Fos expression in response to dopamine D3-preferring phenylpiperazine drugs given with and without cocaine
ABSTRACT
WC 44 and WC 10 are phenylpiperazines with low (23 fold) to moderate (42 fold) selectivity for dopamine D3 receptors (D3Rs) over D2Rs, respectively. WC 44 is a full D3R agonist in the forskolin-stimulated adenylyl cyclase (AC) assay, whereas WC 10 has little efficacy. In contrast to their opposite effects in the AC assay, these drugs often produce similar behavioral effects, suggesting that the AC assay does not predict the efficacy of these drugs in vivo. Here, we examined whether Fos protein expression induced by these drugs would be more consistent with their behavioral effects in vivo. Rats received either vehicle, WC 10 (5.6 mg/kg, i.p.), WC 44 (10.0 mg/kg, i.p), cocaine (10.0 mg/kg, i.p.), or cocaine with WC 10 (5.6 mg/kg, i.p.) or with WC 44 (10.0 mg/kg, i.p). Locomotion was monitored for 90 min and the brains were harvested for immunohistochemistry. Both WC 10 and WC 44 decreased spontaneous and cocaine-induced locomotion. Both compounds also increased Fos expression relative to saline in the dorsal striatum and nucleus accumbens core and shell, and relative to cocaine alone in the nucleus accumbens shell. The findings suggest that even though these compounds have different efficacy in the AC bioassy, they produce similar brain activation and attenuation of cocaine hyperlocomotion. Together with our previous research demonstrating that these compounds down-shift the cocaine self-administration dose-effect function, the findings support the idea that D3R-selective compounds may be useful for cocaine dependence medications development. Synapse 67:847–855, 2013. © 2013 Wiley Periodicals, Inc.
INTRODUCTION
There are five subtypes of dopamine receptors grouped into two families, the D1-like family (D1 and D5) and the D2-like family (D2, D3, and D4) (Missale, 1998; Vallone, 2000). The dopamine D3 receptor (D3R) subtype has garnered attention as a target for development of new therapeutic agents to treat psychostimulant addiction, as well as Parkinson's disease, L-DOPA-induced dyskinesia, and schizophrenia (Heidbreder and Newman, 2010; Joyce and Millan, 2005; Kumar et al., 2009; Le Foll et al., 2005; Levant, 1997; Luedtke and Mach, 2003; Newman et al., 2005). This is partially due to the fact that up-regulation of D3Rs occurs in response to administration of many drugs of abuse, including cocaine (Le Foll et al., 2003; Spangler et al., 2003; Vengeliene et al., 2006). Human cocaine overdose fatalities exhibit increases in D3R binding and mRNA expression (Segal et al., 1997; Staley and Mash, 1996). In rodents, increases in motivation to seek cocaine correspond to up-regulation of D3Rs during the course of abstinence from cocaine (Conrad et al., 2010; Neisewander et al., 2004).
Another important feature of D3Rs is that they show differential expression across brain regions. D3Rs have higher expression in Isles of Calleja in the olfactory tubercle and the nucleus accumbens (collectively referred to as the ventral striatum) relative to other dopamine terminals (Sokoloff et al., 1990). The nucleus accumbens is thought to play a critical role in the development of addiction especially during the early stages, whereas the dorsal striatum (dSt) may be critically involved during the later stages of addiction (Belin and Everitt, 2008; Di Chiara and Bassareo, 2007; Di Ciano et al., 2008; Ito et al., 2002; Koob and Volkow, 2010; Porrino et al., 2007). For instance, functional activation observed in rhesus monkeys using 2-[14C]deoxyglucose imaging shifts from the ventral striatum early during cocaine self-administration to the dSt with chronic cocaine self-administration (Porrino et al., 2007). The nucleus accumbens is critical for reward learning (Apicella et al., 1991; Ikemoto, 2007; Schultz et al., 2000), and is specifically involved in translating the value of a reward into appropriate goal-directed behaviors (Roesch et al., 2009).
