Briquetting of Fine-Grained Residues from Iron and Steel Production Using Organic and Inorganic Binders
Abstract
The Midrex process produces metallurgical residues in the form of dust, sludge, and fines. As these have high iron content, herein, the aim is to recycle the residues and use them as an educt in the Midrex process, thus closing the material cycle and increasing raw material efficiency. Briquetting of these materials with binder is one possibility to prepare them for the use as an educt in the Midrex process. Experiments are conducted to test the suitability of the organic binders starch and cellulose for briquetting. Furthermore, tests with the inorganic bentonite are included for comparison. Briquettes are generally characterized by high strength. However, compared with iron oxide pellets, they have a low porosity and thus a higher apparent density, and consequently, a worse reducibility. The use of organic binders should improve the reducibility. The iron oxides are in close contact with the C-carrier of the organic binder so that a solid–solid phase direct reduction can take place. Furthermore, the solid carbon reacts to CO, and thus, increases the presence of reducing gas in the enlarged pores of the briquettes, and should therefore increase the degree of reduction.
1 Introduction
During the production of hot briquetted iron (HBI) from raw iron oxide pellets in the Midrex process, a wide variety of residues are generated. These residues are sludges, screened fines, and dust and are generally rich in iron oxides.[1, 2] Hence, they have the potential of being recycled back to the Midrex shaft after having been agglomerated. For agglomerates to be considered suitable as feed material for the Midrex direct reduction shaft, they should have sufficient cold strength, thermal stability, and reducibility of iron containing species.[3] The cold bond pressure agglomeration process offers a possible method for achieving this. For cold bond pressure agglomeration, a binder is necessary.[4] Therefore, this article presents results of the experiments conducted to study the effect of various parameters on the quality of briquettes prepared using the Midrex residues and different binders. The binders were organic (starch and cellulose) or inorganic (bentonite). The experiments were based on a statistical experimental design and analysis strategy (design of experiments [DOE]) and take a look at the influence of water and binder content of the mixture and the used binder on briquette strength. As briquettes are characterized by high density and low porosity, they have a worse reducibility compared with iron oxide pellets, for example. The use of organic binders is intended to improve reducibility. The iron oxides are in close contact with the C-carrier of the organic binder so that a solid–solid-phase direct reduction can take place. Furthermore, the solid carbon reacts to CO, and thus, increases the presence of reducing gas in the enlarged pores of the briquettes. All in all, this increases the degree of reduction.
2 Midrex Process and Residues
The dominating technology for the production of direct reduced iron (DRI) as a preproduct for crude steel production is the Midrex process. The Midrex process consists of a shaft furnace containing a packed bed of iron oxide pellets. Pellets are fed at the top and exit at the bottom as hot or cold DRI. Hot reducing gas (H2 and CO) is fed countercurrently to the shaft and passes upward and reduces the iron oxide pellets (these consist mainly of hematite) to the metallic phase. As DRI pellets have the potential to reoxidize and generate heat, the hot DRI is briquetted to make a much more stable product called HBI.[1] During the various stages of HBI production, a number of iron bearing residues are generated. To reduce material losses to the off-gas and minimize flow problems in the shaft furnace, the fed iron oxide pellets are screened at 5–6 mm, resulting in net fines losses of up to 10 wt%. Moreover, the HBI is screened (losses 1–3 wt%), and there are fines losses from the reduction shaft to the off gas (1–1.5 wt%). The off gas goes to a scrubber and the scrubber residue (sludge) is dried.[2] As these residues cannot be recycled back, they need to be agglomerated. The aim is to collect the fines, dusts, and sludge generated in the Midrex process, briquetted it, and charged the briquettes directly back to the shaft together with the iron oxide pellets. The main benefit is to substitute raw material and avoid the disposal of the residues.
3 Quality Requirements for Feed Materials Used in Midrex Process
Briquettes to be considered suitable as feed material for the Midrex process should have sufficient strength for handling, transportation, and storage (physical properties), sufficient thermal stability to avoid premature disintegration, complete reducibility of iron oxides to metallic iron (metallurgical properties), and the content of gangue should be as low as possible (chemical properties). The only major chemical change to pellets in the direct reduction process is the removal of oxygen, because there is no melting or refining. As a result, impurities and gangue in the DRI briquettes are present. Therefore, the iron content of the feed materials should be as high as possible (preferred 67 wt%) and the gangue content as low as possible. Especially, acid gangue constituents like silica and alumina should amount to max. 2 wt%. The total amount of gangue in feed material generally should not exceed 3–4 wt%, as gangue will require additional electric power in the electric arc furnace (EAF) and increase refractory wear.[3] Physical characteristics are defined for iron oxide pellets for use as Midrex feedstock. A preferred Midrex feedstock will be of consistent size to allow homogeneous feeding and sufficient mechanical strength to prevent degradation and fines generation during handling and transport. About 95 wt% of iron oxide pellets should be in the size range of 9–16 mm, whereas the fraction <3 mm should be minimized. The pellets should have a tumble strength of 90–95% > 6.73 mm. Cold compressive strength for pellets should be 250 kg or greater.[3] The requirements of pellets cannot be transferred directly to briquettes, nevertheless adequate mechanical strength of the briquettes is necessary.[4] Furthermore, the complete reduction of the metallic oxides in the briquettes to metallic iron in the Midrex shaft is important.
4 Briquetting with a Binder
Cold bond briquetting may offer a method for recycling the residues. Fines, dusts, and sludge can be briquetted using a binder to a form that is suitable for charging into the Midrex shaft. To get briquettes with sufficient mechanical strength and thermal stability, a binder is needed. With the selection of a binder, the mechanical and metallurgical properties of the briquettes can be influenced. A distinction between organic and inorganic binder materials is possible.[5] Carbonaceous organic materials can be included in the briquettes to improve the reduction kinetics (reduction rate and reducibility) due to the presence of a larger number of reaction sites simultaneously and due to shorter diffusion paths compared with inorganic binders.
