Side-chain symmetry-breaking strategy on porphyrin donors enables high-efficiency binary all-small-molecule organic solar cells
Wentao Zou and Xu Zhang contributed equally to this work.
Abstract
Side-chain symmetry-breaking strategy plays an important role in developing photovoltaic materials for high-efficiency all-small-molecule organic solar cells (ASM OSCs). However, the power conversion efficiencies (PCEs) of ASM OSCs still lag behind their polymer-based counterparts, which can be attributed to the difficulties in achieving favorable morphology. Herein, two asymmetric porphyrin-based donors named DAPor-DPP and DDPor-DPP were synthesized, presenting stronger intermolecular interaction and closer molecular stacking compared to the symmetric ZnP-TEH. The DAPor-DPP:6TIC blend afforded a favorable morphology with nanoscale phase separation and more ordered molecular packing, thus achieving more efficient charge transportation and suppressed charge recombination. Consequently, the DAPor-DPP:6TIC-based device exhibited superior photovoltaic parameters, yielding a champion PCE of 16.62% higher than that of the DDPor-DPP-based device (14.96%). To our knowledge, 16.62% can be ranked as one of the highest PCE values among the binary ASM OSC filed. This work provides a prospective approach to address the challenge of ASM OSCs in improving film morphology and further achieving high efficiency via side-chain symmetry-breaking strategy, exhibiting great potential in constructing efficient ASM OSCs.
1 INTRODUCTION
As one of the leading research topics in photovoltaic technology, organic solar cells (OSCs) are considered to have broad application prospects for semi-transparency and wearable photovoltaics, demonstrating significant development in recent decades.1-13 Among different kinds of OSCs, all-small-molecule (ASM) OSCs have attracted the attention of researchers by virtue of their well-defined chemical structures and remarkable reproducibility of small molecular materials.14-18 As a main challenge, small molecule donors (SMDs) possess similar structures compared to non-fullerene acceptors (NFAs), which indicates their excellent miscibility, possessing challenges in controlling their blend film morphology.19, 20 Weak or strong miscibility may cause self-aggregation or over-mixing rather than forming interpenetrating network morphology, which will lead to reduced exciton dissociation and carrier transfer efficiency as well as increased probability of charge recombination, limiting the power conversion efficiency (PCE) improvement of ASM OSCs.21, 22
Previous studies have clarified that the regulation of molecular structure contributes to improving the properties of molecules in diverse aspects, such as energy levels, absorption, aggregation, and crystallinity.23, 24 The subtle structural optimization of core electron-donating units in acceptor‒donor‒acceptor (A‒D‒A)-type photovoltaic materials has been considered as one of the key points in designing SMD materials,25, 26 demonstrating the great importance in optimizing the optical and electrochemical properties of materials.27, 28 Besides, the functionalization of side chains on electron-donating units is also considered an important structural-tuning strategy for high-efficiency photovoltaic materials, and some studies have demonstrated excellent achievements.29-31 Encouragingly, among side-chain engineering, the side-chain symmetry-breaking strategy has already been employed in high-efficiency photovoltaic materials. The asymmetric molecular structures may not only improve the photophysical properties but also provide materials with stronger intermolecular interaction, triggering more ordered molecular packing.32-34 For instance, an NFA named TOBDT has been synthesized with asymmetric side chains of alkoxy and alkyl thiophene, which induced optimized carrier transport and reduced energy loss in ternary devices.35 An SMD namely TB-F was developed and a maximum PCE of 17% was achieved in TB-F:L8-BO-based devices, which can be attributed to the improved bi-continuous networks and favorable domain size.36 However, as can be seen from Table S1, for high-performance binary ASM OSCs (>16.5% PCE), all the donors and the acceptors are based on BTR analogs and Y6 or Y6 analogs. Thus, there is an urgent need to enrich the diversity of small molecular materials to further enhance the performance of ASM OSCs. Porphyrin, as one of the attractive electron-donating units, has been widely studied in the OSC field,37 displaying great potential in constructing efficient ASM OSCs.38-40 Nevertheless, the effects of asymmetric side chains for porphyrin-based SMDs on the photoelectric behavior, active layer morphology, and device performance lack comprehensive investigation. Therefore, we expected that the porphyrin-based SMDs obtained by the side-chain symmetry-breaking strategy can improve the photoelectric behavior and active layer morphology, and eventually achieve satisfactory device performance.
