Topological network design toward high-performance vegetable oil–based elastomers
Abstract
As a kind of bio-derived feedstock, vegetable oil (VO) shows great potential to replace petroleum-based monomers to develop sustainable polymer materials because of its easy availability, low cost, bio-renewable, and environmentally friendly nature. However, due to the high cross-linking density and amorphous nature, directly cured VOs generally tend to be brittle and weak. To date, it is still difficult to adopt VOs and their derivatives as structural materials to prepare high-performance elastomers. To address this important issue, a multi-scale topology design strategy was proposed in this work. First, topology regulation and functionalization of VO-based networks were realized by managing functional groups proportion during the bulk polymerization of epoxidized soybean oil with dimer fatty acids. Furthermore, a second polymer (SN) network was introduced into the VO-based network as a protective layer via interfacial cross-links. The generated VO-based elastomers (VOEs) exhibit unprecedented comprehensive properties (VO content ≥70 wt.%, Tg as low as −24.4°C, toughness up to 6.8 MJ/m3). Besides, the VOEs also exhibit excellent reprocessability and self-healing capability. Overall, this work developed a novel kind of VOEs with significant comprehensive advantages and provided important inspiration for the preparation of high-performance elastomers through multi-scale topology regulation.
1 INTRODUCTION
Elastomers play an indispensable role in human daily life and many industrial fields. Most synthetic elastomers are based on fossil resources. The development and applications of various petroleum-based materials not only promote the vigorous development of productivity but also bring a series of environmental and resource problems.1, 2 On the one hand, petroleum-based feedstocks are nonrenewable and often cause some negative effects on living organisms that cannot be ignored.3 On the other hand, petroleum-based thermoset wastes are difficult to recycle and degrade naturally, thus putting great pressure on the environment. Therefore, in order to meet the increasing demand for sustainable development, it is imperative to explore renewable raw materials to synthesize bio-based polymers. Lignin, starch, protein, vegetable oil (VO), and other renewable resources have been paid more and more attention because of their wide sources, high yield, low cost, non-toxicity, and environmental friendliness.4-6 Great efforts have been made to produce various renewable materials from biomass instead of petroleum-based monomers.7, 8 It is foreseeable that the increasingly wide application of bio-based materials will greatly alleviate the current global shortage of petrochemical resources and severe climate problems, which is of great significance for achieving “carbon neutrality.”9
Soybean oil (SO), a major VO, contains more than 99% triglycerides, of which esterification products of glycerol and various unsaturated fatty acids are the main components.10 As the most important plant oil derivatives, epoxidized soybean oil (ESO) has higher reactivity than other biomass and can be directly polymerized as a monomer. ESO has been widely used in various applications, such as plastics, coatings, and adhesives.11-13 Unfortunately, due to the network with high cross-linking density and amorphous nature, the directly cured ESO born with high brittleness and low strength is often used as a nonstructural material.14, 15 At present, most of the studies on ESO-based materials focus on improving the glass transition temperature (Tg) of such materials aiming at higher mechanical strength and modulus. For example, incorporating petroleum-based epoxy resins into ESO,16 or using rigid crosslinkers to cure ESO, were adopted to increase the stiffness of the network.17-19 However, increasing the rigidity of the network often led to a decrease in toughness. In addition, the introduction of crystallizable cross-linking agents into the ESO network,20, 21 or blending ESO with crystalline polymers, was effective to improve the toughness of the overall networks.22 However, the Tg of the ESO thermosets prepared by the above methods was too high to be used as elastomers.
Elastomers are important soft materials, the high elasticity and high fracture toughness of which are critical for their applications.23, 24 In recent years, toughening elastomers by designing double networks has received extensive attentions.25, 26 Generally speaking, a dual network is formed by interpenetrating two networks with very different properties, one of which is highly cross-linked and brittle, and the other network is low-cross-linked and flexible, and often the latter has a significant content higher than the former. During the deformation, the molecular chains of the brittle network break preferentially and dissipate a large amount of energy, so that the crack propagation does not occur prematurely or too fast. In order to achieve higher mechanical properties, more complex multiplex networks, such as triple networks and quadruple networks, have been proposed to improve the nonuniformity of the network.27 Different from the abovementioned interpenetrating networks, our group recently designed a series of heterogeneously cross-linked multiphase networks with distinct microscopic phase separation based on an interfacial co-cross-linking strategy, which led to a significant increase in the toughness of the overall network.28, 29 In addition, Suo et al. combined hard blocks with soft matrix by sequential polymerization and photolithography to prepare structured polymer networks and realized interfacial bonding through topological entanglement to obtain topological polymer networks both with high elasticity and high toughness.30 In short, network topology design is an effective means to improve the mechanical properties of elastomers.