In the literature, there are mainly studies on the production of self-reducing or composite pellets with organic substances.[6-15] However, the addition of carbon usually lowers the strength of agglomerates. Therefore, briquettes normally contain less than 10 wt% carbon.[15, 16] Another disadvantage of organic binders is the volatility of the organic substance during thermal treatment (begins at around 300 °C). The most important binder for production of iron oxide pellets is inorganic bentonite.[17] Bentonite is a silicate with a low melting temperature. The silicate components melt, pull particles together, and promote sintering of iron oxide grains. Upon cooling additional solid bonds are added (recrystallization processes). Thus, a high thermal stability of the pellets is achieved.[18] However, the disadvantage is, that bentonite increases the silica content in the HBI (gangue).[19]
Concerning briquettes, produced with organic or inorganic binders, it can be assumed that they have a worse reducibility than iron oxide pellets due to their larger size and lower porosity. In contrast, the briquettes from Midrex residues partly contain already reduced material. It is expected that using organic binders, the reducibility can be improved compared with briquettes with inorganic binders.
5 Experimental Section
5.1 Materials
The materials for briquetting were the Fe carrier (a mixture of residues from Midrex process), binder and water. The mixture of Fe carriers includes screened oxide fines, HBI screened fines, HBI classifier dust, dried sludge, process dust, and remet fines. Table 1 shows the composition of the Fe carrier used for the tests. The results of the analysis for chemical composition of the different residues are shown in Table 2 and the water content in Table 3. The residues were already prereduced to varying degrees. The HBI screened fines were characterized by the highest content of metallic iron (Femet = 74.9 wt%) and total iron (Fetot = 88.7 wt%). Table 4 shows the cumulative particle size distribution Q3(d), where d is the grain diameter, the median particle size d50, and the mean particle size dm of the different residues. Two organic and one inorganic binders were used for the briquettes. These were preswelled wheat starch, cellulose glue (dried, preswollen, and ground cellulose), and a bentonite clay.
| Component | Oxide fines | Dried sludge | Process dust | HBI (screened fines) | HBI (classifier dust) | Remet fines |
|---|---|---|---|---|---|---|
| Content [wt%] | 30 | 40 | 5 | 15 | 5 | 5 |
| Component | Fetot [wt%] | Fe2O3 [wt%] | FeO [wt%] | Femet [wt%] | C [wt%] | SiO2 [wt%] | CaO [wt%] | Al2O3 [wt%] | MgO [wt%] | K2O [wt%] | TiO2 [wt%] | P [wt%] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxide fines | 67.20 | 96.10 | 0.00 | 0.00 | 0.03 | 1.59 | 0.91 | 0.53 | 0.141 | 0.014 | 0.075 | 0.029 |
| Dried sludge | 74.00 | 63.60 | 0.00 | 29.53 | 2.01 | 2.18 | 1.03 | 0.85 | 0.244 | 0.019 | 0.103 | 0.061 |
| Process dust | 70.08 | 79.40 | 0.00 | 14.51 | 0.26 | 3.07 | 1.00 | 1.01 | 0.114 | 0.090 | 0.096 | 0.030 |
| HBI classifier dust | 83.94 | 34.51 | 2.10 | 58.17 | 0.93 | 2.06 | 1.28 | 0.60 | 0.183 | 0.014 | 0.090 | 0.063 |
| HBI screened fines | 88.71 | 17.79 | 1.73 | 74.92 | 1.02 | 2.12 | 1.03 | 0.54 | 0.250 | 0.020 | 0.097 | 0.030 |
| Remet fines | 84.87 | 30.94 | 4.00 | 59.81 | 0.40 | 2.21 | 1.06 | 0.82 | 0.200 | 0.023 | 0.106 | 0.032 |
| Component | Oxide fines | Dried sludge | Process dust | HBI (screened fines) | HBI (classifier dust) | Remet fines | Bentonite | Cellulose | Starch |
|---|---|---|---|---|---|---|---|---|---|
| Amount of water [wt%] | 1.0 | 1.3 | 8.5 | 0.41 | 10.8 | 5.4 | 16.2 | 5.83 | 7.86 |
| Unit | Oxide fines | Dried sludge | Process dust | HBI (screened fines) | HBI (classifier dust) | Remet fines | |
|---|---|---|---|---|---|---|---|
| Q 3(10 mm) | wt% | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Q 3(6.3 mm) | wt% | 99.6 | 100.0 | 99.5 | 100.0 | 98.6 | 99.6 |
| Q 3(4 mm) | wt% | 97.4 | 99.9 | 99.3 | 99.8 | 98.0 | 98.4 |
| Q 3(2 mm) | wt% | 86.1 | 99.0 | 92.5 | 48.1 | 85.5 | 71.7 |
| Q 3(1 mm) | wt% | 71.4 | 97.2 | 55.2 | 28.1 | 21.5 | 55.1 |
| Q 3(0.63 mm) | wt% | 63.5 | 96.1 | 16.3 | 19.5 | 18.6 | 14.7 |
| Q 3(0.5 mm) | wt% | 60.9 | 95.1 | 3.0 | 16.8 | 0.9 | 14.5 |
| Q 3(0.315 mm) | wt% | 60.8 | 94.5 | 1.2 | 16.7 | 0.2 | 13.8 |
| Q 3(0.125 mm) | wt% | 44.4 | 74.1 | 0.1 | 5.3 | 0.1 | 0.1 |
| Q 3(0.09 mm) | wt% | 34.7 | 61.9 | 0.0 | 4.1 | 0.0 | 0.1 |
| Q 3(0.063 mm) | wt% | 24.8 | 43.8 | 0.0 | 2.9 | 0.0 | 0.1 |
| d m | mm | 0.764 | 0.140 | 1.122 | 1.865 | 1.416 | 1.401 |
| d 50 | mm | 0.190 | 0.072 | 0.951 | 2.074 | 1.445 | 0.953 |
5.2 Feed Preparation and Briquetting
The materials, at first the Fe-carrier components, then the binder and at the end water, were fed into an Eirich-Mixer “R02” with a volume of 2 L and mixed for 10 min. The amount of water needed was calculated based on the water content of the Fe carriers and the binder. After mixing, the water content of the mixture was analyzed and, if needed, more water was added. The temperature of the material in the mixer was 35 °C up to 40 °C. The mixture was then preheated prior to briquetting to 60 °C. The preheated material was pressed with a hydraulic stamp press (Raster Zeulenroda, PYXE 250F) into cylindrical briquettes, each having a diameter of 5 cm and a height of 2 cm and a briquette mass of ≈150 g. A pressure of 140 MPa as well as a pressing time of 3 s and a pressing temperature of 60 °C were determined and used for the experiments. The briquettes were hardened under ambient conditions for 1 day.