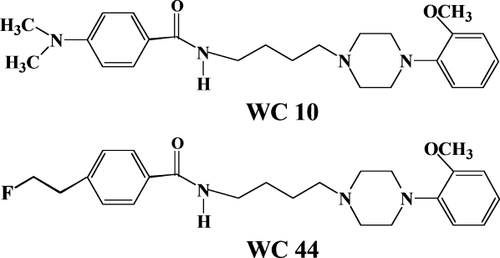
We have recently developed 2-methoxy substituted phenylpiperazine compounds with varying degrees of selectivity and intrinsic activity in the forskolin-stimulated adenylyl cyclases (AC) assay including: WC 10, a low efficacy partial agonist (which can function like an antagonist) with 42-fold selectivity for D3Rs versus D2Rs; and WC 44, a full agonist with 23-fold selectivity for D3Rs versus D2Rs (Chu et al., 2005; Kumar et al., 2009; Xu et al., 2009). The structures of these compounds are shown in Figure 1. WC 10 has a log P value of 3.09 and WC 44 has a log P value of 2.94 (Chu et al., 2005), which predicts that they should readily cross the blood-brain barrier (Dishino et al., 1983). These compounds represent the first series in an ongoing effort to develop useful D3R-selective drugs with varying intrinsic activity.
Surprisingly, our research on behavioral effects of WC 10 and WC 44 has revealed only subtle differences in their effects despite their opposite effects in the forskolin-stimulated AC assay. We have found that both compounds have therapeutic potential for the treatment of L-DOPA-induced dyskinesia (Kumar et al., 2009) and are effective in attenuating deficits in pre-pulse inhibition of acoustic startle, a model of sensory integration deficits observed in schizophrenia (Weber et al., 2009). We have also found that WC 10 and WC 44 decrease cocaine and sucrose intake and that WC 10 increases the latency to perform an operant response for both reinforcers, while WC 44 selectively increases the latency to respond for cocaine only (Cheung et al., 2012). These results suggest that both WC compounds may reduce the motivation to seek cocaine and that the forskolin-stimulated AC assay may not be predictive of the effects of the drugs in vivo (Cheung et al., 2012).
To further examine the potential differences in the mechanism of action of these drugs, this study used Fos protein expression as a marker of the brain functional activation induced by WC 10 and WC 44 given either alone or with cocaine. Fos is the protein product of the immediately early gene c-fos, and its expression has been used as a tool for mapping functional activity in the brain (Chaudhuri, 1997; Herdegen and Leah, 1998). We measured Fos in the nucleus accumbens core (NAcC) and shell (NAcS), dSt, and anterior cingulate cortex (ACC) with the latter included as a control region where we did not expect the compounds to alter Fos expression. Additionally, we examined the effects of WC 10 and WC 44 on spontaneous and cocaine-induced locomotor activity.
MATERIALS AND METHODS
Animals
Adult male Sprague-Dawley rats, weighing 250 ± 25 g at the beginning of the experiment, were housed individually in a climate-controlled colony room with a reversed 12-h light/dark cycle. Housing conditions were in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, 1996). Animals were acclimated to handling daily for 3 days immediately prior to starting the experiments.
Drugs
Cocaine hydrochloride (RTI International, Research Triangle Park, NC) was dissolved in saline. WC 10 and WC 44 were dissolved in a small amount of 1.0 N hydrochloric acid and then brought to proper concentration with 5% dimethyl sulfoxide in saline (0.9% NaCl). An identical solution without drug was used as vehicle. All injections were given intraperitoneally (i.p.) at a volume of 1.0 ml/kg.
Behavioral testing
Animals were habituated to the procedure by placing them into the test chambers for 30 min after receiving an acute injection of saline on each of three consecutive days immediately preceding the test day. The test chambers were clear Plexiglas chambers (48.3 × 26.7 × 15.6 cm) with a thin layer of pine bedding on the floor and stainless steel wire lids.