Herein, we design a class of VO-based elastomers (VOEs) with a multiphase cross-linked network structure by multi-scale topological regulation. First, ESO was directly cured by dimeric acid derived from plant oil. In this process, the polymerization behavior of the monomers (chain extension, grafting, acidolysis, cross-linking, etc.) was regulated by managing the molar ratio of carboxyl with epoxy groups (f) in the network to realize the control of network topology. Besides, combined with the trapping effect of the polymerization network on functional groups, the first network (FN) with tunable mechanical properties and functional group content was obtained. Further, a small amount of “interfacial binder” containing epoxy groups was mixed into the FN by mechanical mixing and constructed a second network (SN) based on interfacial chemical cross-linking. The construction of the SN further regulates the topology of the overall network at the mesoscopic scale. By virtue of the ductile SN protective layer to passivate cracks and the high deformation ability of the FN with special molecular topology, the prepared dual-network elastomer exhibits an unprecedented combination of high toughness and low-temperature resistance. The entire network is cross-linked by thermally activated dynamic ester bonds,31, 32 conferring self-healing capability and reprocessability, thus improving the sustainability of the developed materials.
2 EXPERIMENTAL SECTION
2.1 Materials
ESO (average functionality of epoxy is 4.2) without double bonds left was supplied by Aladdin. Dimer fatty acid (DFA, 98%, 191 mg KOH/g of acid value) was purchased from Guangzhou Yuanda New Material Co.,Ltd. ELVALOY PTW (copolymer of ethylene–butylacrylate–glycidyl methacrylate) was manufactured by Dow Chemical. 1,2-Dimethylimidazole (DMI, 99%) and 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD, 98%) were purchased from Sigma-Aldrich. Toluene was purchased from Tianjin Fuyu Fine Chemical Co. LTD.
2.2 Synthesis of functionalized vegetable oil–based (VO-based) network (ED-f) with ESO and DFA
The chemical structures and functionalities of ESO and DFA were characterized by 1H NMR and FT-IR (Figures S1 and S2). ESO reacts with DFA to form hydroxyl-functionalized polyesters through the reactions between epoxy and carboxyl groups. A typical procedure of the ring-opening reaction of ESO and DFA was carried out in a 100 mL single-necked flask equipped with a magnetic stirrer. DFA and ESO in the determined carboxyl/epoxy molar ratio, f, were added into the sealed flask, and then, the catalysts for ring-opening reactions (DMI) and transesterifications (TBD) were added into the mixture of monomers. After mixing thoroughly, the mixture was poured into a Teflon mold and keep it in an oven at 150°C for 3 h. According to f, the resulting transparent cured biopolymers were named ED-f, f = 0.40, 0.50, 0.60, 0.70, 0.75, 0.80, 0.85, 0.90, and 1 (Table S1).
2.3 Preparation of dual-network VOEs and control sample
Briefly, a predetermined amount of PTW, an epoxy-functionalized ethylene–acrylate–glycidyl ether acrylate copolymer (Figure S3), as an interfacial adhesive, was directly admixed with ED-f by the two-roll mill and then molded by hot pressing to prepare VOEs, PED-f-g (f = 0.70, 0.75, 0.80, 0.85, 0.90, 1; g = 70, 80, 90, 95, 100), where f and g represent the carboxyl/epoxy molar ratios and the mass percent of VO ingredients in whole networks, respectively. All the above samples were subjected to hot pressing at 180°C for the optimum curing times, as determined by a vulcameter. Detailed formulations of these samples were tabulated in Table S1.
For the control sample, 20 wt.% PTW was dissolved in hot toluene at 90°C and then directly admixed with ESO and DFA (their total mass was 80 wt.% of the whole sample) accompanied with the catalysts to prepare the control sample, which was recorded as Control-80. The mixture was poured into a Teflon mold and keep it in an oven at 150°C for 3 h after removing the solvent.