5.3 Briquette Testing Procedure
The apparent density ρapp of the briquettes was determined by measuring the briquette (height and diameter) with caliper gauge in addition to weighing it. The apparent density is the ratio of the mass of the briquette to the volume. The low-temperature disintegration test was carried out based on ISO 4696. The briquettes were isothermally reduced in a fixed bed, at 550 °C, using a reducing gas consisting of 20.5 vol% H2, 13.5 vol% CO, 10 vol% CO2, and 44 vol% N2 (adapted for conditions in Midrex shaft), for 30 min. The reduced sample was then tumbled in a specific tumble drum for 900 revolutions and then sieved. Furthermore, a reduction test was carried out for suitable briquettes in accordance with ISO 4695. The briquettes were first heated in an N2 atmosphere, then reducing gas consisting of 42.5 vol% H2, 31.5 vol% CO, 15.5 vol% CO2, and 10.5 vol% N2 (also adapted for conditions in Midrex shaft) was introduced at 800–850 °C, and the briquettes were isothermally reduced. During the reduction process, the decrease in mass is continuously recorded.
5.4 Design of Experiments
As a first step, the influence of water content and binder content on the cold strength of the briquettes was investigated for different binders. A sufficient cold strength of the briquettes is an essential requirement for the use in the Midrex process. In most cases, the compressive strength, apparent density, and the abrasion or shatter resistance go not on the same trend line so that a global parameter optimum had to be found which meets all strength requirements. This was done by means of statistical design of experiments with the software Statgraphics 18. In the second step, briquettes with suitable strength values were then tested for low-temperature disintegration. If the briquettes passed this test, a reduction test could be carried out.
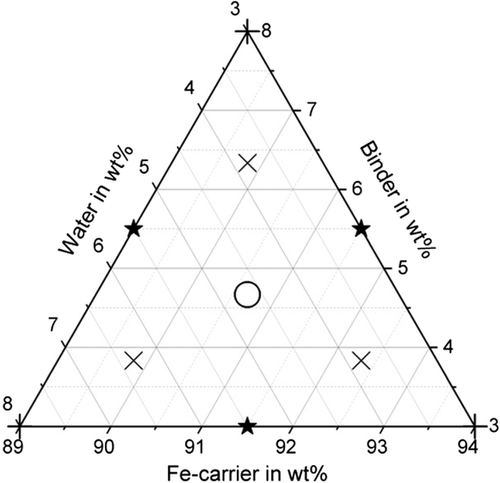
The values margin of the variables was: Fe carrier: 89–94 wt%, binder: 3–8 wt%, and water: 3–8 wt% (the amount of water includes also the water in the binder and Fe carrier). The total for controllable mixture components was 100 wt%. For the tests, a Simplex Centroid Design was chosen that runs at all primary blends, binary blends, and the tertiary blend (7-run simplex centroid design) and additional runs are located along axial lines running from the pure blends through the centroid (three additional checkblends). The ten design points are shown in Figure 1. The tests were carried out for three different binders. Bentonite as inorganic binder as well as cellulose and starch as organic binder. With the design of experiments an optimum mixture composition can be determined for each binder.
