Residual water and interfacial bonding effects on the mechanical performance of CNT/fly ash geopolymer binder
Abstract
Nowadays, geopolymers are advanced alternatives to cementitious materials, where their excellent chemical and fire resistance are some of its most appealing properties. Fly ash geopolymers enable the use of industrial waste materials while converting them into a novel binding material. Their production is accompanied by a much lower CO2 emission when compared to Portland cement. Reinforcing fly ash geopolymers with carbon nanotubes would significantly strengthen its microstructure and with this enhancing the long-term mechanical and durability performance. The aim of this work is to use reactive molecular dynamics simulation method to optimize the mechanical properties of fly ash geopolymers with nano-reinforced carbon nanotubes (CNTs). During this study, the impact of humidity and interfacial bonding strength between carbon nanotube and geopolymer is investigated. Our findings show that structural transformations under hydration process comes from the weakening of AlO bond, leading to the elongation of AlO and NaOH bonds, forming aluminum and sodium hydroxyls. Conversely, silicate is not sensitive to water and exhibits hydrophobic behavior. In addition, our results show that there is an optimal value of water content (7.17 wt.%) that makes the geopolymer nanostructure strengthen. The related elastic modulus rises by 21.56%, 20.60%, and 18.41% for Si/Al ratio of 1, 2, and 3, respectively. Inserting carbon nanofillers to fly ash nanostructure has remarkably shown an interesting strength enhancement. More precisely, when interfacial bonding concentration is around 19.36%, it is observed a positively increasing of the compressive strength, shear, indentation and elastic modulus with 39%, 65.2%, 72.3%, and 144.85%, respectively. Reduced density gradient supports that the interaction between carbon nanotube and fly ash geopolymer is dominated by a van der Waals one.
1 INTRODUCTION
Geopolymer cements are nowadays an advanced alternative to construction materials1 and the only binder with an environmentally friendly application since their production is accompanied by much lower CO2 emissions.2, 3 Nowadays, geopolymer nanocomposites, an alternative binder to ordinary Portland cement (OPC), are drawing considerable attention for their great prospective to withstand heat and sound exposure beside to their outstanding properties including high early strength, little creep and shrinkage and a good resistance to corrosion and abrasion. The role of such materials is to bind unreacted aggregates to form a stable and solid hardened mass. They are synthesized by mixing aluminosilicate sources such as metakaolin4 and industrial by-products5 (fly ash and ground granulated blast furnace slag) with alkali activators (NaOH, KOH) and water. The role of alkali activators is to induce the dissolution of the precursors (aluminosilicate sources) and the polymerization of AlO2 and SiO2 tetrahedra into a three-dimensional network on the molecular scale. Some water remains physisorbed while the cations (Na+ or K+) are bound ionically, providing positive charges that maintain overall neutrality and allow for the Al to be tetracoordinated. During geopolymerization, there are mainly three steps before the formation of the final product: dissolution of silicon and aluminum atoms from the source material, reorientation of precursor ions, and finally their condensation process. The final obtained product is sodium aluminosilicate hydrate (NASH) gel, which is the main hydration product of geopolymer. The structure of NASH is a three-dimensional network composed of silicon–oxygen tetrahedral and aluminum–oxygen tetrahedral. Geopolymers exhibit excellent chemical and fire resistance as well as high mechanical strength and durability compared to the OPC.6-9 Besides, they promise an excellent toxic metal immobilization property.10 Geopolymers have complex structure due to the formation of the cross-linked silicate and aluminate tetrahedrons connected by bridging oxygen atoms.11 X-ray diffraction patterns reveal the presence of regions with various degrees of crystallinity: from highly crystalline to nanocrystalline, polycrystalline to amorphous.12 Besides, it is found that under raw material composition and curing conditions, the structure of geopolymers is similar to that of zeolite.1, 13-21 Many works have shown that hydroxysodalite is the easily formed phase in the case of fly ash geopolymers.22-27 The mechanical properties of geopolymer composites have been widely investigated experimentally.28-31 These studies indicate that the strength of geopolymers increases with increasing Si/Al or Na/Al ratio up to a certain level and then starts to decrease, meaning that there is an optimum value for both the Si/Al and Na/Al ratios. Belena and Zhu32 reported that the Young's Modulus and hardness of geopolymer gels cured under ambient conditions was in the range of 7–14 and 0.2–0.5 GPa, respectively.