3 RESULTS AND DISCUSSION
3.1 Design and construction of dual-network VOEs
Herein, SO-based elastomeric materials were prepared by a two-step method. As shown in Figure 1A, two derivatives derived from soybeans, ESO and DFA, were selected as raw materials for direct bulk polymerization to prepare primary cross-linked networks. SO can be epoxidized efficiently with a conversion rate of up to 98%. The 1H NMR spectroscopy of ESO reveals that the molar ratio of the epoxy to ester carbonyl groups was 1.4 based on the ratio of the peak area of the epoxy group proton (2.8–3.2 ppm) to the α-CH2 peak area of the carbonyl group (2.3–2.4 ppm). (Figure S1). Therefore, ESO has an average functionality of 4.2 (1.4 × 3). DFA is a long-chain dicarboxylic acid derived from unsaturated fatty acids (Figure S2), which may endow the prepared polymers with high elasticity, flexibility, hydrophobicity, and low Tg.33 The bulk polymerization of DFA (A2 type) and ESO (B3 type) may include chain extension, grafting, cross-linking, cyclization, and other reaction processes.34, 35 These processes were complexly affected by factors such as f and reaction degree. The ESO network directly cured by diacid was very fragile. Generally, it has been attributed to the following origins: (1) Excessive cross-linking density reduces the ductility of the network, making the network very fragile; (2) irregular flexible molecular chains result in low Tg and amorphous networks.14, 36, 37 In fact, the flexibility of the molecular chain of ESO is not the cause of its poor toughness, which instead confers low Tg for use as an elastomer. Therefore, it is crucial for ESO-based elastomers to moderately reduce the cross-linking density of the ESO network to improve its toughness.
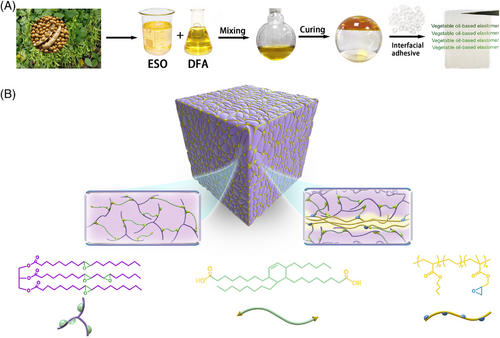
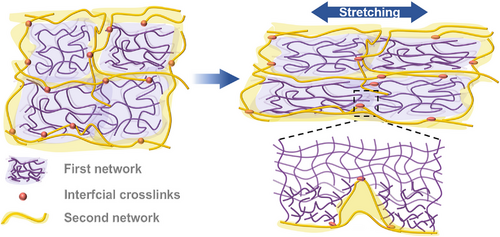
In general, functionalized oligomers are initially formed after the polymerization reaction of ESO and DFA begins. As the step-growth polymerization proceeds, a mixed system of polymers with different branching degrees are generated, and finally, a cross-linked network is formed. By controlling the proportion of the monomers, we can manage the cross-linking density and topology of the cross-linked networks, thereby preliminarily improving the toughness of the elastomer. Further, the primary network (FN) is mechanically mixed with PTW-containing epoxy groups (Figure S3). By the virtue of the reactions between the residual carboxyl groups of FN exposed at the interface and the epoxy groups of PTW, PTW chains attach to the FN, and a protective layer for the VO network is formed. By the virtue of interfacial cross-linking, the PTW protective layer constructs a spatial topological strip structure (Figure 1B). Therefore, a dual-network elastic network with multi-scale topology is prepared by a two-step method.
The primary VO-based network is prepared by ring-opening polymerization of ESO and DFA under the catalysis of DMI. The two raw materials are directly mixed according to a predetermined f for bulk polymerization at 150°C (Table S1). First, heat release curves during isothermal curing for different systems were monitored by DSC. It can be seen that all the polymerization reactions are completed within 90 min for all systems (Figure S4). Furthermore, the curing behaviors of mixed systems with different f were observed by small amplitude oscillation scanning of the rheometer (Figure S5). The gel points and equilibrium storage moduli (G′) of the networks are shown in Figure 2. It can be seen that, with increasing f, the gelation rate of the networks decreases, and the cross-linking degree also decreases. This indicates that during the reaction between DFA and ESO, more DFA is involved in the grafting and chain extension reactions, rather than directly forming more cross-linked structures with the increase of DFA content. This further weakens the trapping effect of the cross-linking network on functional groups, so that more functional groups can participate in the polymerization process. Thus, an increase in f leads to a systematic change in the polymerization behavior of the monomers, enabling a systematic management of the molecular network topology. Besides, a lower DFA proportion also was attempted to reduce the cross-linking density of the network. As shown in Figure S6, the storage modulus (G′) of cured ED-f (f = 0.4–0.7) decreases with decreasing f, which implies consistently decreasing cross-linking degree of ED-f.
