Facile Synthesis of Zinc Indium Oxide Nanofibers Distributed with Low Content of Silver for Superior Antibacterial Activity
Abstract
Exploring new antibacterial materials is of great significance for limiting the transmission of germs and protecting human health. Although Ag and oxide nanoparticles have both been extensively used in the field of antibacterial, it is still urgently need to figure out how to combine their characteristics for sterilization. Herein, ZnO/In2O3-Ag nanofibers are prepared with uniformly dispersed Ag through a facile method of electrospinning and subsequent sintering. The obtained nanofibers are of high purity and have diameters from 90 to 110 nm. The silver is in a zero-valent state, which is beneficial for sterilization. The quantitative test of bacterial activity shows that the nanofibers are bactericidal for both Staphylococcus aureus and Escherichia coli, with an especially strong inhibitory effect on E. coli. At relatively low concentrations, 98% of E. coli can be killed. Herein, light is shed on exploring more complexes of oxide nanomaterials and Ag for sterilization.
1 Introduction
In the history of human survival and development, epidemics caused by pathogens such as viruses and bacteria have always posed a severe threat to human health.[ 1-4 ] Therefore, the development of efficient antibacterial materials is imminent. In addition, due to the overuse of antibiotics, especially against Gram-negative bacteria like Escherichia coli (E. coli) and Gram-positive bacteria like Staphylococcus aureus (S. aureus), bacterial resistance to currently available bacteriostatic agents is increasing.[ 5-8 ] Therefore, completely eliminating superbugs and addressing the growing serious problem of bacterial drug resistance have long been a research hotspot in the medical field.[ 9-12 ] Silver is a natural antibiotic that has the ability to kill more than 650 different kinds of bacteria and viruses.[ 13 ] Its bactericidal ability has been discovered and applied by humans thousands of years ago.[ 14 ] Although silver has unique antibacterial capabilities, it is not entirely harmless as an antimicrobial agent and is more expensive than other fungicides.[ 15 ]
Much research has focused on how to reduce the use of silver.[ 16-19 ] For example, Wang et al.[ 17 ] prepared Ag-doped carbon quantum dots and found that a trace amount of Ag can achieve excellent antibacterial properties. Liang et al.[ 18 ] prepared Pebax nanofiber-modified Ag nanoparticles and found that ultralow Ag loadings could effectively kill E. coli and S. aureus. Additionally, some oxide nanomaterials, such as ZnO, CuO, and their derivatives, have been developed as effective antibacterial agents.[ 20-25 ] By combining Ag and these oxides, not only can the amount of Ag utilized be lowered, but also the characteristics of the two can be combined to achieve a more effective sterilization effect. Peng et al.[ 26 ] constructed Ag/Ag2O/ZnO heterostructure decorating cellulose–chitosan films, which exhibits excellent antibacterial activity against E. coli and S. aureus. The width of the inhibition zone around the film reaches 10.0–19.6 and 12.4–15.0 mm, respectively. Li et al.[ 27 ] synthesized Ag@CuO nanohybrids with a core-shell structure. After being exposed to light for 10 min, the nanohybrids at a concentration of 11.0 mg L−1 reduce E. coli and S. aureus colony-forming units by at least seven orders of magnitude. Therefore, exploring the hybrid of oxide and Ag is an effective strategy for further improving the bactericidal performance.
The morphology and dispersion effect of Ag also affect the bactericidal activity. Sadeghi et al.[ 28 ] found that the surface area of silver, which is dependent on the shape and size of silver particles, is the key factor affecting the antibacterial activity. Jing et al.[ 29 ] prepared Ag nanoparticles with an average diameter of less than 10 nm using a “molecular cage” approach, which show better antibacterial activity against both E. coli and S. aureus than conventional chemical precipitation. Truong et al.[ 30 ] distributed spherical silver nanoparticles and silver nanoprisms over graphene oxide nanosheets and found that the composite's antibacterial properties are superior to Ag or graphene oxide alone. Therefore, the sterilization effect will be further improved if a greater dispersion of Ag can be obtained on the basis of creating a hybrid of oxide and Ag.
In this work, we prepared ZnO/In2O3-Ag nanofibers with uniformly dispersed Ag by electrospinning and subsequent sintering. Benefit from the nanofibrous structure and good dispersion of Ag, more surface area of zero-valent Ag that can be used for antibacterial purposes, is exposed. The produced nanofibers are effective at killing S. aureus. More notably, ZnO/In2O3-Ag nanofibers show a particularly prominent killing impact on E. coli. At relatively low concentrations, ZnO/In2O3-Ag-5% nanofibers can eliminate 98% of E. coli. Considering the method's simplicity, it is applicable to the preparation of other oxide nanomaterials and Ag composites for sterilization.