Rats were randomly assigned to 1 of 4 test conditions for each compound, using a between-subject design. For WC 10, the four conditions were tested in two experiments that were conducted with separate cohorts of rats run at different times. Experiment 1a tested the effect of vehicle versus WC 10 (5.6 mg/kg, i.p., n = 10 for both groups). Experiment 1b tested the effect of cocaine (10.0 mg/kg, i.p.) alone or given with WC 10 (5.6 mg/kg, i.p., n = 8 for both groups). Experiment 2 examined the effects of WC 44 on spontaneous and cocaine-induced locomotion using a single cohort of rats in a 2 × 2 factorial design with the resulting four groups (n = 10 per group): vehicle (i.e., vehicle + saline), WC 44 (i.e., 10.0 mg/kg WC 44 + saline), Coc (i.e., vehicle + 10.0 mg/kg cocaine), and Coc + WC 44 (10.0 mg/kg of both WC 44 and cocaine). The doses of WC 10 and WC 44 were chosen based on our previous findings that they flatten the dose-effect curve for cocaine intake (Cheung et al., 2012). All drug injections were given 5 min before the start of the test session. Locomotor activity was measured using a computer-automated system (Clever sys, Restyon,VA) that continuously tracked the animals throughout the 90-min test period. The conclusion of the 90-min test session coincides with previous observations of peak Fos expression after an acute stimulus (Herdegen and Leah, 1998; Nestler, 2004).
Tissue preparation
Immediately after the test for locomotion, rats were deeply anesthetized with 50–100 mg/kg, i.p., Euthasol and intracardially perfused with ice cold phosphate buffered saline (PBS, 0.1M, pH 7.4) followed by ice cold paraformaldehyde (PFA, 4% in 0.1M PBS, pH 7.4). Brains were then removed and allowed to post-fix in PFA for 24 h at 4°C. Next, brains were cryoprotected in a 10% sucrose solution dissolved in 0.1M phosphate-buffered saline (PBS) for 24 h followed by 30% sucrose dissolved in 0.1M PBS for 48 h. A freezing microtome was then utilized to cut sections (40 μm) at +1.6 mm ± 0.2 mm from bregma (Paxinos and Watson, 1998). Serial sections were stored at −20°C in cryoprotectant solution consisting of 30% ethylene glycol, 30% sucrose, 10% polyvinyl pyrrolidone, and 0.1M PBS until labeled using immunohistochemistry.
Immunohistochemistry
Sections from rats in each group were processed simultaneously for Fos protein expression using methods adapted from Kufahl et al. (2009). Sections were first thawed for 20 min at room temperature followed by extensive washes in 0.1M phosphate buffer (PB, pH 7.3, 3 times for 30-min). This was followed by incubation in hydrogen peroxide (3% for 30 min) and then another incubation in normal goat serum (3% NGS, Vector Labs, Burlingame, CA). Sections were then incubated in primary antibody (rabbit polyclonal anti-Fos antibody, Santa Cruz Biotech., Santa Cruz, CA: sc-52; 1:5000) dissolved in phosphate buffered goat serum (0.1% bovine serum albumin, 0.2% Triton X-100, 2% NGS and 0.1M PB 0.1M) for 72 h at 4°C with agitation. The tissue was then washed 3 times for 10 min in 0.1M PB and allowed to incubate for 1 h in secondary antibody (biotinylated goat anti-rabbit IgG antibody, Vector Laboratories, Burlingame, CA; 1:500), dissolved in 0.1% bovine serum albumin (Sigma, St. Louis, MO), 0.2% Triton-X (Sigma, St. Louis, MO), 2% NGS, and 0.1M PB. This was followed by three washes for 10 min in 0.1M PB and subsequent incubation in extravidin peroxidase (1:1000) dissolved in 0.1% bovine serum albumin, 0.2% Triton-X, 2% NGS, and 0.1M PB for 90 min. Once again the sections were washed 3 times for 30 min in 0.1M PB. They were then incubated in 3,3′-diaminobenzidine tetrahydrochloride (Sigma, St. Louis, MO) containing ammonium chloride (0.4%), D-Glucose (20%), and nickel ammonium sulfate (2%) for 20 min. The reaction was visualized using glucose oxidase for 10 min and immediately terminated by two washes for 30 min in 0.1M PB. Finally, sections were stored in 0.1M PB at 4°C until they were mounted onto slides coated with gelatin chrom-alum, dried, dehydrated, and cover-slipped.