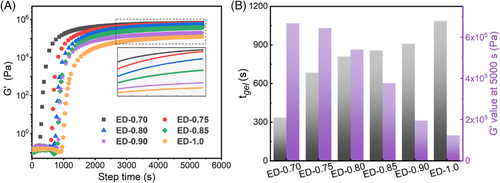
Furthermore, the curing reaction of the network was monitored by in situ infrared spectroscopy. Specifically, the mixed system with different f is placed in the test window of 150°C, and then, the infrared spectrums are recorded at different reaction times (Figure S7). The same baseline correction and normalization are performed on the spectra (Figure S8). The results show that as the reaction proceeds, the intensity of the C=O absorption peak (∼1743 cm−1) belonging to the ester bonds increases, and that of the C=O peak (∼1715 cm−1) belonging to the carboxyl groups in the DFA monomer gradually decrease (Figure 3A). In addition, the absorption peak of the epoxy group in ESO is gradually reduced, and the change rate slows down gradually (Figure 3B), which is similar to the changes of carboxyl content. Because the wave number of the absorption peak at 1715 cm−1 attributed to C=O in the carboxyl group is relatively unaffected, the change in the intensity of this absorption peak is suitable for the kinetic study of the ring-opening reaction. However, the C=O absorption peak assigned to the carboxyl group partially overlaps with that of the ester bonds, so the deconvolution of the C=O absorption peak is performed (Figure 3C and Figure S9). Taking ED-0.90 as an example, as the reaction proceeds, the carboxyl groups gradually are transformed into ester bonds. Furthermore, the changes in the content of carboxyl groups in systems with different f along the increase of reaction time are calculated and fitted (Figure S10 and Figure 3D). It can be seen that, as the reaction proceeds, the carboxyl groups are consumed rapidly and then gradually reach an equilibrium state, which is consistent with the abovementioned changes in the exothermic rate during isothermal curing and the storage modulus in rheological tests. This is because, as the reaction proceeds, the concentration of functional groups decreases. In addition, as the reaction proceeds, the viscosity of the system increases, and gradually, the system gels, which further impedes the reactions between carboxyl and epoxy. It was worth noting that when the equilibrium is reached, the carboxyl groups remained in the network, which is attributed to the fact that some DFA participates in the network in a grafting manner, and the residual functional groups are trapped in the formed 3D networks. The primary network is, therefore, functionalized by the residual carboxyl groups, which is the prerequisite for the subsequent construction of a second protective network through interfacial cross-linking.
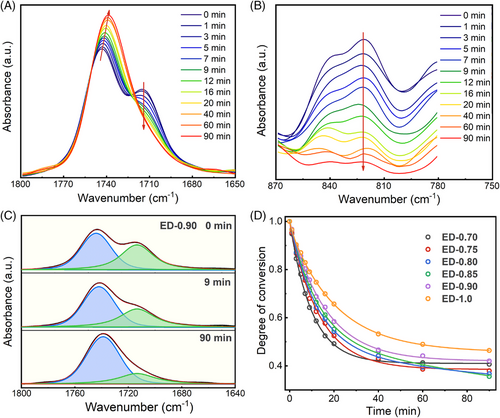
As a result, it is believed that the obtained cross-linked network undergoes an evolution of the network topology as f increases (Figure 4). It can be found that the DFA-cured ESO network possesses the highest cross-link density when f is about 0.7 (Figure 2 and Figure S6). At f = 0.70, the 3D network is formed rapidly with high cross-link density and tight structure. The rapid gelation and sufficiently high cross-linking density lead to a significant “trapping effect,” which prevents the complete reaction between the carboxyl and epoxy groups. As f increases, on the one hand, the rate of polymerization reaction decreases, and the network is gelled at a longer time, which provides an opportunity for the full reaction between functional groups; on the other hand, some carboxyl groups extend the molecular chain between the cross-links by the acidolysis of the glycerol center of the ESO molecules,38 which allows more monomers to participate in the formation of the 3D network in the form of chain extension. When the carboxyl group fraction is too high (f ≥ 1), the integrity of the cross-linked network decreases due to the presence of superabundant uncured branched oligomers or even diacid monomers in the network. In short, the polymerization reaction behavior of the ESO/DFA and the effect of f on the network formation and the final network topology are systematically revealed.
