23 (0.00–0.41) | 0.13 (0.00–0.31) | 0.22 (0.00–0.38) | 0.21 (0.00–0.35) | 0.26 (0.12–0.37) |
| Wet | 0.20 (0.00–0.38) | n/a | 0.36 (0.12–0.70) | 0.23 (0.00–0.40) | 0.20 (0.13–0.26) | |
| 2. Pomposo Castellanos | Dry | 0.09 (0.00–0.33) | 0.05 (0.00–0.26) | 0.21 (0.00–0.38) | 0.29 (0.07–0.55) | 0.27 (0.15–0.35) |
| Wet | 0.33 (0.13–0.46) | 0.06 (0.00–0.28) | 0.37 (0.16–0.55) | 0.12 (0.00–0.33) | 0.04 (0.00–0.17) | |
| 3. Cintalapa | Wet | n/a | 0.30 (0.06–0.45) | 0.23 (0.04–0.35) | 0.10 (0.00–0.31) | 0.38 (0.30–0.47) |
| 4. El Aguacero | Dry | 0.12 (0.00–0.28) | 0.30 (0.06–0.77) | 0.23 (0.00–0.40) | 0.22 (0.00–0.35) | 0.07 (0.00–0.24) |
| Wet | 0.15 (0.00–0.37) | 0.23 (0.00–0.46) | 0.26 (0.02–0.43) | 0.27 (0.02–0.44) | 0.06 (0.00–0.22) | |
| 5. Emilio Rabasa | Dry | 0.06 (0.00–0.31) | 0.05 (0.00–0.27) | 0.35 (0.04–0.68) | 0.34 (0.02–0.61) | 0.05 (0.00–0.29) |
| Mollies Poecilia spp. | ||||||
| 1. Roberto Barrios | Dry | 0.28 (0.01–0.50) | 0.03 (0.00–0.20) | 0.24 (0.00–0.44) | 0.36 (0.11–0.59) | 0.08 (0.00–0.18) |
| Wet | 0.21 (0.00–0.38) | n/a | 0.37 (0.12–0.70) | 0.23 (0.00–0.40) | 0.19 (0.13–0.26) | |
| 2. Pomposo Castellanos | Dry | 0.26 (0.01–0.47) | 0.08 (0.00–0.29) | 0.29 (0.02–0.52) | 0.30 (0.02–0.55) | 0.05 (0.00–0.17) |
| Wet | 0.18 (0.00–0.32) | 0.05 (0.00–0.30) | 0.43 (0.19–0.72) | 0.16 (0.00–0.46) | 0.03 (0.00–0.15) | |
| 3. Cintalapa | Wet | n/a | 0.07 (0.00–0.37) | 0.38 (0.08–0.65) | 0.09 (0.00–0.38) | 0.32 (0.11–0.52) |
| 4. El Aguacero | Dry | 0.03 (0.00–0.26) | 0.38 (0.04–0.59) | 0.20 (0.00–0.48) | 0.27 (0.00–0.57) | 0.02 (0.00–0.20) |
| Wet | 0.08 (0.00–0.35) | 0.22 (0.00–0.43) | 0.27 (0.00–0.52) | 0.27 (0.00–0.51) | 0.05 (0.00–0.31) | |
| 5. Emilio Rabasa | Wet | 0.05 (0.00–0.23) | 0.06 (0.00–0.28) | 0.26 (0.00–0.47) | 0.53 (0.21–0.74) | 0.04 (0.00–0.22) |
| Largelip Killifish | ||||||
| 1. Roberto Barrios | Dry | 0.24 (0.00–0.44) | 0.05 (0.00–0.28) | 0.29 (0.02–0.48) | 0.34 (0.10–0.57) | 0.05 (0.00–0.15) |
| Wet | 0.12 (0.00–0.37) | n/a | 0.32 (0.06–0.65) | 0.29 (0.02–0.46) | 0.20 (0.13–0.28) | |
| 2. Pomposo Castellanos | Dry | 0.09 (0.00–0.40) | 0.05 (0.00–0.24) | 0.29 (0.01–0.50) | 0.37 (0.09–0.67) | 0.07 (0.00–0.16) |
| 3. Cintalapa | Wet | n/a | 0.27 (0.00–0.49) | 0.28 (0.00–0.50) | 0.27 (0.00–0.49) | 0.28 (0.00–0.50) |
| 4. El Aguacero | Dry | 0.05 (0.00–0.19) | 0.30 (0.01–0.55) | 0.30 (0.02–0.51) | 0.29 (0.02–0.47) | 0.03 (0.00–0.17) |
| Wet | 0.07 (0.00–0.32) | 0.11 (0.00–0.38) | 0.32 (0.02–0.58) | 0.31 (0.01–0.57) | 0.04 (0.00–0.23) | |
| 5. Emilio Rabasa | Dry | 0.03 (0.00–0.13) | 0.02 (0.00–0.11) | 0.48 (0.12–0.82) | 0.39 (0.08–0.70) | 0.03 (0.00–0.13) |
| Wet | 0.04 (0.00–0.18) | 0.04 (0.00–0.20) | 0.07 (0.00–0.34) | 0.72 (0.39–0.83) | 0.04 (0.00–0.17) | |
| Tailbar Cichlid | ||||||
| 1. Roberto Barrios | Dry | 0.24 (0.00–0.42) | 0.20 (0.01–0.36) | 0.27 (0.04–0.45) | 0.27 (0.05–0.42) | 0.10 (0.01–0.17) |
| Wet | 0.27 (0.04–0.44) | n/a | 0.32 (0.07–0.57) | 0.21 (0.00–0.37) | 0.23 (0.17–0.28) | |
| 2. Pomposo Castellanos | Wet | 0.22 (0.00–0.39) | 0.22 (0.00–0.38) | 0.24 (0.00–0.41) | 0.23 (0.00–0.40) | 0.18 (0.00–0.37) |
| 3. Cintalapa | Wet | n/a | 0.27 (0.00–0.49) | 0.27 (0.00–0.49) | 0.26 (0.00–0.48) | 0.29 (0.00–0.48) |
| 4. El Aguacero | Dry | 0.06 (0.00–0.27) | 0.45 (0.10–0.78) | 0.12 (0.00–0.36) | 0.17 (0.00–0.37) | 0.04 (0.00–0.23) |
| Wet | 0.21 (0.00–0.38) | 0.23 (0.00–0.43) | 0.23 (0.00–0.39) | 0.23 (0.00–0.39) | 0.16 (0.00–0.33) | |
| 5. Emilio Rabasa | Dry | 0.04 (0.00–0.15) | 0.03 (0.00–0.12) | 0.48 (0.14–0.77) | 0.38 (0.10–0.67) | 0.03 (0.00–0.14) |
| Wet | 0.05 (0.00–0.24) | 0.06 (0.00–0.28) | 0.22 (0.00–0.44) | 0.52 (0.22–0.70) | 0.05 (0.00–0.25) | |