2 Results and Discussions
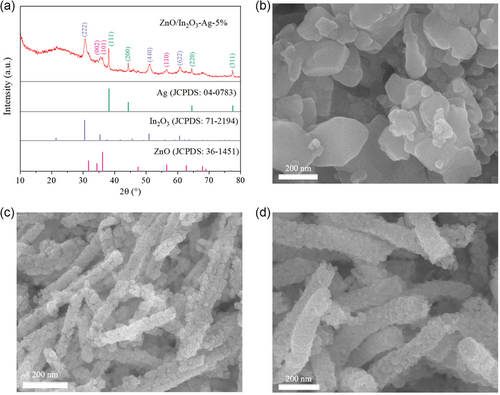
The sample ZnO/In2O3-Ag-5% nanofibers were prepared by electrospinning and subsequent calcination in air. X-ray diffraction (XRD) data (Figure 1a) indicate that the sample consists of ZnO (JCPDS: 36-1451), In2O3 (JCPDS: 71-2194), and Ag (JCPDS: 04-0783). The crystal planes corresponding to the diffraction peaks were obtained by indexing. The most exposed crystal plane of silver is (111), which is consistent with the results in the literature.[ 30 ] The diffraction peaks of silver are sharp, indicating good crystallinity. According to the Scherrer formula,[ 31 ] the crystal size of Ag is calculated to be about 35 nm based on half-peak width data, which is comparable to the size of Ag with good bactericidal effect described in the literature.[ 32 ] No other impurities were observed, indicating high purity of the sample. As shown in Figure 1d, the nanofibrous morphology of the samples is evident. These nanofibers are about 90–110 nm in diameter and consist of tiny nanoparticles. ZnO, In2O3, and ZnO/In2O3 were all prepared using similar methods. Their compositions were confirmed by XRD data (Figure S1–S3, Supporting Information). ZnO exhibits nanoparticle morphology (Figure 1b). Both In2O3 and ZnO/In2O3 exhibit nanofiber morphology (Figure 1c, Figure S3, Supporting Information). The diameter of In2O3 nanofibers is typically 40–60 nm, while the diameter of the nanofibers of ZnO/In2O3 is mostly between 80 and 100 nm. This shows that the addition of ZnO and Ag to In2O3 increases the size of the nanofibers.
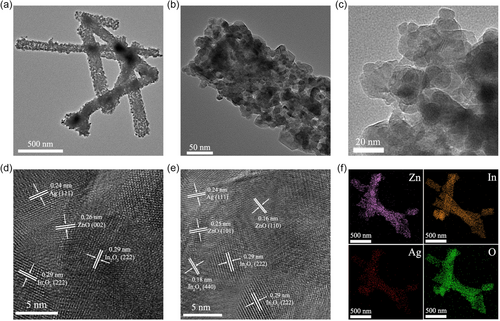
The morphology and microstructure of the ZnO/In2O3-Ag-5% sample were further investigated using transmission electron microscopy (TEM). As shown in Figure 2a–c, clear nanofibers can be observed. The diameter of the nanofibers is between 90 and 110 nm, which is consistent with the SEM results. In addition, it was noted that the nanofibers are made up of particles that are between 10 and 20 nm in size. According to the high-resolution TEM (HRTEM) images (Figure 2d–e), many clear lattice fringes can be observed. The crystal plane with a spacing of 0.24 nm corresponds to Ag (111).[ 33 ] The crystal planes with spacings of 0.26, 0.25, and 0.16 nm are the (002), (101), and (110) crystal planes of ZnO, respectively.[ 34 ] The planes with spacings of 0.29 and 0.18 nm are the (222) and (440) planes of In2O3,[ 35 ] respectively. The aforementioned observed crystal plane indices are consistent with the indexed results in the XRD pattern. The TEM elemental mapping of Zn, In, Ag, and O elements for ZnO/In2O3-Ag-5% shows that these four elements are uniformly distributed in the sample. The relatively dark color of Ag is due to the lower content.