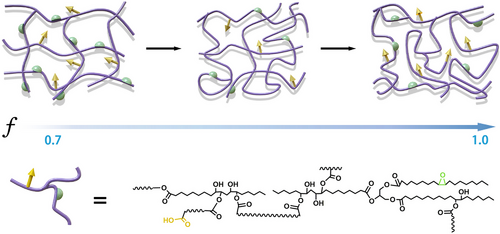
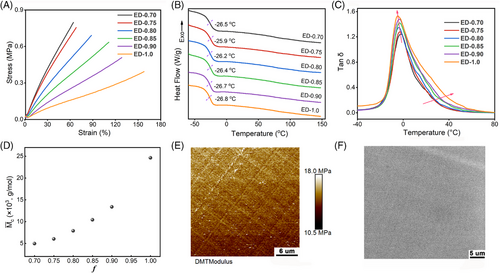
With the evolution of the network topology, the macroscopic properties were also manipulated by f. As shown in Figure 5A, with increasing f, the final strength and modulus of the cross-linked ED-f decrease, whereas the elongation at break gradually increases. When f increases from 0.70 to 1.0, the elongation at the break of ED-f is nearly twofolded. This is caused by the aforementioned change in the topology of the network. However, the tensile property of ED-f is not improved despite the decrease in cross-linking density with decreasing f (0.40–0.70) (Figure S11). The thermal analysis results show that the Tg of the network decreases only slightly from ED-0.70 to ED-1.0, whereas the loss factor (tan δ) of ED-f increases with increasing f (Figure 5B,C). Along the increase in f, the tan δ peak is widened, whereas the storage modulus (E′) of the rubbery state is decreased (Figure S12). In addition, with increasing f, the swelling ratio of ED-f gradually increases, and the gel fraction decreases (Figure S13). We attributed the above results to the fact that as f increases, more branching reactions will occur during polymerization, and more highly branched oligomers with wide molecular weight distributions will form in the resulting ED-f.35 Besides, the molecular weight between adjacent cross-links increases, leading to a decrease in the overall cross-linking density of the network and increased molecular chain motility (Figure 5D). It is obvious that the viscoelasticity and tensile properties of the DFA-cured ESO network are regulated by modulating the ratio of monomer functional groups. Especially, as f increases, the extensibility of the ESO network is improved. Further, taking ED-0.90 as an example, the microscopic morphology of the network is observed by AFM and SEM (Figure 5E,F). The relatively uniform modulus distribution and the flat and smooth morphology indicate that the prepared ED-0.90 is essentially a homogeneously structured elastomer.
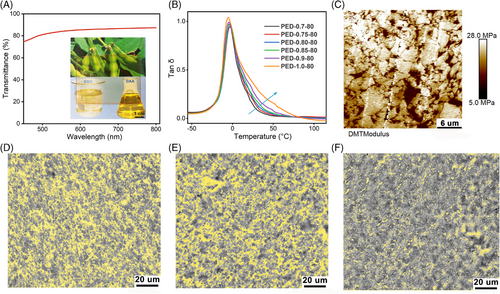
Although the deformability of the cross-linked ESO network is improved by modulating the molar ratio of polymerized monomers, its toughness is still not sufficient for practical applications. As mentioned above, the functionalization of ESO network is also achieved through topological modulation; a small amount of PTW was further introduced to construct an SN via interfacial cross-linking, which also acted as a “protective layer” for the VO-based network (Figure 1). First, we prepared PED-f-80 series samples by introducing 20 wt.% of PTW directly into ED-f by general rubber processing method (mechanical mixing and compression molding). Remarkably, the cross-linked VO network (ED-f) acquires mechanical processability, which renders it great potential for subsequent engineering applications. As shown in Figure 6A, the prepared VOE (PED-0.90-80) possesses excellent transmittance in the most visible light ranges. Besides, the Tg of the prepared PED-f-80 sample is as low as −24°C (Figure S14), which is only slightly higher than the initial ED-f (∼−26°C), indicating excellent low-temperature resistance. The endothermic peak of PED-f-80 near 67°C originated from the melting of crystallites in the PTW-based SN (Figure S15). The significantly reduced crystallinity of PTW in the VOE compared with that for the neat PTW also provides additional implications for sufficient interfacial cross-linking. Figure 6B presents the temperature dependence of the tan δ for PED-f-80, showing only one broad tan δ peak for all samples. Compared to ED-f, tan δ at Tg for PED-f-80 is significantly lower because the overall network motion is further constrained by interfacial cross-linking. E′ of PED-f-80 at elevated temperature decreases with increasing f, which indicates that the overall cross-linking density of the dual network is mainly determined by ED-f (Figure S16). The results of the equilibrium swelling show that the swelling ratio of PED-f-80 increases, and the gel fraction decreases as f increases. Compared to ED-f, the introduction of the SN further reduces the sol fraction of the overall network (Figures S13 and S17). As shown in Figure 6C–F, the microscopic morphology of PED-f-g indicated that the