Developing a higher strength fly ash geopolymer material is actually a challenge and the promising way to develop this new generation of materials is the incorporation of nanomaterials. Experimental studies show that the incorporation of nano-SiO2 and nano-Al2O3 improve the mechanical strength of the microstructure.33-36 SEM tests reveal that filling 5% of nano-TiO2 to the geopolymer matrix makes the specimen more compact and very dense with less cracks. For a higher amount of TiO2, the porosity was decreased significantly due to the denser microstructure.37 In the other hand, it is shown that the effect of nano-materials and geopolymeric reactions produce a dense matrix resulting in a lower water absorption.38 It is reported that for geopolymer mortars specimens a noticeable decrease in water absorption value with increasing time of hydration.39 Regarding carbon nanotubes (CNT), they still have extraordinary mechanical properties, with an elastic modulus of the individual nanotube of around 1 TPa, and a tensile strength in the range of 65–93 GPa.40 In the work of Saafi et al.41 it is shown that the addition of a concentration up to 0.5 wt.% of MWCNTs increased the flexural strength, young modulus and flexural toughness by 160%, 109%, and 275%, respectively. It also enhanced the fracture energy and increased the electrical conductivity by 194%. Abbasi et al.42 studied the reinforcement of metakaolin-based geopolymer with MWCNTs at concentrations of 0, 0.5, and 1 wt.%. They found that only 0.5 wt.% of MWCNTs gives an increase of the flexural strength and elastic modulus by 128% and 109%, respectively. Luz et al.43 observed that the addition of 0.1 wt.% of pristine CNT in metakaolin geopolymer improves the Young's modulus, compressive and flexural strengths with 3.8%, 13.2%, and 28.7%, respectively. Experiments measurements of Yuan et al.44 reported that 3 wt.% of functionalized COOH-MWCNT enhances the flexural strength, the elastic modulus and the fracture toughness with 42.3%, 29.6%, and 38.5%, respectively. Interesting results were observed when graphene and its derivatives (graphene oxide and reduced graphene oxide) are filled.45 Zhang et al.46 found that substitutional doping of graphene with silicon inserted into metakaolin geopolymer gives the elastic modulus double than graphene/geopolymer matrix. As discussed in other works,47-49 the dispersion problem of CNT and interfacial chemical bonding have a great impact on the structure and mechanical properties of the reinforced nanocomposite. More recently, experimental measurements50 on the evolution of mechanical properties of metakaolin-red mud/carbon nanotubes show that when adding 2% of carbon nanotubes, the compressive and the flexural strengths increased to 37.05% and 36.06%, respectively due to the bridging mechanism and filling the cavities and porosity. On the other hand, scanning electron microscopy51 show that the addition of 0.045 wt.% of multi-walls CNTs significantly increased the compressive strength and flexural strength of slag-based geopolymer. Recent modeling and simulation research52 address the subject of the improvement of mechanical properties of metakaolin geopolymer structure through the incorporation of CNT nanofillers. They found that the incorporation of 1.08 wt.% of pristine CNT enhance the elastic and the indentation modulus up to 74% and 94%, respectively. While 10% doped CNT-oxide lead to a raise of the elastic modulus up to 165% making denser the nanostructure. As a conclusion, the exploitation of CNT geopolymer is still limited due to a poor understanding of the linkage between chemical composition and macroscopic properties.
The scope of the present paper is to develop a higher strength fly ash geopolymer reinforced by carbon nanotubes. Using modeling and simulations tools, our aim is to analyze deeply the role of water content and interfacial chemical bonding between CNT and fly ash geopolymer on the structural transformations and the mechanical properties of the nanostructure. Reactive molecular dynamics simulation is used to gain insight into the bonding transformation under humidity and chemical composition variations. Carbon nanotubes are inserted into the sample with various bonding concentrations. Mechanical properties for the CNT/geopolymer nanostructure will be evaluated including elastic and indentation modulus, compressive and shear strength. A particular attention will be paid to the nature of the interaction energy between carbon nanotubes and fly ash geopolymer.
2 MODELING AND SIMULATION PROCEDURE
In this section we present schematically the procedure of the model construction. Fly ash molecular structure is represented, following the protocol below: we build a cubic simulation box containing the experimental sodalite structure of Hassan et al.53 (Figure 1a). Then, we replace Cl atoms with hydroxide ions (OH−) (Figure 1b). The obtained structure is relaxed based on the ReaxFF force field54 and the conjugate gradient algorithm in NPT ensemble at normal conditions of temperature and pressure. Afterwards, water molecules are introduced to the structure with various contents using the Grand Canonical Monte Carlo technique. The obtained NASH configuration is relaxed (Figure 1c) again giving the final structure with the chemical formulation (Na2O)4(Al2O3)3(SiO2)6(OH)2(H2O)9 (Figure 1d).