Microscopy and analysis
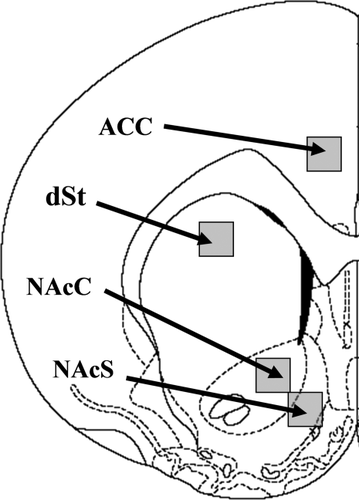
Regions sampled at +1.6 ± 0.2 mm from bregma (Paxinos and Watson, 1998) were the NAcC, NAcS, the dSt, and ACC (Fig. 2). The ACC served as a control region, as we did not expect Fos expression to be affected in this region by changes in D3R transduction (Southam et al., 2007). These regions were sampled bilaterally in two different brain sections for each subject at 20× magnification using a Nikon Eclipse E600 microscope (Nikon Instruments, Melville, NY). Digital images were stored as .tiff files for later analysis. The photomicrographs were captured by individuals blind to group assignment. The software Image J (public domain imaging software developed at the National Institutes of Health) was used to analysize the unmodified images. Qualitative judgment was used to set a threshold (in Image J) for detecting Fos based on size and darkness. Quantification was then performed automatically using the Image J software. Fos-positive nuclei in each region were counted by individuals blind to group assignment. For each region in each subject, the counts were averaged across quadruplicate samples (i.e., 1 sample per hemisphere per duplicate sections), and these averages were analyzed statistically as described below.
Statistical analysis
For WC 10, locomotion and Fos-labeled nuclei were analyzed separately for Experiments 1a and 1b (vehicle vs. WC 10 and cocaine vs. cocaine + WC 10, respectively) because these experiments were conducted at different times. Independent sample t-tests were used to test differences between groups. For WC 44 (experiment 2), these dependent variables were analyzed using a 2 × 2 factorial ANOVA (vehicle/WC 44 × vehicle/cocaine). Newman-Keuls tests were used for post-hoc analyses.
RESULTS
Effects of WC 10
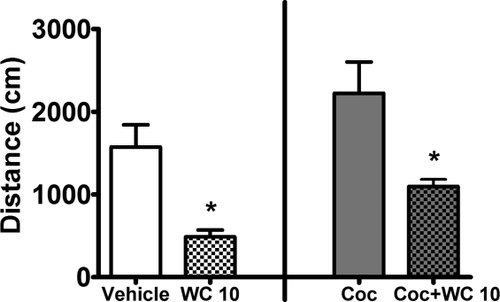
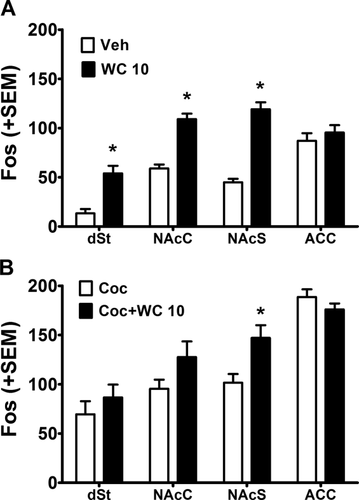
WC 10 (5.6 mg/kg, i.p.) decreased spontaneous and cocaine-induced locomotor activity (Fig. 3) and increased Fos expression in all brain regions examined except the ACC (Fig. 4A). Independent sample t-tests comparing the effect of vehicle versus WC 10 alone in Experiment 1a revealed that WC 10 reduced spontaneous locomotor activity (t(18)= 3.87, P < 0.01) and increased Fos expression in the dSt, NAcC and NAcS (t(18)=4.57, P < 0.01, t(18) = 7.30, P < 0.01, and t(18) = 9.34, P < 0.01, respectively). Fos expression in the ACC did not differ between groups (t(18) = 0.77, n.s.), demonstrating that the effect of WC 10 was region-specific. Similarly, comparing the effect of cocaine (10 mg/kg, i.p.) alone versus cocaine + WC 10 (5.6 mg/kg, i.p.) in Experiment 1b revealed that WC 10 reduced cocaine-induced locomotor activity (t(14) = 2.92, P < 0.02). WC 10 further increased Fos expression relative to cocaine alone in the NAcS (t(14) = 2.91, P < 0.02; Fig. 4B). Fos expression in the other three regions did not differ between groups.