Following the inspiration of the above considerations, we designed and synthesized two porphyrin-based SMDs, namely DDPor-DPP and DAPor-DPP. Both SMDs possess identical π-bridges and end groups as the symmetric counterpart ZnP-TEH, but differ only in asymmetric alkoxy benzene and alkyl pyrazole side chains attached to the porphyrin core, respectively. In contrast to their symmetric counterpart ZnP-TEH,41 these asymmetric structures displayed enlarged dipole moments. Such characteristic enhanced intermolecular interactions, which may result in modified molecular aggregation and crystallization, offering possibilities for morphology optimization. Grazing incidence wide-angle X-ray scattering (GIWAXS) characterization displayed that both DDPor-DPP and DAPor-DPP performed stronger crystallinity. As a result, a tighter and more ordered molecular packing as well as suitable bi-continuous phase separation were evolved in the DAPor-DPP-based active layer, leading to lower charge recombination and longer carrier lifetime. Consequently, the DAPor-DPP:6TIC-based device achieved an encouraging PCE of 16.62% along with simultaneously improved photovoltaic parameters of short-circuit current density (JSC), VOC, and fill factor (FF) compared to the DDPor-DPP-based OSCs (14.96%). To the best of our knowledge, 16.62% can be ranked as one of the highest PCE values among the ASM OSC studies. Our work demonstrated that introducing asymmetric side chains on SMDs is an excellent structural tuning strategy to optimize molecular packing and nanoscale morphology for high-efficiency ASM OSCs, paving a new way to develop more efficient donor materials for ASM OSCs.
2 RESULTS AND DISCUSSION
The chemical structures of ZnP-TEH, 5,15-bis(2,5-bis(2-ethylhexyl)-3,6-dithienyl-2-yl-2,5-dihydro-pyrrolo[3,4-c]pyrrole-1,4-dione-5′-yl-ethynyl)-(10-(5-(2-ethylhexyl)thienyl)-20-(4-(octyloxy)phenyl))porphyrin zinc(II) (DDPor-DPP), and 5,15-bis(2,5-bis(2-ethylhexyl)-3,6-dithienyl-2-yl-2,5-dihydro-pyrrolo[3,4-c]pyrrole-1,4-dione-5′-yl-ethynyl)-(10-(5-(2-ethylhexyl)thienyl)-20-(1-(2-ethylhexyl)-1H-pyrazol-4-yl))porphyrin zinc(II) (DAPor-DPP) are displayed in Figure 1A. The detailed synthetic routes of DDPor-DPP and DAPor-DPP are shown in Scheme 1, and ZnP-TEH was synthesized as previously reported.41 First, we synthesized and purified asymmetric porphyrins 2a and 2b through a one-flask reaction using dipyrromethane and two different arylaldehydes. DDPor-DPP and DAPor-DPP were obtained after bromination, zinc coordination and two steps of Sonogashira reaction. Figures S1‒S8 summarize the detailed experimental methods and nuclear magnetic resonance spectra. As shown in Figure S9, thermogravimetric analysis showed that thermal decomposition temperatures of DDPor-DPP and DAPor-DPP exceeded 300°C, indicating their good thermal stability.