6 Results and Discussion
6.1 Mechanical Strength of Briquettes
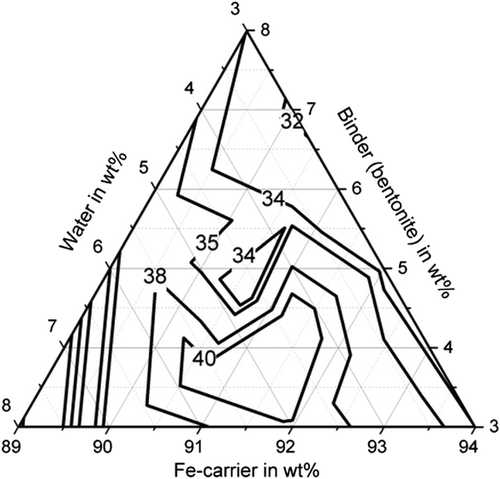
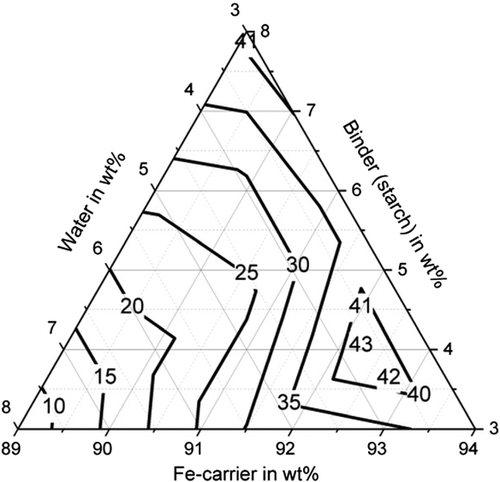
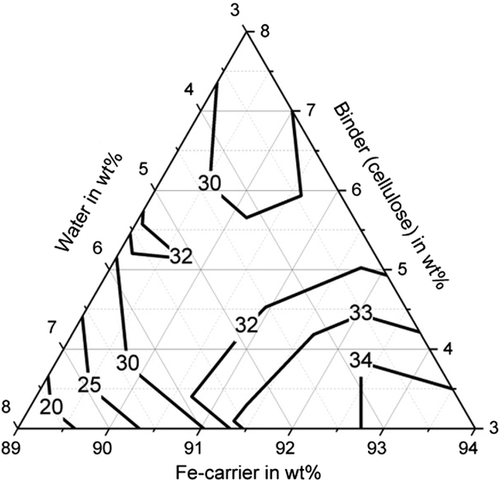
Table 5 shows the tests carried out and the strengths achieved for the binder starch (A1–A10), the binder cellulose (B1–B10), and the binder bentonite (C1–C14). The compressive strength of the briquettes when using the various binders is shown in Figure 2–4. With all binders, high compressive strengths of over 30 MPa can be achieved with a suitable mixture composition, whereby the influence of the mixture composition is greatest with cellulose as a binder. Compressive strengths of up to 42.1 MPa (C4) can be achieved for bentonite and up to 43.2 MPa (A2) for starch. The compressive strength of the briquettes can be described with sufficient accuracy of R2 = 70.7% by a quadratic model for the binder bentonite and by a quadratic model for the binder starch (R2 = 90.89%). An overview of the model equations for describing the mechanical properties of the briquettes is shown in Table 6. The compressive strengths achieved with the binder cellulose tend to be lower than with starch and bentonite. Nevertheless, compressive strengths of up to 34.7 MPa (B1) are achieved. The compressive strength can also be described by a quadratic model with an R2 of 93.87%. Particularly high compressive strengths of greater than 35 MPa are achieved for the binder bentonite at a binder content of 3–5.5 wt% and a water content of 3.7–7 wt%. For the binder starch, the water content is decisive. For compressive strengths above 35 MPa, this should be between 3 and 4 wt%. For cellulose, the highest strengths (greater than 33 MPa) are achieved at a binder content of 3–5 wt% and a water content of 3–6 wt%, provided that the Fe-carrier content is greater than 91 wt%.
| Number | Binder | Fe carrier [wt%] | Binder [wt%] | Water [wt%] | σ P [MPa] | R30(100) [%] | S 20 [%] | ρ app [g cm−3] |
|---|---|---|---|---|---|---|---|---|
| A1 | Starch | 94.00 | 3.00 | 3.00 | 36.9 | 91.9 | 99.0 | 3.33 |
| A2 | Starch | 92.33 | 3.83 | 3.83 | 43.2 | 96.5 | 99.6 | 3.42 |
| A3 | Starch | 91.50 | 3.00 | 5.50 | 30.2 | 97.0 | 99.6 | 3.29 |
| A4 | Starch | 89.83 | 6.33 | 3.83 | 30.6 | 97.5 | 99.7 | 3.49 |
| A5 | Starch | 89.00 | 5.50 | 5.50 | 23.3 | 97.1 | 99.6 | 3.38 |
| A6 | Starch | 90.67 | 4.67 | 4.67 | 23.9 | 98.5 | 99.7 | 3.47 |
| A7 | Starch | 89.00 | 3.00 | 8.00 | 6.5 | 98.2 | 99.6 | 3.36 |
| A8 | Starch | 91.50 | 5.50 | 3.00 | 37.5 | 95.8 | 99.2 | 3.46 |
| A9 | Starch | 89.00 | 8.00 | 3.00 | 41.8 | 97.2 | 99.6 | 3.42 |
| A10 | Starch | 89.83 | 3.83 | 6.33 | 17.7 | 98.4 | 99.7 | 3.45 |
| A11a) | Starch | 93.40 | 3.00 | 3.60 | 42.1 | 95.5 | 98.8 | 3.52 |
| B1 | Cellulose | 94.00 | 3.00 | 3.00 | 34.7 | 66.4 | 89.6 | 3.31 |
| B2 | Cellulose | 91.50 | 3.00 | 5.50 | 33.2 | 86.2 | 97.6 | 3.25 |
| B3 | Cellulose | 89.00 | 5.50 | 5.50 | 32.3 | 92.1 | 97.7 | 3.17 |
| B4 | Cellulose | 89.83 | 6.33 | 3.83 | 28.9 | 87.4 | 83.2 | 3.18 |
| B5 | Cellulose | 92.33 | 3.83 | 3.83 | 34.0 | 82.4 | 87.0 | 3.29 |
| B6 | Cellulose | 90.67 | 4.67 | 4.67 | 31.6 | 83.3 | 75.9 | 3.23 |
| B7 | Cellulose | 91.50 | 5.50 | 3.00 | 31.2 | 80.9 | 85.5 | 3.22 |
| B8 | Cellulose | 89.00 | 3.00 | 8.00 | 15.6 | 90.4 | 87.8 | 3.07 |
| B9 | Cellulose | 89.00 | 8.00 | 3.00 | 29.2 | 85.4 | 94.2 | 3.12 |
| B10 | Cellulose | 89.83 | 3.83 | 6.33 | 30.6 | 90.9 | 97.8 | 3.21 |
| B11a) | Cellulose | 92.40 | 3.00 | 4.60 | 41.4 | 91.9 | 98.1 | 3.36 |
| C1 | Bentonite | 94.00 | 3.00 | 3.00 | 34.0 | 0.0 | 15.5 | 3.51 |