dual-network VOEs constructed by stepwise curing exhibit a microscopically phase-separated structure, which is attributed to the significantly different cross-linking density between the two networks. The reticulated phase (the yellowish phase in the SEM photograph and the low-modulus region in the AFM stress diagram [Figure S18]) corresponds to the SN with lower cross-linking extent, which is uniformly adhered to the surrounding highly cross-linked VO-based network (ED-f). Visibly, the microscopic phase of dual-network VOEs is not only related to the cross-linking degree of FN (Figure S19) but also affected by the mass ratio of FN and SN. As shown in Figure 6D–F, with the decrease of SN content, the continuity of VO-based network increases, and the microstructures of PED-0.90-g gradually evolve into the continuous two-phase structure from the analogous “sea–island” structure. The tiny and homogeneous phase size and excellent interfacial bonding enable the phase-separated PED-0.90-80 highly transparent (Figure 6A).
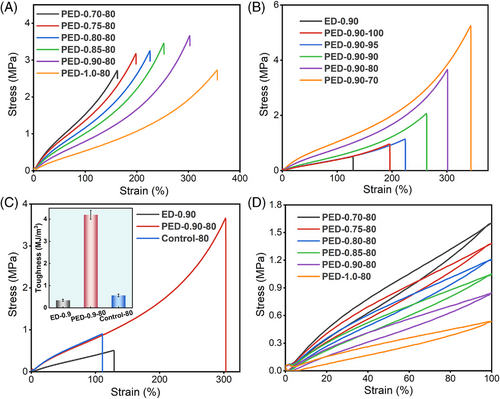
3.2 Mechanical performance and toughening mechanism of dual-network VOEs
As mentioned before, the toughness of elastomers can be significantly improved by introducing an SN into the elastomer. Here, the chemical gluing of the brittle and weak VO-based FNs by a “protective layer” is realized by the virtue of the interfacial reaction of the carboxyl-functionalized SN with the epoxidized polymer. As shown in Figure 7A and Figure S20, the introduction of PTW significantly improves the stretchability of the networks. Compared to ED-f, the elongation at break and the ultimate strength of PED-f-80 are significantly enhanced with the incorporation of only 20 wt.% of the second component. The mechanical properties of PED-0.9-g improved significantly with the increase in SN content (Figure 7B). Interestingly, the mechanical properties of PED-0.90-100 obtained by simply hot pressing without introducing PTW are also improved. We speculate that this is due to the changes in network structure during compression molding. The residual carboxyl groups in the ED-0.90 network are able to acidolyze part of the triglycerides in the network, lengthening the molecular chains between the adjacent cross-links. Meanwhile, the released carboxyl groups can further react with the residual epoxy groups (Figure S21), which further increases the overall cross-linking density. In addition, the Tg of the overall network is slightly increased, and the gel fraction is observably improved as the SN content increases (Figures S22 and S23). Control-80 was obtained by one-step mixing and curing of all components (ESO/DFA/PTW/catalyst) with the same formula as PED-0.90-80. Figure 7C compares the mechanical properties of ED-0.9, Control-80, and PED-0.90-80. It is clear that the introduction of 20 wt.% SNs significantly improves the mechanical properties of ED-0.90. Compared to ED-0.90, the fracture toughness of PED-0.90-80 is increased by 1200%. In contrast, the stretchability of Control-80 deteriorated, although Control-80 had a similar initial modulus and similar network viscoelastic behavior as PED-0.90-80 (Figure S24). It should be noted that Control-80 has higher crystallinity than PED-0.90-80 (Figure S25), yet its mechanical properties are incredibly poor, which suggested that the excellent mechanical properties of PED-0.90-80 do not originate from the crystallization of PTW. To further exclude the influence of PTW microcrystals on toughness, high-temperature tensile tests of ED-0.9, Control-80, and PED-0.90-80 samples were also conducted, and the results also demonstrated the above conclusion (Figure S26). The low residual strain (<4%) and hysteresis energy of PED-f-80 after loading–unloading cycling at 100% strain indicate excellent resilience of the dual-network VOE samples (Figure 7D). In addition, the introduction of SNs effectively improves the thermal stability of the VOE network (Figure S27). Compared to ED-0.90, the incorporation of 20 wt.% SNs (PED-0.90-80) leads to an increase of nearly 10°C in T5% (the temperature at 5 wt.% weight loss) (Figure S27a). It is attributed to the higher thermal stability of SNs, which acts as a protective layer for FNs to improve the temperature tolerance of the VO network (Figure S27b).