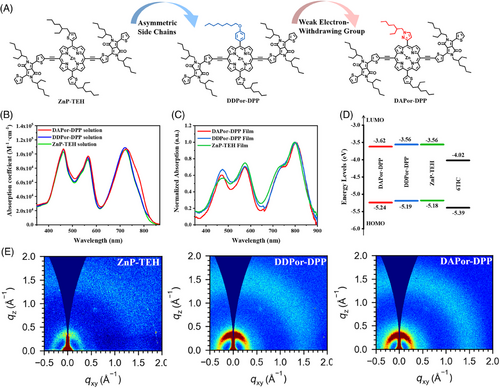
Ultraviolet‒visible spectra were characterized to explore optical physical properties of SMDs (Figure 1B,C). For porphyrin-based A‒D‒A-type SMDs, typical absorption peaks can be discovered in both short- and long-wavelength regions, which were caused by structural units and intramolecular π-electron delocalization, respectively.42, 43 From solution to solid state, significant red shifts of over 75 nm were distinguished from all three SMDs, perhaps owing to the optically active aggregation led to tight parallel stacking.44 We also performed cyclic voltammetry measurement (Figures 1D and S10). The results showed that DAPor-DPP demonstrates slighter down-shifted highest occupied molecular orbital (HOMO), which may potentially lead to improved VOC in photovoltaic devices. Detailed optical and electrochemical data are summarized in Table 1.
| SMDs | λmaxa (nm) | λmaxb (nm) | HOMO (eV) | LUMOc (eV) | EgCV (eV) |
|---|---|---|---|---|---|
| DAPor-DPP | 462, 566, 726 | 470, 572, 802 | −5.24 | −3.62 | 1.62 |
| DDPor-DPP | 460, 565, 720 | 474, 572, 800 | −5.19 | −3.56 | 1.63 |
| ZnP-the | 466, 568, 725 | 472, 573, 801 | −5.18 | −3.56 | 1.62 |
- a In chloroform solution.
- b In neat film.
- c Lowest unoccupied molecular orbital.
Density functional theory calculation provided further insights into the effect of asymmetric side chains in molecular structure modification. As shown in Figure S11, both DAPor-DPP and DDPor-DPP exhibited satisfying planarity such as ZnP-TEH, indicating that the side-chain functionalization has little effect on the molecular planarity at the molecular level. Besides, DAPor-DPP showed a larger dipole moment of 2.52 D than that of DDPor-DPP (1.67 D). The enlarged dipole moment can bring about strong intermolecular interaction, which will cause tight and orderly molecular stacking, exhibiting the potential to optimize the morphology of active layers.45, 46
GIWAXS has shown comprehensive demonstrations of the molecular orientation and crystalline properties of pure SMD films. Figures 1E and S12 display the 2D patterns and 1D curves. Table S2 summarizes the corresponding data. DAPor-DPP and DDPor-DPP exhibited similar orientations with the symmetric ZnP-TEH, which means the asymmetric side-chain functionalization would not change the molecular packing orientation. Broad rings at 1.55, 1.52, and 1.51 Å−1 are shown in DAPor-DPP, DDPor-DPP, and ZnP-TEH pure films, with the corresponding π‒π stacking distances of 4.05, 4.13, and 4.16 Å, respectively, indicating a tighter π‒π stacking in neat films of asymmetric molecules. In addition, the calculated crystal coherence lengths (CCLs) for DAPor-DPP and DDPor-DPP films are 10.10 and 8.08 Å, respectively, which are larger compared with ZnP-TEH film (7.34 Å). Notably, DAPor-DPP exhibited stronger crystallinity at ∼0.32 Å−1 than the other two counterparts, which may improve the crystallinity of the blend film. These results can be attributed to that asymmetric structural building blocks enhanced intermolecular interaction, and endowed molecules with tighter and more orderly stacking mode, agreeing well with the above theory calculation.36
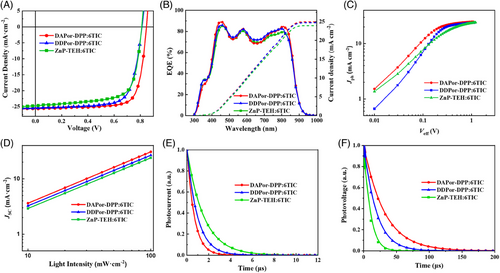