Effects of WC 44
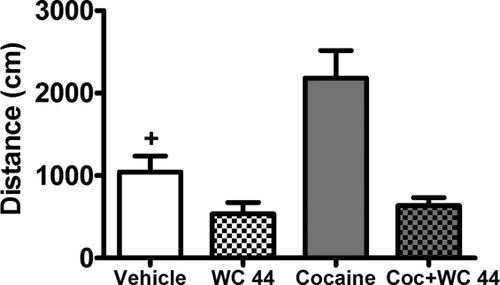
There was an interaction between cocaine and the full agonist WC 44 whereby cocaine increased locomotion but only in rats pretreated with vehicle and not those pretreated with WC 44, and WC 44 reduced locomotion regardless of vehicle versus cocaine pretreatment (Fig. 5). Due to a technical error, locomotor activity data from two rats in each group were lost. The final group size for the behavioral measure was therefore eight rats per group. The ANOVA of distance travelled revealed a significant interaction between WC 44 (10 mg/kg, i.p.) and cocaine (10 mg/kg, i.p.; F(1,28) = 6.11, P < 0.05). Subsequent post-hoc analysis indicated that animals given cocaine alone exhibited an increase in locomotion relative to all other groups (P < 0.05, Newman-Keuls tests). WC 44 decreased locomotor activity relative to vehicle-treated controls regardless of whether animals received cotreatment with vehicle or cocaine (P < 0.05, Newman-Keuls tests).
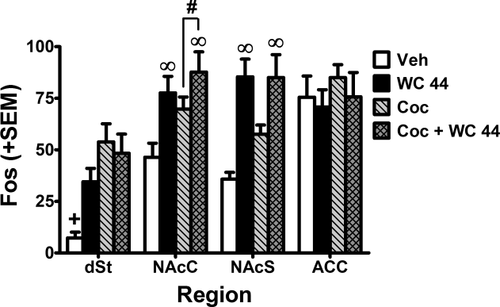
Region-specific increases in Fos protein expression by both cocaine and WC 44 were observed in the striatum but not the ACC (Fig. 6). For the dSt, ANOVA found a significant interaction between the effect of WC 44 and cocaine (F(1,36) = 4.97, P < 0.05). Post-hoc analysis revealed that all three experimental groups showed significant increases in Fos expression relative to vehicle in the dSt (P < 0.05, Newman-Keuls tests). For the NAcC, ANOVA found significant main effects of WC 44 (F(1,36) = 10.07, P < 0.01) and cocaine (F(1,36) = 4.62, P < 0.05), indicating that both drugs increased Fos expression; the WC 44× cocaine interaction was not significant. For the NAcS, ANOVA found a main effect of WC 44 (F(1,36) = 26.04, P < 0.01), with increased Fos expression in animals treated with WC 44 regardless of cotreatment with vehicle versus cocaine. Although ANOVA failed to detect a main effect of cocaine (F(1,36) = 1.98, n.s.) or a WC 44 × cocaine interaction in the NAcS (F(1,36) = 2.14, n.s.) a previous study has found that cocaine alone increases Fos expression in this region (Uslaner et al., 2001). Thus, it is worth noting that a two-tailed t-test suggested that Fos expression was increased in the cocaine alone group compared with vehicle (t(18) = 3.86, P < 0.01). For the ACC, there were neither main effects nor an interaction, suggesting that Fos expression in this area was not affected by any of the treatments.