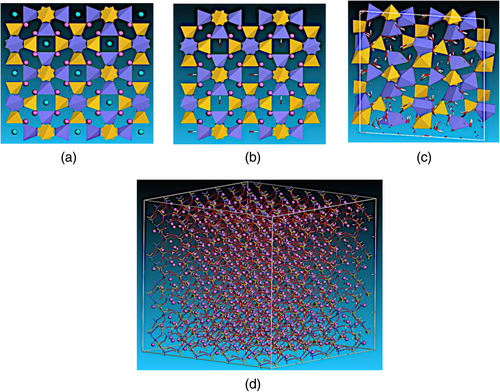
To investigate the tensile tests for the dry and wet structures, supercells with size of 40 x 40 x 40 Å are built containing 5402 atoms. In Table 1, it is presented the chemical constituents of dry and wet NASH model for various Si/Al molecular ratios of 1, 2, and 3, water contents (0–14.89%) and corresponding densities.
| Structure | SiO2 (wt.%) | Al2O3 (wt.%) | Na2O (wt.%) | Water content (wt.%) | Density (g/cm3) |
|---|---|---|---|---|---|
| Dry (Si/Al = 1) | 38.62 | 32.83 | 26.60 | 0 | 2.20 |
| Dry (Si/Al = 2) | 51.39 | 21.84 | 26.55 | 0 | 2.21 |
| Dry (Si/Al = 3) | 57.75 | 16.36 | 26.52 | 0 | 2.21 |
| Wet (Si/Al = 1) | 32.90 | 27.97 | 22.66 | 14.89 | 2.59 |
| Wet (Si/Al = 2) | 43.79 | 18.61 | 22.62 | 14.78 | 2.59 |
| Wet (Si/Al = 3) | 49.22 | 13.94 | 22.60 | 14.76 | 2.60 |
In the present work, reactive molecular dynamics method is employed based on ReaxFF forcefield developed by van Duin et al.55 and molecular dynamics method. ReaxFF forcefield is a bond-order dependent potential making possible the simulation of chemical reactions. The atomic charges are calculated from its charge equilibration nature. The total energy of the nanostructure is of a sum of the bond formation energy, the van der Waals energy, the Coulombic contribution, the angular and torsional energy, the lone pair energy and finally the energy penalty for under/over coordination stability of atoms. The ReaxFF parameters used in this work were developed by the group of van Duin55 containing data on Na/Si/Al/O/H systems. The used ReaxFF forcefield has been applied in geopolymer52, 56 and for calcium silicate hydrate.57-66
These structures are dynamically equilibrated using molecular dynamics simulations under NPT ensemble for 1 ns at 300 K and followed by equilibration phase in NVT ensemble at 300 K for one more 1 ns. Temperature and pressure are controlled by The Nose–Hoover thermostat and barostat algorithms.67 Periodic boundary conditions are applied to all directions during the simulation procedure. When equilibrium is reached, structural and bonding analyses are first investigated, followed by elastic properties calculations for the final structure using the constant strain method.68 In this method, a series of strains are applied to the simulation boxes in one direction with constant strain rate fixed at 0.01%/ps. The obtained stresses are then calculated by the virial stress method. During the simulation, the structure dimension along a loading direction changes linearly with time.
3 RESULTS
3.1 Mechanical properties of fly ash geopolymer nanostructure: humidity and chemical composition effects
The study will focus first on the analysis of the model used and its efficiency under composition and water content effects. As displayed in Figure 2a,b the variation of the density and the total energy of the relaxed structures with chemical composition and humidity variations. It is observed that wet NASH structure is more stable than the dry one since the total energy decreases with increasing water content from 0 to 14.89 wt.%. Further, it is noticed that varying molecular Si/Al ratio from 0 to 3 does not affect this tendency. The density variation presented in Figure 2a shows a slight increase in the beginning of the hydration process followed by a decrease with relatively a same slope for the three samples (Si/Al = 1, 2, 3). Specifically, for lower water content, H2O molecules fills the pore structures leading to a denser system with a higher density.