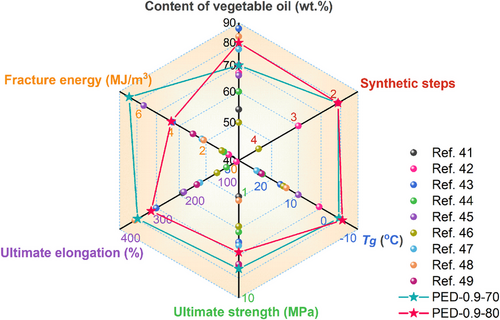
To comprehensively evaluate the performance of the prepared VOEs in this work, we compared the VOEs with the reported VOEs,39-47 in terms of VO content, synthesis process, Tg, and mechanical properties (Figure 8 and Table S2). Notably, the VOEs prepared in this work possess significant comprehensive advantages, including high VO content (≥70 wt.%), simple and green synthesis process (no complex chemical synthesis, no solvent involved, and general processing facilities and procedure), and transcendent low-temperature resistance (Tg significantly lower than all reported systems), excellent mechanical properties (final strength up to 5.3 MPa, elongation at break up to 343%, fracture energy up to 6.8 MJ/m3).
The excellent comprehensive performances of the dual-network VOEs are closely related to their unique multi-scale topological structures. Unlike the common interpenetrating multi-networks, the prepared dual-networks in this work consist of two-phase networks with significantly different cross-linking densities. In addition, the construction of the SN entirely depends on the interfacial reaction provided by the FN, resulting in the formation of a withy coating on the surface of the fragile FNs. As a result, the performances of VOEs can be improved significantly only by mixing a low content of SNs, ensuring the high renewability of overall materials. Besides, a relatively independent structure is maintained in the dual network, so VOEs exhibit a low Tg. More importantly, excellent interfacial bonding is crucial to the excellent mechanical properties of the material. Based on the reaction between the carboxyl and epoxy, the SN is attached to the FN to form excellent interfacial bonding between the two networks (Figure 6C,D). When the network is deformed, the interfacial cross-linking ensures the effective transfer of the stress, and the overall network integrity is maintained under small strain. When the deformation is increased further, the FN is highly stretched, and then, part of the network is damaged, and the cracks are initiated. The flexible layer (SNs) effectively prevents the extension of cracks and maintains the elasticity and structural integrity of the material (Figure 9). The above plausible toughening mechanism can be implicated by the results of loading–unloading cycling experiments of VOE samples. At lower strains (50%), the modulus of PED-0.90-80 is maintained during the multiple loading–unloading cycling. However, after cycling at larger strains (200%), the modulus decreased significantly with the increasing number of cycling (Figure S28). This may be because such small deformation mainly induces the reversible extension of network chains in the SN. As the deformation increases, the network stress further transfers to the highly cross-linked FN. Due to the considerable difference in the modulus between the two networks, a local strain amplification effect will also be achieved and, hence, leads to a larger local deformation of the SN.48, 49 Accordingly, the reversible hydrogen-bonding linkages in the SN network certainly will be dissociated, followed by the irreversible rupture of the covalent linkages due to the poor extensibility of the SN network. Thus, an obvious stress softening occurs upon reloading. After storing the specimen at 25°C, the dissociated hydrogen-bonding linkages can spontaneously reform again, leading to a certain recovery of the hysteresis loop of the sample (Figure S29). However, the rupture of the covalent linkages in the SN network still contributes an irreversible hysteresis even after sufficient storage. As the SN content increases, the interfacial layer became thicker and developed into a more continuous grid, further reinforcing the overall network (Figure 6D–F). In contrast, Control-80, also with a completely different dual-phase structure, does not exhibit similar excellent properties. It is because SNs distributed in micron-sized spherical particles in the FNs, which could not effectively protect the fragile FNs (Figures S30 and S31).