DISCUSSION
The purpose of this study was to determine whether WC 10 and WC 44 produce differential changes in functional neuronal activity as predicted from their differential effects on D3R transduction through the adenylate cyclase pathway measured in a D3R cell line in vitro (Chu et al., 2005). Functional activity in this study was measured by Fos immunoreactivity in striatal brain regions that are associated with the motor stimulant effects of cocaine. In contrast to that predicted by their in vitro effects on adenylate cyclase, the pattern of Fos expression in dorsal and ventral striatal subregions was similar across the two compounds when given either alone or with cocaine. The present results thus parallel previous findings that several behavioral effects of WC 10 and WC 44 are similar, such as the ability to decrease spontaneous and cocaine-induced locomotion, and to reduce operant responding for cocaine and sucrose (Cheung et al., 2012; Kumar et al., 2009).
The decrease in spontaneous and cocaine-induced locomotion observed with acute administration of WC 10 (5.6 mg/kg, i.p.) and WC 44 (10 mg/kg, i.p.) suggest that even though these drugs suppress cocaine self-administration, they also produce motor side-effects (Cheung et al., 2012; Kumar et al., 2009). This differs from the D3R-selective antagonist SB-277011-A, which does not alter psychostimulant-induced locomotor activity at doses up to 40 mg/kg (Southam et al., 2007) but is effective in reducing cocaine abuse-related behaviors (Di Ciano et al., 2003; Le Foll et al., 2002; Vorel et al., 2002). A likely explanation for the differences is that WC 10 and WC 44, at the doses tested here, may also block D2Rs since both drugs have lower selectivity for D3Rs versus D2Rs than SB-277011-A (43 fold for WC 10, 23 fold for WC 44, 100 fold for SB-277011-A; Kumar et al., 2009; Reavill et al., 2000) and act as low efficacy partial agonists or antagonists at the D2R according to the AC assay (Kumar et al., 2009). Similar to WC 10, other D3R-preferring antagonists, such as U99194A, attenuate locomotor activity at high doses (Carr et al., 2002; Rodriguez-Arias et al., 1999). In contrast, nonselective and D3R-preferring agonists have biphasic effects on locomotion where low doses decrease locomotion and higher doses first decrease then increase locomotion (Fuchs et al., 2002; Jackson et al., 1995; Khroyan et al., 1995, 1999; Li et al., 2010). It is controversial whether or not the hypolocomotion produced by D3R-preferring agonists is due to a post-synaptic D3R effect or to preferential stimulation of D2/D3 autoreceptors resulting in a decrease in dopamine release (Ackerman and White, 1990; Pierce et al., 1995). Thus, either or both mechanisms may have contributed to the effects of WC 44 and WC 10 on locomotion in this study.
Both WC compounds, when given alone, increased Fos expression relative to vehicle in the NAcS, NAcC, and dSt, but not in the ACC. The regions affected by the drugs correspond to those that have moderate to high D3R expression (Diaz et al., 2000; Sokoloff et al., 1990; Surmeier et al., 1996; Xu et al., 2010). A significant enhancement of cocaine-induced Fos expression was also observed with both WC 10 and WC 44 in the NAcS, which has the highest density of D3Rs of all regions examined. Our results are consistent with a previous report that the D3R-preferring antagonist U99194A increased Fos expression in the NAcS, the NAcC, and the dSt (Carr, 2002). However, another study found that the D3R-preferring antagonist GR103691 failed to alter c-fos mRNA levels in either the dorsal or ventral striatum (Hurley et al., 1996). With the more the selective D3R antagonist SB-277011-A, Southam et al. (2007) found that Fos expression is selectively increased in the NAcS and NAcC, but not in the dSt. The increase in dSt Fos expression with the WC compounds in contrast to SB-277011 suggests that this effect involves an action at D2Rs, which also likely accounts for the motor side-effects of the WC compounds in comparison to SB-277011-A. Indeed, D2R antagonists such as haloperidol (>15 fold selectivity for D2 vs. D3) are capable of inducing Fos in the medial and lateral regions of the dSt, as well as the NAc (Robertson, 1994).