| C2 | Bentonite | 90.00 | 7.00 | 3.00 | 31.7 | 59.4 | 39.8 | 3.43 |
| C3 | Bentonite | 90.00 | 3.00 | 7.00 | 35.8 | 85.7 | 73.0 | 3.69 |
| C4 | Bentonite | 91.33 | 4.33 | 4.33 | 42.1 | 85.8 | 84.2 | 3.64 |
| C5 | Bentonite | 92.00 | 5.00 | 3.00 | 33.6 | 22.3 | 32.9 | 3.33 |
| C6 | Bentonite | 92.00 | 3.00 | 5.00 | 39.9 | 86.1 | 78.2 | 3.70 |
| C7 | Bentonite | 90.00 | 5.00 | 5.00 | 34.8 | 86.0 | 84.3 | 3.63 |
| C8 | Bentonite | 92.67 | 3.67 | 3.67 | 35.7 | 5.2 | 28.3 | 3.54 |
| C9 | Bentonite | 90.67 | 5.67 | 3.67 | 34.2 | 68.0 | 49.3 | 3.49 |
| C10 | Bentonite | 90.67 | 3.67 | 5.67 | 41.2 | 88.5 | 88.3 | 3.66 |
| C11 | Bentonite | 89.00 | 8.00 | 3.00 | 34.0 | 25.6 | 26.4 | 3.49 |
| C12 | Bentonite | 89.00 | 3.00 | 8.00 | 24.4 | 86.5 | 66.5 | 3.67 |
| C13 | Bentonite | 89.00 | 5.50 | 5.50 | 36.3 | 91.5 | 97.6 | 3.71 |
| C14 | Bentonite | 90.67 | 4.67 | 4.67 | 32.9 | 83.9 | 88.9 | 3.54 |
| C15a) | Bentonite | 90.00 | 5.00 | 5.00 | 41.5 | 87.1 | 92.9 | 3.68 |
- a) Briquettes used for tests on metallurgical properties.
| Binder | Compressive strength σP [MPa] | Abrasion resistance R30(100) [%] | Shatter strength S20 [%] | Apparent density ρapp [g cm−3] |
|---|---|---|---|---|
| Bentonite | = 33.9 ⋅ Fe + 33.6 ⋅ B + 25.6 ⋅ W + 8.1 ⋅ Fe ⋅ B + 44.4 ⋅ Fe ⋅ W + 20.3 ⋅ B ⋅ W (R2 = 70.7%) | =11.6 ⋅ Fe + 36.9 ⋅ B + 82.5 ⋅ W + 92.9 ⋅ Fe ⋅ B + 187.5 ⋅ Fe ⋅ W + 130.5 ⋅ B ⋅ W (R2 = 83.2%) | = 11.4 ⋅ Fe + 29.0 ⋅ B + 63.7 ⋅ W + 48.4 ⋅ Fe ⋅ B + 164.7 ⋅ Fe ⋅ W + 202.5 ⋅ B ⋅ W (R2 = 90.2%) | = 3.49 ⋅ Fe + 3.51 ⋅ B + 3.69 ⋅ W + 0.78 ⋅ Fe ⋅ B + 0.42 ⋅ Fe ⋅ W + 0.35 ⋅ B ⋅ W (R2 = 84.9) |
| Starch | = 39.6 ⋅ Fe + 41.2 ⋅ B + 6.3 ⋅ W + 12.6 ⋅ Fe ⋅ B + 29.6 ⋅ Fe ⋅ W + 14.4 ⋅ B ⋅ W (R2 = 90.9%) | = 91.9 ⋅ Fe + 97.0 ⋅ B + 98.1 ⋅ W + 7.8 ⋅ Fe ⋅ B + 10.7 ⋅ Fe ⋅ W (R2 = 93.6%) | p-Value > 0.05 (description with model not useful) | = 3.47 ⋅ Fe + 3.29 ⋅ B + 3.46 ⋅ W + 0.01 ⋅ Fe ⋅ B + 0.09 ⋅ Fe ⋅ W + 0.08 ⋅ B ⋅ W (R2 = 97.8%) |
| Cellulose | = 34.9 ⋅ Fe + 28.6 ⋅ B + 16.5 ⋅ W + 8.6 ⋅ Fe ⋅ B + 30.2 ⋅ Fe ⋅ W + 35.4 ⋅ B ⋅ W (R2 = 93.9%) | = 67.4 ⋅ Fe + 85.7 ⋅ B + 90.5 ⋅ W + 14.3 ⋅ Fe ⋅ B + 34.4 ⋅ Fe ⋅ W + 9.4 ⋅ B ⋅ W (R2 = 92.9%) | p-Value > 0.05 (description with model not useful) | = 3.31 ⋅ Fe + 3.12 ⋅ B + 3.08 ⋅ W + 0.01 ⋅ Fe ⋅ B + 0.30 ⋅ Fe ⋅ W + 0.33 ⋅ B ⋅ W (R2 = 98.1%) |
Furthermore, sufficiently high abrasion resistance of more than 85% could be achieved with all binders (with suitable water and binder contents), shown in Figure 5–7. For bentonite, a very strong influence of the water and binder contents on the abrasion resistance was found, as values for abrasion resistance between 0% and 91.5% were determined. The determined maximum value is 91.5% with 5.5 wt% water and 5.5 wt% binder (C13). High abrasion resistance of more than 85% is achieved with a water content of the mixture between 5 and 8 wt%. The abrasion resistance for the binding agent bentonite can be determined with sufficient accuracy of R2 = 83.2% with a quadratic model. For the binder starch, all determined abrasion resistance values are between 91.9% and 98.5%. The abrasion resistance for the binder starch can be described with a quadratic model with a high accuracy of R2 = 93.6%. Good abrasion resistance between 80.9% and 92.1% is also achieved with the binding agent cellulose, with the exception of test point B1 (abrasion resistance 66.4%). For an abrasion resistance of more than 85%, a water content between 5 and 8 wt% is particularly important. The abrasion resistance can be described with an accuracy of R2 = 92.9% using a quadratic model.