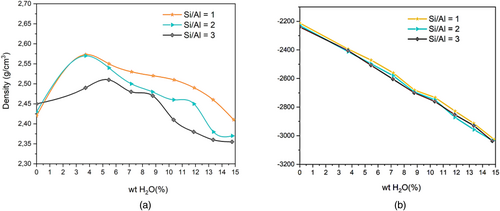
However, for higher water content, the skeleton of NASH undergoes structural transformations leading to a decrease of the density of the system, as will be explained below. Moreover, we notice that for a given water content, the skeleton density decreases with the increase of Si/Al ratio from 0 to 3. For more comparison, we report in Table 2 our data of densities for the dry and wet samples with Si/Al ratios variations from 1 to 3, with those from previous simulation calculations.69-72 The obtained data for the wet densities are between 2.35 and 2.41 g/cm3.
| Si/Al ratio | Density of dry sample (g/cm3) | Density of wet sample (g/cm3) | H2O (wt.%) |
|---|---|---|---|
| 1: Lyngdoh et al.69 | 2.53 | 2.37 | 16.17 |
| 1: Sadat et al.70 | 2.51 | 2.05 | 6.63 |
| 1: Our data | 2.42 | 2.41 | 14.89 |
| 1.22: Smilauer et al.71 | 2.372 | ||
| 1.15–1.65: Duxson et al.72 | 1.8-1.7 | ||
| 2: Lyngdoh et al.69 | 2.42 | 2.11 | 16.96 |
| 2: Sadat et al.70 | 2.45 | 2.03 | 6.65 |
| 2: our data | 2.43 | 2.37 | 14.78 |
| 3: Lyngdoh et al.69 | 2.24 | 1.99 | 19.78 |
| 3: Sadat et al.70 | 2.41 | 2.00 | 6.66 |
| 3: our data | 2.45 | 2.35 | 14.76 |
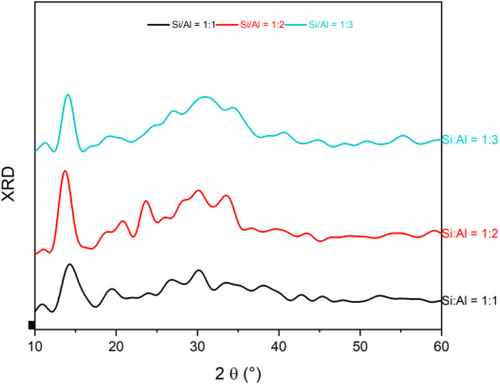
Figure 3 shows the simulated X-ray diffraction patterns of NASH configurations with Si/Al = 1, 2, 3, obtained from MD calculations. In fact, a principal broad peak (typical of disordered alkali aluminosilicate gels) is located between 2θ = 20° and 2θ = 35°, more pronounced in the sample with Si/Al = 3. Moreover, in the sample with Si/Al ratio of 2, it is noticed the presence of mainly two peaks around 24° and 30° related to the sodalite structure as found in experiments.63
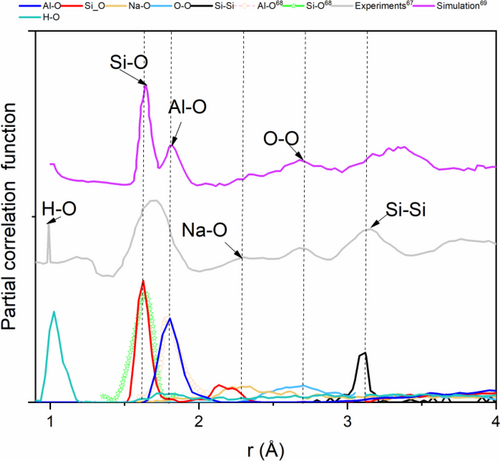
For a deeper analysis, a partial correlation function is presented in Figure 4 in comparison with experiments74 and other theoretical works.75 The lengths of different atomic bonding obtained from various peaks are summarized in Table 3. It is seen that our model gives data in good agreement with experiments73, 74 (0.61%–6.25%) and other theoretical calculations75, 76 (0.62%–7.36%). More precisely, SiO/AlO bond lengths of 1.62 and 1.81 Å are comparable with Raman spectroscopy and neutral diffraction measurements,73, 74 which confirms the current simulation results.