The effects of D3R-preferring agonists on c-fos induction have been mixed. For example, the D3/D4R agonist cis-8-OH-PBZI (90-fold D3R selectivity vs. D2R; 10-fold selectivity vs. D4) increases Fos expression in vivo in the rat dorsal and ventral striatum and medial prefrontal cortex (Scheideler et al., 1997). The increase in Fos in the medial prefrontal cortex suggests an action of cis-8-OH-PBZI at D4Rs since there are very few D3Rs in this region. The D3R-preferring agonists 7-OH-DPAT (16-fold D3R selectivity vs. D2R) and PD 128907 (56-fold D3R selectivity vs. D2R) both increase c-fos mRNA expression in in vitro cultures of dorsal and ventral striatal neurons (Morris et al., 2000). However, several other studies report that in vivo administration of D3R-preferring agonists does not increase c-fos mRNA in the dSt or nucleus accumbens (Guo et al., 1995; Ishibashi et al., 2002; Ridray et al., 1998) but blocks Fos expression induced by haloperidol or by the atypical antipsychotic clozapine in both regions (Guo et al., 1995; Vahid-Ansari and Robertson, 1996). Furthermore, clozapine-induced Fos expression in the nucleus accumbens can be blocked by a low dose of 7-OH-DPAT that is thought to act mainly at D3Rs (Vahid-Ansari and Robertson, 1996). These results suggest that D2/D3R agonists do not increase Fos expression in the nucleus accumbens and can attenuate the induction of Fos expression by other drugs in this area. This differs from the present finding that WC 44 increases Fos expression in both nucleus accumbens and dSt, which is more similar to the Fos expression pattern induced by D2/D3R antagonists.
Enhancement of cocaine-induced Fos expression has been associated with lower D3R activity. For instance, Zhang et al. (2004) found that mutant mice lacking D3Rs showed increased cocaine-induced Fos expression in the NAc and dSt. Furthermore, Carta et al. (2000) found that D3R knockout mice showed enhanced behavioral responses and increased Fos expression in the dorsal and ventral striatum in response to acute administration of cocaine compared with wildtype mice. In contrast to D3R knockout mice, Welter et al. (2007) reported that D2R knockout mice show a lack of cocaine-induced c-fos response and decreased cocaine-induced motor activity. These findings suggest that cocaine-induced Fos expression involves stimulation of D2Rs whereas stimulation of D3Rs may inhibit mechanisms involved in cocaine-induced Fos expression. Thus, the increased Fos expression in the NAc in rats given cocaine plus WC 10 or WC 44 relative to those given cocaine alone may be due to antagonism of D3Rs. Importantly, the similar patterns of Fos expression induced by WC 44 and WC 10 suggest that the action of these drug in vivo is likely the same in contrast to their characterization as an agonist and an antagonist, respectively, in D3R-expressing cell in vitro.