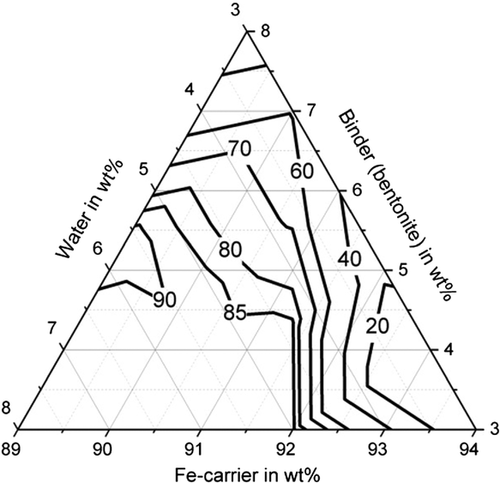
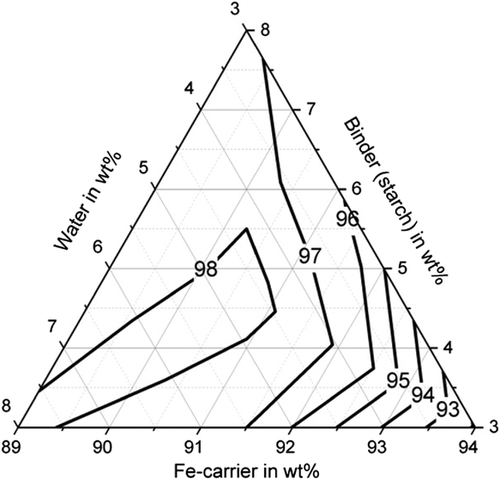
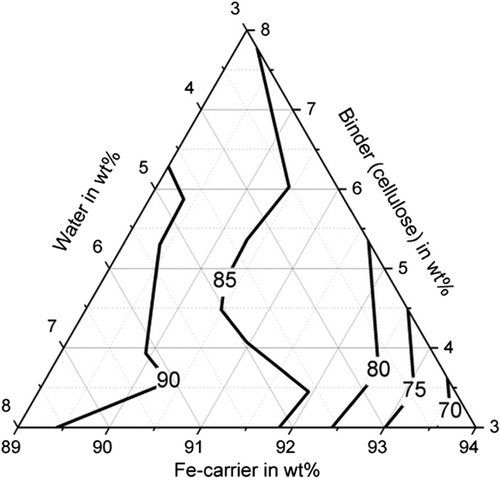
With regard to shatter resistance, sufficient strengths of more than 85% can also be achieved for all binders, as shown in Figure 8–10. In this context, the adjustment of a suitable water and binder content for the briquettes with bentonite is again of particular importance. The shatter strength of the briquettes with bentonite lies between 15.5% and 97.6% for the investigated briquettes. A water content of ≈5 wt% is decisive for sufficiently high shatter resistance. The shatter strength of the bentonite briquettes can be described with a quadratic model with sufficient accuracy of R2 = 90.2%. For the binder starch, all shatter strengths are between 99% and 99.7% and thus reach the maximum possible in practice. The influence of binder and water contents is not visible (p-value > 5%). With the binding agent cellulose, shatter strengths of a maximum of 97.8% (B10) are achieved. As the p-value is also higher than 5%, a description based on a model is not possible. A water content of the mixture of 5–6 wt% favors a high abrasion resistance.

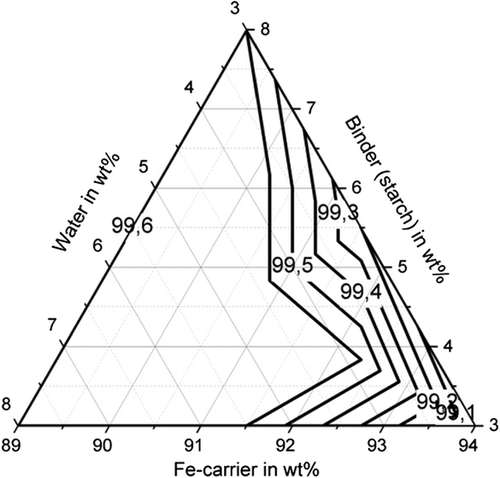
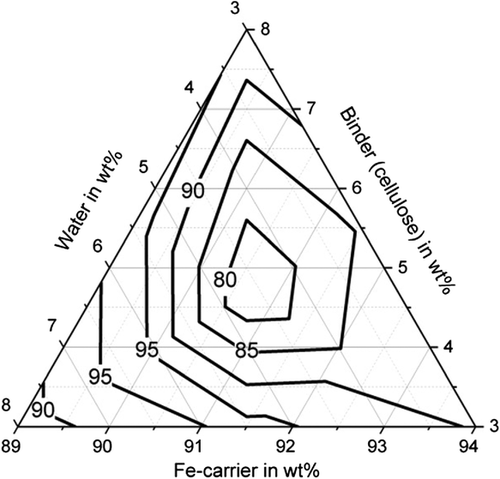
Figure 11–13 shows the apparent density of the briquettes. The apparent density of the briquettes can be represented with high accuracy (R2 > 84%) for all three binders by a quadratic model as a function of the mixture composition. The apparent density of the briquettes is in the range of 3.4–3.7 g cm−3 for the binding agent bentonite, in the range of 3.3–3.5 g cm−3 for the binding agent starch and in the range of 3.1–3.3 g cm−3 for cellulose. Thus, higher apparent densities are achieved with the inorganic binder than with the two organic binders. But this does not correspond to the porosity of the briquettes due to the higher weight of the inorganic binder particles. When using the binder starch, the apparent density is mainly dependent on the binder content. For a low binder content of 3 wt%, the highest apparent densities are achieved. When using bentonite as a binder, the apparent density is determined by the water content. For a high water content of more than 5 wt%, the highest apparent densities are achieved. For cellulose as a binder, the Fe-carrier content is decisive. A high content of Fe carrier results in a high apparent density. As a result, the apparent density for the three binders investigated behaves differently. The bulk density of the briquetting mixtures of Fe carrier, binder, and water are similar for all mixtures (1.6–1.8 g cm−3) and do not correlate with the apparent density of briquettes. Bentonite is a silicate with a low melting temperature. The particles are encased by the bentonite, the silicate components melt, and thus contract the particles, increasing the apparent density of the briquettes in the Midrex shaft.[19] The lower apparent density of briquettes containing starch and cellulose can be explained by the lower weight of the macromolecular binder particles in the first place. These binders promote plastic compaction as they act like a lubricant. It, therefore, can be assumed that they lead to less pore volume of the briquettes despite the fact of a lower apparent density.