Now we turn our attention to the structural transformation under hydration process. For the present NASH model, silicon and aluminum are predominantly tetrahedral, with 80% of Q4 species (four bridging oxygen atoms connected to the center Si or Al atoms) (Figure 5a). Water molecules in NASH structure are physically and chemically bounded to SiAl skeleton. Adsorbed water is mainly bounded to aluminosilicate skeleton to form H-bond but also as terminal hydroxyl, as found experimentally.73 In Figure 5b, we present the configurations before and after water penetration, respectively. Our results agree with those obtained with the defective model proposed recently the beginning, oxygen in water molecule interacts with tetrahedral aluminum to form the weakening OH bond. Then, one hydrogen atom dissociates from water molecule leading OH bond attached to tetrahedral aluminum. Regarding silicate skeleton, almost all silicon atoms are four-coordinated.
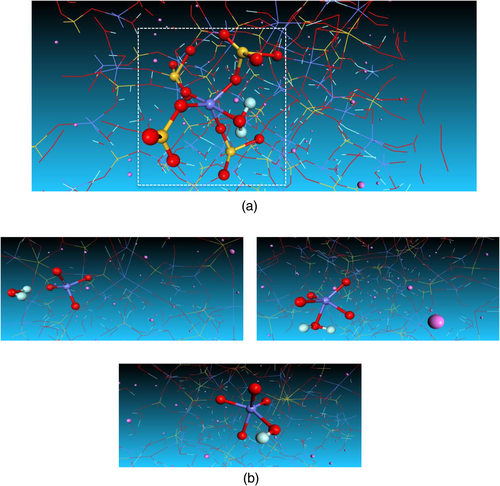
Partial correlation functions are used to probe the interatomic correlations in the NASH structure. Figure 6 shows the calculated curves for AlO, SiO and NaO bonds in the case of dry and wet nanostructures with water content of 14.89%. A significant increase for AlO bond is observed in Figure 6a, from the first peak enhanced from 1.77 to 1.81 Å (2.25%) when hydrating the sample. For NaO bond, it is noticed from Figure 6b that the position of the first peak changes with an amount of 4% from 2.20 to 2.29 Å. In contrast, it is shown from Figure 6c that SiO bond is rather unchanged against the hydration process since the position of the first peak is equal to 1.62 Å before and after hydration. That means that the reaction with water molecules comes from the weakening of Al-O bond, since most of bridging oxygen in silicate tetrahedron did not react with water molecules.77 Aluminate tetrahedron having a weak ionic-covalent bond has the ability to interact with neighboring water molecules to form H-bond and aluminate hydroxyls. This induces a reduction of the attractive electrostatic interaction between Al and their bonding O, which increases the bond distance. Further, sodium cations appear to interact with water molecules and stabilize to form NaOH bond.
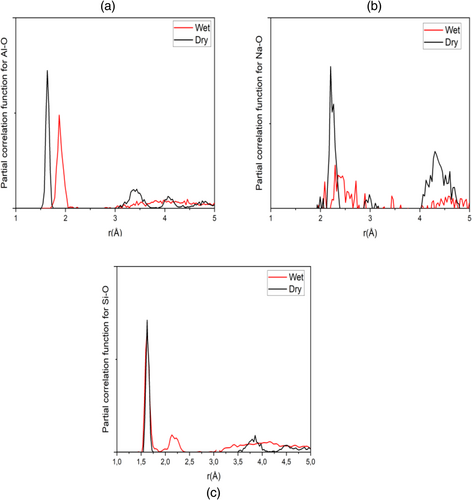
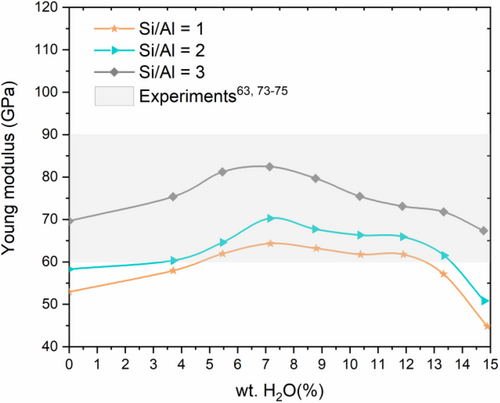
Figure 7 illustrates the variation of the elastic modulus with water content and chemical compositions variations (Si/Al = 1, 2, 3). The obtained data (between 44 and 82 GPa) are reported in Table 4. They are in the range of experimental measurements64 and data calculated from the glassy model.70, 82 As illustrated in Figure 7, the strength of the geopolymer paste increases with increasing Si/Al ratio, consistent with the experimental findings of Duxson et al.30 and other modeling calculations.70 For a given Si/Al ratio, increasing water content up to 7.17 wt.% leads to an increase of Young modulus by 21.56%, 20.60%, and 18.41% for Si/Al ratio of 1, 2, and 3, respectively. For further hydration up to 14.89 wt.