There are several possible explanations for why WC 44 and WC 10 produced similar effects in vivo despite their different efficacies in modulating AC in vitro. First, WC 44 may become defluorinated in vivo and the resulting metabolite may act as an antagonist at the D3R (Tu et al., 2011). Second, the drugs may produce opposite effects in regions other than the ventral striatum. D3Rs are found in high levels in the cerebellum (Stanwood et al., 2000) where local infusion of the D3R-preferring agonist 7-OH-DPAT reduces locomotor activity, an effect that is reversed by the D3R antagonist S33084 (Kolasiewicz, 2008), which has 100-fold D3R selectivity over D2R (Millan, 2000). Consistent with this idea, systemic treatments with 7-OH-DPAT have been found to increase c-fos mRNA in the rat cerebellum, whereas changes in the dSt or the nucleus accumbens were negligible (Ishibashi et al., 2002). Third, the D3R-related behavioral effects of WC 10 and WC 44 in vivo may not be mediated via an AC pathway. D3R-selective phenylpiperazines exhibit functional selectivity such that the intrinsic efficacy differs for the AC versus the mitogenic signaling pathways (Taylor et al., 2010). Therefore, the intrinsic activity of WC 10 and WC 44 may differ for AC inhibition, but may be similar for another pathway. Fourth, some of the in vivo effects of WC 10 and WC 44 may be due to an action at the 5-HT1AR since both compounds have affinity for these receptors (Chu et al., 2005). 5-HT1AR agonists do not increase Fos expression in the dSt or the nucleus accumbens, but attenuate the induction of Fos expression in both areas (Ohno et al., 2008, 2009; Tilakaratne and Friedman, 1996; Tremblay et al., 1998; Tsuchida et al., 2009). In contrast, the 5-HT1AR antagonist WAY-100635, which also acts as an agonist at D4Rs (Chemel et al., 2006; Marona-Lewicka and Nichols, 2009), increases Fos expression in the dSt and nucleus accumbens (Jongsma et al., 2002). WAY-100635 has also been reported to attenuate cocaine-induced locomotion (Carey et al., 2000, 2001; Müller et al., 2002) and cocaine seeking behavior (Burmeister et al., 2004; Schenk, 2000). It is therefore possible that blockade of 5-HT1ARs contributes to some of the effects of WC 10 and WC 44.
Although we have highlighted the similarities in Fos expression and behavioral effects of WC 10 and WC 44, it is important to note that some differences exist including: (1) their ability to reverse prepulse inhibition (PPI) of startle deficits induced by the nonselective dopamine agonist apomorphine, for which only WC 10 is effective even though both drugs reverse PPI deficits produced by the D3R-preferring agonist pramipexole (Weber et al., 2009) and (2) a more selective effect of WC 44 to increase latency to respond for cocaine but not for sucrose, whereas WC 10 increases latency to respond for both reinforcers (Cheung et al., 2012). An important goal of future research is to determine the dopamine signaling pathways involved in the above behavioral effects in an effort to establish in vitro assays with validity for predicting potential therapeutic and side effects of novel D3R drugs.
In conclusion, the results provide further information regarding effects of the D3R-preferring phenylpiperazines WC 10 and WC 44 on cocaine-induced behaviors. Moreover, the similarity in the pattern of Fos immunoreactivity and changes in spontaneous and cocaine-induced locomotion following WC 10 and WC 44 administration suggests these compounds may have the same action in vivo despite being classified as an antagonist and an agonist, respectively, in the in vitro adenlyate cyclase inhibition assay (Chu et al., 2005; Kumar et al., 2009). Further research aimed at understanding the relationship between efficacy and selectivity of drugs that act at D2-like receptors is essential for developing medications for psychostimulant dependence. At present, the literature suggests that selective D3R antagonists may be useful for promoting abstinence without producing unwanted side-effects; however, they may not be effective in decreasing cocaine intake should relapse occur (Heidbreder and Newman, 2010). Less selective D3/D2R partial agonists and antagonists appear to be more effective in reducing psychostimulant intake in preclinical self-administration models than selective D3R antagonists, but they also produce more side-effects (Millan et al., 2004). It is possible that some degree of co-occupancy of D2 and D3Rs is needed to reduce psychostimulant intake and perhaps what is needed are compounds with a ratio of D3R:D2R occupancy levels that can inhibit cocaine self-administration while minimizing the potential side effects.