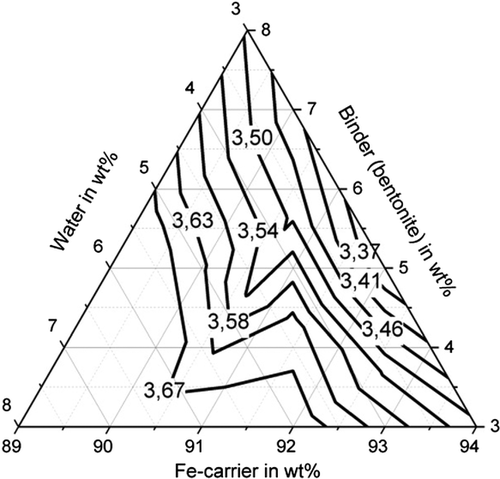
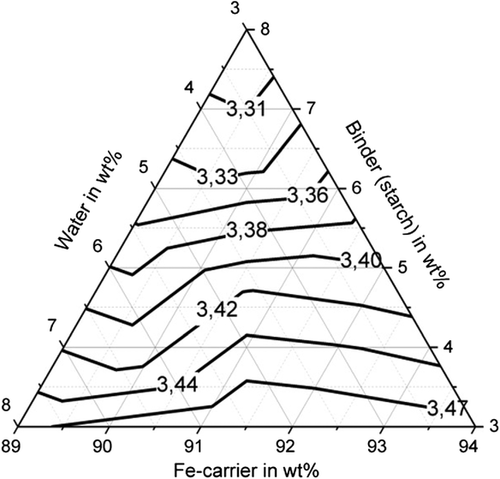
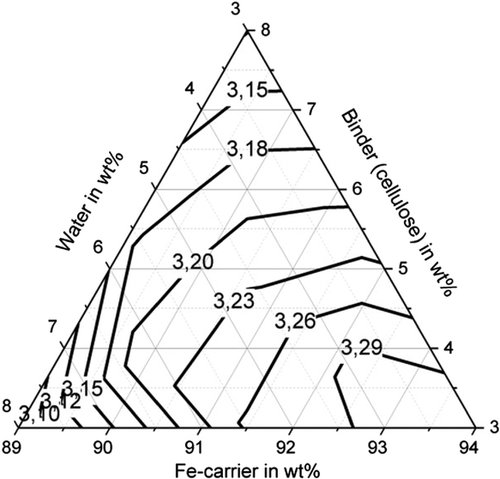
All three investigated binders are characterized by a macromolecular structure, have the property of swelling, and can absorb many times their own weight of water. The water absorption capacity and thus the swelling behavior can be characterized by means of the plate water absorption test (PWAT) value.[5] The exact value depends strongly on the composition of the binder and possible modifications. Basically, cellulose has particularly high PWAT values of up to 3000%, whereas starch and bentonite reach values of ≈1000%. This behavior also influences briquette density.[20]
For the briquettes with the binding agent cellulose, the apparent density correlates with the compressive strength of the briquettes. A high apparent density and a high compressive strength is achieved for low water contents (<5 wt%) (the briquettes behave brittle), whereas high shatter and abrasion resistance are achieved for water contents greater than 5 wt%. Due to the higher water content, the briquettes have a pressure plastic behavior. For the briquettes with bentonite and starch, the correlation between apparent density and compressive strength is not observed. In terms of strength, a high apparent density is desirable. However, a high apparent density of briquettes also results in lower porosity of briquettes, which can be a disadvantage especially for the reducibility of briquettes.[21]
It is shown that when considering the abrasion and shatter resistance, the water content of the briquette mixture is the decisive factor. With increasing water content, higher strength values tend to be achieved, irrespective of the binder. During the shatter and abrasion test, the briquettes are subjected to dynamic and multiaxial mechanical stress. Therefore, a certain plastic deformability of the briquettes is useful to withstand this stress. In this case, the addition of water ensures a certain plastic behavior of the briquettes. The pressure stress, in contrast, is a quasistatic load. The lower the water content, the more brittle the briquettes are, which is ultimately reflected in the higher compressive strength. For high water contents, in contrast, the lubricating effect of the water comes into play, so that the briquettes are deformed under load. This is mainly visible for the binders starch and cellulose. With bentonite, compressive strengths of higher than 30 MPa are achieved in almost the entire area under investigation.
In terms of cold strength, the inorganic binder bentonite can be used to achieve a higher compressive strength, whereas the briquettes with the organic binders show better abrasion and shatter resistance. In general, however, briquettes with sufficient strength for transport and handling can be produced with all three binders investigated.
The advantage of briquettes with organic binders concerning reduction efficiency can be taken for granted. Nevertheless, this quality parameter has to be tested. The same applies to the thermal stability of the briquettes which is also an important requirement for the briquettes to be recycled into the Midrex shaft. As already mentioned, the silicate components of bentonite favor sintering, as a result of which a high thermal stability of the briquettes can be expected.[19] Organic binders decompose at about 300 °C and form solid coke bridges that may also contribute to thermal stability.