3.3 Recyclability and self-repairing capability afforded by dual-network VOEs
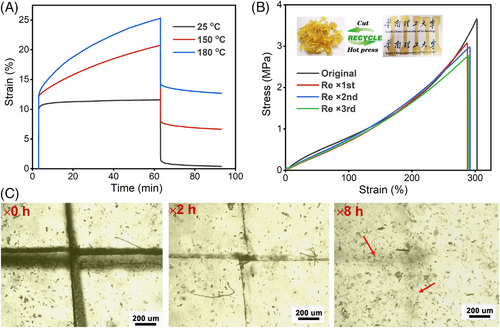
Reprocessability and self-healing capability can effectively prolong the service life of materials, which are features coveted by thermosets. Herein, the construction of overall VOE networks is based entirely on the cross-links of β-hydroxy ester bonds, which could undergo dynamic rearrangement upon heating. Therefore, the reprocessing of VOEs would be feasible. To verify its feasibility, the heat-stimulated network dynamics were first characterized by high-temperature creep and stress relaxation experiments. Figure 10A shows the creep behavior of PED-0.90-80 at different temperatures. When an external force is applied to PED-0.90-80 at room temperature, the sample is instantaneously deformed, and when the external force is removed, the sample returns to its original shape, exhibiting the elasticity of the covalently cross-linked network. When the temperature is elevated to 150 or 180°C, PED-0.90-80 exhibits a completely different viscoelastic response. After the thermal expansion and elastic response of the sample, viscous deformation occurs due to the reshuffling of the ester bonds at elevated temperatures. The dynamic feature of the network is further confirmed by stress relaxation tests (Figure S32). As shown in Figure 10B, a smooth recycled PED-0.9-80 sample can be obtained by hot pressing its scraps at 180°C for 10 min. The PED-0.90-80 sample can recover most of the mechanical properties (recovery ratio >85%) of the original sample despite multiple reprocessing. The FT-IR, viscoelastic behavior, and gel fraction of the PED-0.90-80 sample do not change significantly before and after the reprocessing (Figures S33–S35), indicating an almost unchanged network structure before and after recycling. Besides, the self-healing ability of PED-0.90-80 is demonstrated by scratch-healing experiments as shown in Figure 10C. The sample with cross scars made by the scalpel was placed in an oven, and after being treated at different times, the wounds were observed with a microscope. Obviously, the wounds are healed gradually with increasing treatment time, and almost no obvious marks are observed after healing for 8 h. The demonstrated good self-healing ability of PED-0.90-80 is beneficial to extend its service life.
4 CONCLUSIONS
In summary, this study demonstrates a novel strategy to prepare high-performance VOEs based on multi-scale topological network design, and the resulting VOEs exhibited outstanding comprehensive properties. First, the incipient full VO-based network was prepared by ring-opening polymerization of dimeric acid with ESO. In this way, the topology design and carboxyl-functionalization of the cross-linked network were achieved by regulating the proportion of monomers to control the polymerization behavior and trapping effect. Further, a small amount of withy polymer (PTW) was introduced into the FN as the SN by a facile mechanical compounding process. VOEs with dual-network structures were yielded by the virtue of interfacial cross-linking between FN and PTW. The strength and stretchability of the overall network were significantly improved by blending only 20 wt.% SN, and the fracture toughness could be improved by one order of magnitude. Meanwhile, the introduction of SN with higher thermal stability also improved the overall network thermal stability. Besides, VOEs exhibited unprecedented cold resistance (low Tg) compared to previously reported VO-based elastomers. The excellent overall performance of the dual-network VOEs was due to their unique multiphase network structure, in which the SN could effectively prevent the crack expansion of the FN upon deformation and maintains the integrity of the overall network. In addition, the heat-activatable dynamic ester bond cross-links endow VOEs with reprocessability and self-healing capability, further highlighting the sustainability of these bio-based thermosets. Overall, this study provides a new approach for the design of high-performance bio-based thermosetting elastomers, which has guiding significance for the synthesis of sustainable thermosetting elastomers and will also promote the practical application of VOEs.
ACKNOWLEDGMENTS
The work was funded by the National Science Fund for Distinguished Young Scholars (51825303) and National Natural Science Foundation of China (52130305, 52073097, 51903085, and 52003024).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.