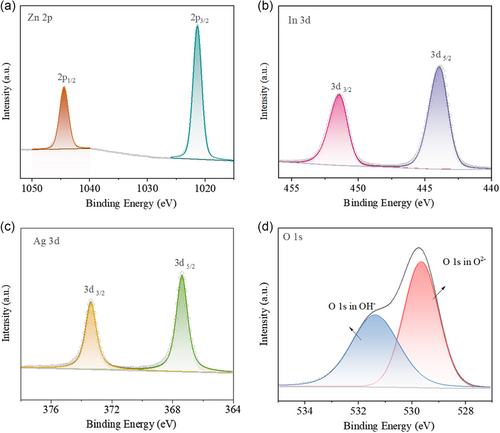
The chemical states of elements in the samples were analyzed using X-ray photoelectron spectroscopy (XPS). Only the signals of Zn, In, Ag, O, and C elements were found in the XPS survey spectra of ZnO/In2O3-Ag-5% (Figure S5, Supporting Information), where C was purposefully added to correct the data. The results indicate that the samples did not contain any additional contaminants, which is consistent with the XRD results. The high-resolution Zn 2p XPS spectra of ZnO/In2O3-Ag-5% can be fitted by two peaks located at 1044.5 and 1021.4 eV (Figure 3a), which correspond to the 2p1/2 and 2p3/2 electrons of Zn2+, respectively.[ 36 ] As for the In 3d spectra (Figure 3b), the fitted peaks at 451.4 and 443.9 eV originate from the 3d3/2 and 3d5/2 electrons of In3+, respectively.[ 37 ] For the Ag 3d spectra (Figure 3c), the peaks around 367.4 and 373.4 eV are assigned to the 3d3/2 and 3d5/2 electrons of Ag0, respectively.[ 38 ] This result, together with the XRD results, indicates that the sample surface is free of silver oxide contamination, maximizing the amount of silver that is accessible for sterilizing.[ 32 ] The O 1 s spectra of oxygen can be fitted with two peaks located at 531.4 and 529.7 eV (Figure 3d). The former correlates to the OH− groups adsorbed on the surface of the sample, whereas the latter corresponds to O2− in the lattice.[ 39 ] Similar results of chemical states can also be observed for ZnO, In2O3, and ZnO/In2O3 (Figure S1–S4, Supporting Information).
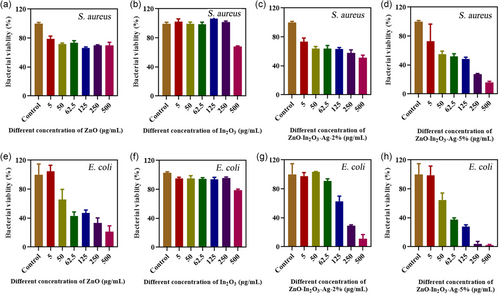
Gram-positive bacteria represented by S. aureus and Gram-negative bacteria represented by E. coli were used for the quantitative test of bacterial activity to obtain the antibacterial properties of the samples. For comparison, ZnO/In2O3-Ag-2% was also synthesized to show the impact of Ag content on the bactericidal performance. As shown in Figure 4a–d, ZnO alone has a limited bactericidal impact on S. aureus. Even at a concentration of 500 μg mL−1, over 70% of S. aureus are still present. In2O3 alone also has a limited bactericidal effect on S. aureus. More than 60% of the bacteria are still present at maximal concentrations. When ZnO is compounded with In2O3 and 2% Ag, the bactericidal effect is improved to a certain extent, but more than half of the bacteria remain at the concentration of 500 μg mL−1. For ZnO/In2O3-Ag-5%, the bactericidal effect of S. aureus is significantly improved. Only about 15% of S. aureus remain at the maximum set concentration. As shown in Figure 4e–h, ZnO alone has a certain killing effect on E. coli bacteria. After increasing the concentration to 500 μg mL−1, only about 20% of E. coli remains. While for In2O3 alone, approximately 80% of bacteria survive at this concentration. For ZnO/In2O3-Ag-2%, the bactericidal effect is more obvious at higher concentrations. At the maximum concentration, only approximately 10% of E. coli persists. The killing effect of ZnO/In2O3-Ag-5% on E. coli is even more significant. Only 2% of the bacteria are still present when the concentration is 500 μg mL−1. The previous results show that the sterilization effect is greatly improved after increasing the Ag content, and ZnO/In2O3-Ag-5% has a better killing effect on E. coli than S. aureus. It is consistent with what has been reported in the literatures.[ 30, 40 ] Since Gram-positive bacteria have a thicker peptidoglycan layer, S. aureus is generally less sensitive to nano-antimicrobial materials than E. coli.[ 41 ]
3 Conclusion
In summary, we prepared ZnO/In2O3-Ag nanofibers with high purity by electrospinning and subsequent sintering. The nanofibers have a diameter of 90–110 nm. Ag is evenly dispersed throughout the nanofibers at a particle size of around 35 nm. Zinc and indium in the nanofibers are divalent and trivalent, while silver is zero-valent and hence useful for sterilizing. The antibacterial effect of ZnO/In2O3-Ag-5% on S. aureus and E. coli is significantly improved compared with the same dose of ZnO and In2O3. In particular, ZnO/In2O3-Ag-5% has a remarkable bactericidal effect on E. coli. The 98% of the bacteria are killed by the nanofibers at a concentration of 500 μg mL−1. This work provides a facile method to construct oxide nanofibers with Ag for superior antibacterial activity.
Acknowledgements
Z.Z. and W.L. contributed equally to this work. This work was financially supported by the Natural Science Foundation of Zhejiang Province, China (grant no. LTY20E020001).
Conflict of Interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