The strengths of the Midrex residue briquettes with the different binders tested can be described with sufficient accuracy (R2 mostly > 90%) using quadratic models. This is a quality criterion for the tests carried out and indicates a stable briquetting process and a low influence of disturbance variables, such as mixture inhomogeneities. Table 7 shows a summary overview of the necessary composition of the mixtures to achieve sufficient strength. Additional briquettes were produced with each of the three binders for a suitable mixture composition to assess metallurgical properties and thermal stability.
| Compressive strength σP > 35 MPa | Abrasion resistance R30(100) > 85% | Shatter strength S20 > 85% | Apparent density ρapp | |
|---|---|---|---|---|
| Bentonite | Binder 3–5.5 wt% | Water 5–8 wt% | Water 5 wt% | ρ app > 3.6 g cm−3 for water >5 wt% |
| Water 3.7–7 wt% | ||||
| Starch | Water 3–4 wt% | Sufficient strength in the entire area examined | Sufficient strength in the entire area examined | ρ app > 3.4 g cm−3 for binder <5 wt% |
| Cellulose | Binder 3–5 wt% | Water 5–8 wt% | Water 5–6 wt% | ρ app > 3.2 g cm−3 for Fe >91 wt% |
| Water 3–6 wt% | ||||
| Fe carrier >91 wt% |
6.2 Metallurgical Properties of Briquettes
In addition to cold strength, the thermal stability of the briquettes under the corresponding gas atmosphere and the reducibility is also of essential importance. The results of the low-temperature disintegration tests are shown in Table 8. It is obvious that the briquettes with bentonite are also suitable for use at elevated temperatures and corresponding gas atmospheres. The proportion of briquette fragments larger than d = 6.3 mm after loading with reducing gas at temperatures of 550 °C and the subsequent abrasion stress is 91.8 wt%. In contrast, the briquettes with organic binders cannot withstand these stresses. For the use of cellulose as a binding agent, the proportion greater than 6.3 mm is 17.3 wt%, and only 3.3 wt% for starch. This means that the briquettes with starch and cellulose would probably disintegrate when fed into the reduction shaft. This can be explained by the fact that the organic binders decompose at temperatures of ≈300 °C and solid coke bridges form only at temperatures higher than 800 °C. Although, part of the carbon passes to the gaseous phase through the reaction with reducing gas. As partial melt adhesion between the iron oxide particles does not yet occur at these temperatures, the briquettes disintegrate. The disintegration of the briquettes is not necessarily to be seen as negative, but the high dust content (d < 0.5 mm) of 59.7 wt% for cellulose and 68.6% for starch is problematic. This means that the gas flow through the reduction shaft could be disturbed. In contrast, the briquettes have to break up into fragments to some extent to ensure trouble-free combined hot briquetting with the directly reduced iron oxide pellets and not to negatively influence the strength properties of the HBI. Furthermore, the reducibility of smaller briquette pieces is better than that of a whole briquette.
| Binder | d > 6.3 mm | d < 3.2 mm | d < 2.8 mm | d < 0.5 mm |
|---|---|---|---|---|
| Bentonite [wt%] | 91.8 | 8.2 | 8.1 | 7.5 |
| Starch [wt%] | 3.3 | 91.6 | 89.7 | 68.6 |
| Cellulose [wt%] | 17.3 | 78.4 | 76.7 | 59.7 |
For the briquettes with bentonite, a reduction test was still being carried out due to the positive results of the low-temperature disintegration test. This shows that the reduction process is very slow due to the high density of the briquettes and their size. Only after 300 min, a reduction degree of 80% was achieved. As already predicted, briquettes with bentonite are thermally stable, but are not suitable for use in the Midrex reduction shaft with regard to reducibility.
7 Conclusions
The Midrex direct reduction process produces a number of fine-grained residual materials such as dust, sludge, and fines. As these residues have a high iron content, the aim of these investigations was to briquette the residues to use them as feedstock in the Midrex direct reduction process. The suitability of organic and inorganic binders has been investigated with regard to the cold strength and metallurgical properties of the briquettes. In the first part, tests were carried out on the basis of a simplex mixture experimental design. The tests showed that briquettes with sufficient cold strength could be produced with all three investigated binder cellulose, starch, and bentonite. However, it is important to use a suitable water and binder content depending on the binder. The low-temperature disintegration tests have shown that the briquettes with the inorganic binder bentonite withstand the loads when fed into the reduction shaft, whereas the briquettes with the organic binder cellulose and starch disintegrate. However, it is also shown that the briquettes with the inorganic binder bentonite have poor reducibility and are therefore also not suitable for use in the Midrex direct reduction process. Consequently, the procedure of briquetting with organic binder needs further optimization for sufficient thermal stability. Furthermore, the expected effect of the better reducibility of the briquettes with organic binder still has to be proven. One chance in addition to others to make this possible could be the joint use of bentonite and starch or cellulose as a binder.
Acknowledgements
The authors gratefully acknowledge the funding support of K1-MET GmbH, metallurgical competence center. The research programme of the K1-MET competence center was supported by Competence Center for Excellent Technologies (COMET), the Austrian programme for competence centers. COMET was funded by the Federal Ministry for Climate Action, Environment, Energy, Mobility, Innovation and Technology, the Federal Ministry for Digital and Economic Affairs, the Federal States of Upper Austria, Tyrol and Styria as well as the Styrian Business Promotion Agency (SFG). In addition to the public funding from COMET, this research project was partially financed by the scientific partner TU Bergakademie Freiberg (TUBAF), Institute of Thermal-, Environmental- and Resources’ Process Engineering (ITUN), and the industrial partner voestalpine Stahl GmbH Linz.
Conflict of Interest
The authors declare no conflict of interest.