OSCs with a classical structure of ITO/PEDOT:PSS/SMDs:6TIC/C60-bissalt/Ag were fabricated to subsequently investigate the photovoltaic performance of SMDs. As a high-efficiency acceptor, 6TIC has been widely investigated and proved to be suitable for porphyrin-based SMDs in previous studies. The current density versus voltage (J‒V) curves of the devices are shown in Figure 2A and detailed device parameters are shown in Table 2. An efficiency of 14.96% was achieved by the DDPor-DPP-based device, which was higher than that of the ZnP-TEH:6TIC-based device (14.12%). Encouragingly, the DAPor-DPP:6TIC-based device delivered a champion PCE of 16.62%, which is one of the highest efficiencies for binary ASM OSCs. In comparison to DDPor-DPP and ZnP-TEH, the enhancement of VOC in DAPor-DPP (0.845 V) can be assigned to the down-shifted HOMO energy level. Even more delightful, the DAPor-DPP:6TIC-based devices brought about improved JSC and FF values of 25.61 mA cm−2 and 0.768, respectively. To validate the accuracy of the JSC values, external quantum efficiency (EQE) spectra were analyzed as a means of verification. As depicted in Figure 2B, these devices displayed broad photovoltaic responses in the range from 300 to 1000 nm. The integrated JSC (JSC,Cal) from the EQE curves of the DAPor-DPP-based, DDPor-DPP-based, and ZnP-TEH-based devices are calculated to be 24.95, 24.71, and 23.92 mA cm−2, respectively, which conform well to the date measured from J‒V curves (within an error of 5%). Curious about the underlying factors behind this interesting enhancement, a comprehensive range of characterizations was conducted as follows.
| Active layer | VOC (V) | JSC (mA cm−2) | JSC,Cala (mA cm−2) | FF | PCE (%) |
|---|---|---|---|---|---|
| DAPor-DPP:6TIC | 0.845 | 25.61 | 24.95 | 0.768 | 16.62 |
| DDPor-DPP:6TIC | 0.811 | 25.41 | 24.71 | 0.726 | 14.96 |
| ZnP-TEH:6TIC | 0.809 | 24.72 | 23.92 | 0.706 | 14.12 |
- Abbreviations: FF, fill factor; PCE, power conversion efficiency.
- a The integrated current densities were calculated from the EQE curves.
To deeply investigate the charge-carrier dynamics, analyses were carried out to examine the relationship between photocurrent density (Jph) and effective voltage (Veff) as well as JSC against the light intensity (Plight). As shown in Figure 2C, with Veff enhancing, Jph of the DAPor-DPP:6TIC-based device grew most rapidly and reached saturation value (Jsat), which implies the efficient exciton dissociation and charge collection process in the corresponding devices. The probabilities of exciton dissociation (Pdiss) can be calculated from Jph/Jsat to further understand the exciton dissociation process.47, 48 Pdiss were calculated to be 0.922 for DAPor-DPP:6TIC-based devices, 0.903 for DDPor-DPP:6TIC-based devices, and 0.898 for ZnP-TEH:6TIC-based devices. In addition, at the maximum power point, the probability of charge collection (Pcoll) of DAPor-DPP:6TIC was 0.813, which was higher than that of ZnP-TEH:6TIC (0.762) and DDPor-DPP:6TIC (0.807). The results indicate the highest-efficiency exciton dissociation and charge collection in DAPor-DPP-based active layer, resulting in improved photovoltaic performance. JSC‒light intensity curves in Figure 2D can serve as indications of the charge recombination condition within the device. According to the power law JSC ∝ (Plight)α,49, 50 the slope factor α can be calculated to demonstrate the bimolecular recombination property within active layers. DAPor-DPP:6TIC-based, DDPor-DPP:6TIC-based, and ZnP-TEH:6TIC-based devices showed α values of 0.93, 0.91, and 0.90, respectively. The α of the DAPor-DPP-based device is closer to 1, indicating a lower probability of charge recombination. Additionally, to explore the trap-assisted recombination behavior in the devices, VOC as a function of light intensity (VOC ∼ Plight) was measured and is shown in Figure S13. The slope values for DAPor-DPP:6TIC, DDPor-DPP:6TIC, and ZnP-TEH:6TIC devices were 1.18, 1.28, and 1.33, respectively, the smaller slope can efficiently alleviate trap-assisted recombination in the corresponding device. These results probably stem from the morphological improvement, which will be thoroughly discussed below.