%, it is noticed that the strength of the wet sample decreases by an amount of 29.23%, 27.66%, and 18.32% for Si/Al ratio of 1, 2, 3, respectively. Consequently, the loss of the young modulus amount from the dry to the wet sample with 14.86% of water content is around 3.28%, 12.75%, and 15.30% for Si/Al = 1, 2, 3, respectively. Previous study found that water weakens the mechanical properties of the sodium aluminosilicate structure.70 Our work shows, however, that there is an optimum value of water content (7.17 wt.%) for all compositions of Si/Al ratios that enhance the mechanical properties of NASH gel. The observed reduction of the strength of geopolymer paste with increasing water content (from 7.17% to 14.86%) is due the mobility of aluminum. During hydration process, skeleton connectivity is broken, which affects the stability of AlO bonds in AlO4 molecules leading to AlO bond breaking to form AlOH bonds. Since SiO bond is stronger than AlO, the obtained young modulus for the sample with Si/Al ratio of 3 is found to be higher. As a conclusion, our results clearly point that enhancing the mechanical properties of geopolymer paste can be obtained if the sample has low amount of aluminum (Si/Al = 3) combined with a reduced water content of around 7.17 wt.%.84
3.2 Interfacial bonding effect on mechanical properties of NASH/CNT nanostructure
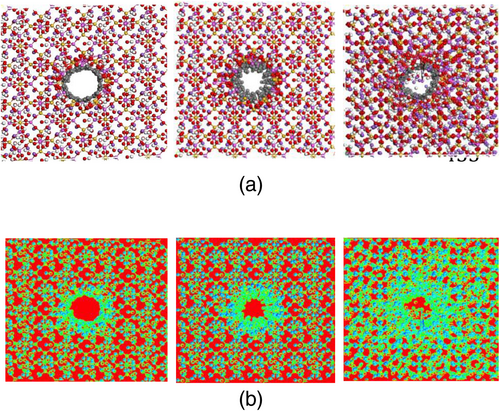
Regarding NASH/CNT model, a supercell with a size of 35 x 35 x 55 Å3 is created then one (6) CNT molecules is put in the middle of the supercell. After that, we pack the aluminosilicate structure into the simulation box with a density of 2 g/cm3. The obtained nanostructure contains 7309 atoms (Figure 8). In order to study the effect of interfacial bonding, carbon atoms are attached to oxygen of the aluminosilicate randomly with a bonding concentration in the range of 0%–19.36%.

Figure 9a,b displays the atomic structure and the bonding density for the NASH/CNT model with bonding concentration of 13.72%, 17.14%, and 19.36%, respectively. It is noticed that increasing the density of the connected atoms increase with increasing bonding concentration between CNT and oxygen atoms of the aluminosilicate structure. This is an important point since it affects the mechanical properties of the nanostructure, as will be discussed below.
In Figure 10a,b, radial distribution function (RDF) is represented for carbon–oxygen and carbon–aluminum/silicon interactions. It is observed from Figure 10a that the first peak for CO is located at around 1.11 Å for 13.72% of bonding concentration and is stabilized around 1.4 for higher concentrations. From Figure 10b, it is found that the location of the first peak is higher than the one of CO interaction. That is because oxygens atoms of the NASH structure are in the first layer around carbon nanotube nanofiller.
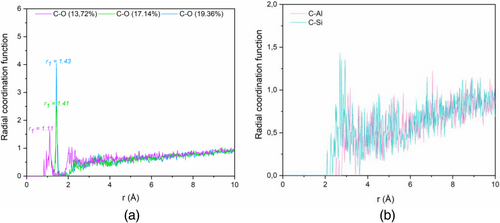

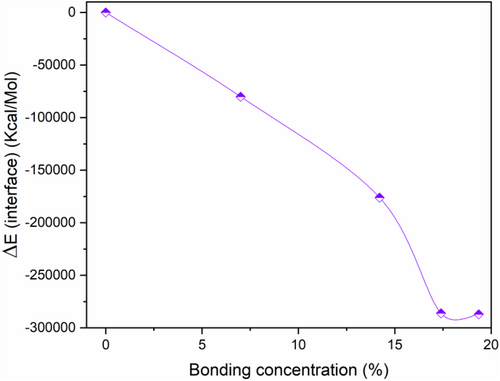
The obtained results displayed in Figure 12 show negative values of ΔE due to the attractive forces between CNT and NASH system. Moreover, all energies decrease with increasing CO bonding concentration, which means that interfacial bonding makes more stability to the CNT/NASH nanostructure.