Furthermore, transient photocurrent (TPC) and transient photovoltage (TPV) measurements were employed to gain a comprehensive understanding of the charge transport behavior (Figure 2E,F). By fitting TPC and TPV curves, carrier extraction time and lifetime can be obtained, respectively. As shown in Table S3, DAPor-DPP-based and DDPor-DPP-based active layers displayed faster carrier extraction times of 0.66 and 0.95 µs, respectively, than the symmetric ZnP-TEH-based device (1.53 µs), which suggests that employing the symmetry-breaking strategy may efficiently improve charge transport behavior. Besides, the carrier lifetimes of DAPor-DPP-based and DDPor-DPP-based devices are 30.97 and 18.59 µs, respectively, which are significantly higher than that of the ZnP-TEH:6TIC-based device (8.71 µs), indicating the suppressed charge recombination in the former two devices.
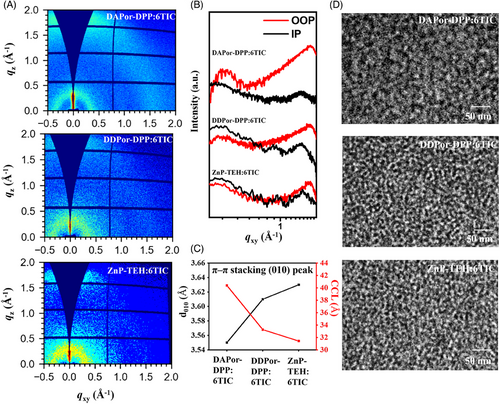
To delve deeper into the impact of asymmetric materials on the stacking behaviors of blended films, morphology characterizations were undertaken. Seeing from the 2D images (Figure 3A), the DAPor-DPP:6TIC-based blend film displays the highest-intensity π‒π (010) stacking signal compared to other counterparts, which can further be proved by the tendency of 1D curves (Figure 3B). Figure 3C and Table S4 show the crystallization behaviors of the blended films. The d010 and CCLs were calculated as 3.55 and 40.39 Å for DAPor-DPP:6TIC-based active layer, 3.61 and 33.26 Å for DDPor-DPP:6TIC-based active layer, and 3.63 and 31.42 Å for ZnP-TEH:6TIC-based active layer, respectively. These values demonstrated the tightest and most orderly molecular packing in the DAPor-DPP:6TIC-based active layer, which suggested that employing asymmetric structures significantly improved the molecular crystallization and orientation behaviors. Furthermore, contact angle tests were performed to investigate the miscibility of 6TIC and the donors. As shown in Figure S14 and Table S5, the calculated interaction parameters (χ) of DAPor-DPP:6TIC, DDPor-DPP:6TIC, and ZnP-TEH:6TIC are 0.0030, 0.0417, and 0.2359, respectively. The results indicate that DAPor-DPP exhibited superior miscibility with 6TIC and thus improved the D/A interaction, which provides a chance to optimize the morphology of blend films. Transmission electron microscopy images are shown in Figure 3D. DAPor-DPP:6TIC-based blend film exhibited a pronounced bi-continuous network with a more favorable phase separation scale around 30 nm, while the other two blends presented a smaller phase separation scale. These results tend to facilitate charge extraction and carrier transport, thus improving the JSC and FF in the corresponding devices.