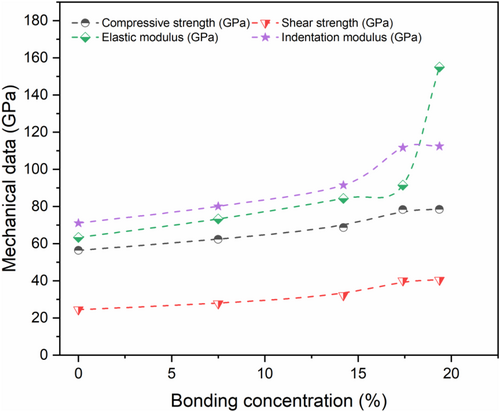
The impact the interfacial bonding is presented in Figure 13 where the results of the elastic properties are displayed with CNT/NASH bonding concentration. It appears that the compressive strength, the shear and the indentation modulus rise with 39%, 45%, and 58%, respectively. A distinguish reinforcement is observed when the bonding concentration is around 19.36% since the enhancement of the elastic modulus is within 145%. That means the nanostructure becomes denser for higher CO concentrations. This result is consistent with the recent work of Sekkal et al.52 finding that the incorporation of CNT-oxide with 10% of COH concentration leads to a reinforcement of the nanostructure since the elastic modulus is increasing with 165%, comparing to 65% in the case of 10% Si-doping graphene.46
4 CONCLUSION
The present work was devoted to atomic-scale investigations of the role of humidity and interfacial bonding between CNT nanofiller and NASH nanostructure. Reactive molecular dynamics simulations have been conducted varying the chemical composition Si/Al from 1 to 3 and the water content up to 14.89 wt.%. The obtained results predict the energetic stability of the three samples and a decrease of the corresponding densities with increasing water content. Under hydration process, our simulations prove that the structural transformations come from the weakening of AlO bond leading to the formation of aluminum and sodium hydroxyls, in contrast to the hydrophobic behavior of silicon. The enhancement of the elastic modulus is obtained for the sample with low aluminum content hydrated with 7.17 wt.%. The maximum improvement is 21.56%, found for the chemical composition ratio Si/Al = 3. Our findings show that the incorporation of carbon nanotube into the aluminosilicate sample makes the nanostructure denser, especially when the CO bonding concentration is around 19.36%. The obtained elastic modulus is enhanced with 145%, which is a promising result. The RDG analysis confirms the van der Waals interaction between CNT and oxygen atoms in the aluminosilicate framework.
AUTHOR CONTRIBUTIONS
WS: Conceptualization, Methodology, Data Curation, Writing - Review & Editing. A. Z: Conceptualization, Methodology, Writing-Review & Editing, Project administration.
ACKNOWLEDGMENTS
The authors would like to thank the French National Research Agency's (ANR) for the financial support of the “NanoGP” project under the number ANR-20-CE92-0016.This work is part of the international bilateral collaborative research project ANR/DFG between Laboratoire de Génie Civil et géo-Environnement (LGCgE) from the university of Lille (France) and the Institute of Construction and Building Materials from Technical University of Darmstadt (Germany).
FUNDING INFORMATION
The French National Research Agency's (ANR) for the financial support of the “NanoGP” project under the number ANR-20-CE92-0016.
CONFLICT OF INTEREST STATEMENT
The authors declare no competing interests.
Biographies

W. Sekkal, Univ. Lille, IMT Lille Douai, Univ. Artois, JUNIA, ULR 4515 - LGCgE, Laboratoire de Génie Civil et géo-Environnement, F-59000 Lille, France.
Email: [email protected].

A. Zaoui, Univ. Lille, IMT Lille Douai, Univ. Artois, JUNIA, ULR 4515 - LGCgE, Laboratoire de Génie Civil et géo-Environnement, F-59000 Lille, France.
Email: [email protected].
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