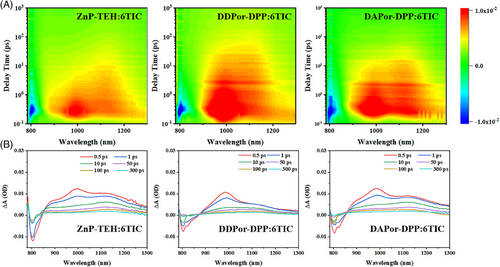
In addition, we further measured transient absorption (TA) spectra, aiming to obtain a deeper awareness of carrier transport mechanisms. TA spectra of blends and neat films are shown in Figures 4 and S15 by employing the pump wavelength of 800 nm. As shown in Figure 4A, three blends present similar TA images (concentrated in the range of 1100‒1300 nm). While regarding 6TIC, a well-defined excited state absorption feature was found at about 995 nm. By fitting decay curves of ground state bleaching peak at around 800 nm in Figure 4B, τ can be obtained to present the charge transfer process in the active layer, which was related to the time scale of exciton dissociation.51 As shown in Figure S16, τ values were calculated to be 1.29 ps for DAPor-DPP:6TIC-based devices, 3.12 ps for DDPor-DPP:6TIC-based devices, and 3.57 ps for ZnP-TEH:6TIC-based devices, which indicated the facilitating influence of introducing asymmetric structures. The results suggested that, in this system, the active layer constructed with asymmetric SMDs exhibited more efficient exciton dissociation compared to the symmetric counterpart ZnP-TEH, and also agreed well with the above-discussed device performances. These results suggest that the asymmetric SMD-based blends have faster hole transfer process compared to ZnP-TEH-based blend, which facilitates charge generation, resulting in higher photovoltaic performance of the corresponding OSCs.
3 CONCLUSION
In conclusion, we successfully synthesized two asymmetric porphyrin-based donors, namely DAPor-DPP and DDPor-DPP, via incorporating an alkyl pyrazole and an alkoxy benzene respectively as two asymmetric side chains on the porphyrin cores. Benefiting from the stronger intermolecular interactions of DAPor-DPP, DAPor-DPP:6TIC blends achieve a favorable morphology with enhanced crystallinity and closer molecular packing, which leads to more efficient charge transport and lower charge recombination in devices. Ultimately, a champion PCE of 16.62% was obtained in DAPor-DPP-based devices, which is significantly superior to the devices based on DDPor-DPP (14.96%). To our knowledge, the PCE of 16.62% is the highest value for ASM OSCs based on non-BTR:non-Y6 systems reported to date. Our study systematically illustrates the significant potential of a symmetric-breaking strategy for developing porphyrin-based SMDs in optimizing film morphology and improving the performance of ASM OSCs, offering valuable insights into designing and synthesizing SMDs for high-performance ASM OSCs.
ACKNOWLEDGMENTS
The authors thank the National Key Research and Development Program of China (2022YFB4200400) funded by MOST, the National Natural Science Foundation of China (52172048, 52103221, 22205130, and 12175298), the Shandong Provincial Natural Science Foundation (ZR2021QB024, ZR2021QB179, ZR2021ZD06, and 2023HWYQ-026), the Qingdao New Energy Shandong Laboratory Open Project (QNESL OP 202309), the Guangdong Natural Science Foundation of China (2023A1515012323, 2023A1515010943, 2022A1515110643, and 2024A1515010023), and the Fundamental Research Funds of Shandong University. The authors sincerely thank the staff of beamlines BL17B1, BL19U, and BL01B1 at SSRF for providing the beam time and User Experiment Assist System of the SSRF for their help. The authors also acknowledge Haiyan Sui of the Core Facilities for Life and Environmental Sciences and the State Key Laboratory of Microbial Technology of Shandong University for the NMR analysis (Bruker AV600 Spectrometer).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in the Supporting Information of this article.




