Carboniferous horseshoe crab musculature suggests anatomical conservatism within Xiphosurida
Abstract
An exceptionally preserved specimen of the horseshoe crab Euproops danae (Xiphosurida, Belinurina) in a siderite concretion from the Carboniferous (Upper Pennsylvanian, Virgilian) Lawrence Formation, Kansas, shows anatomical details of the prosomal musculature. The extrinsic appendicular muscles are comparable to those of Limulus polyphemus (the modern American horseshoe crab), demonstrating anatomical conservatism within Xiphosurida that spans two morphologically disparate subgroups, Belinurina and Limulina. The three-dimensional preservation of muscles highlights how siderite concretion fossils (including those of the Mazon Creek Konservat-Lagerstätte) have better preservational fidelity than previously realized and the potential to reveal new anatomical information, especially with regard to the labile tissues of euarthropods.
Exceptional preservation allows for a stunning array of anatomical features to be observed in the fossil record. Such details are essential for addressing topics regarding the history of life and exploring aspects of organismal evolution (Briggs & McMahon 2016). An underexplored exceptional preservation deposit (or Konservat-Lagerstätte) occurs in the Carboniferous (Upper Pennsylvanian, Virgilian) Lawrence Formation, Kansas, USA (Feldman et al. 1993; Babcock & Merriam 2000). Siderite concretions in the deposit host a fossil assemblage that is comparable to the ‘Braidwood biota’ of the famous Mazon Creek Konservat-Lagerstätte, Illinois, USA (Schram 1979; Shabica & Hay 1997; Clements et al. 2019). The Lawrence Formation represents a marine-influenced, estuarine swamp and floodplain setting (Feldman et al. 1993; Babcock & Merriam 2000), thus giving insight into latest Pennsylvanian ecosystems that are similar to the slightly older Mazon Creek deposit of Middle Pennsylvanian (Moscovian) age.
Examination of the Lawrence Formation biota has provided data on terrestrial and marine euarthropods, especially insects and euchelicerates (Feldman et al. 1993; Babcock & Merriam 2000; Wright & Selden 2011). A representative of the latter group is the xiphosurid, Euproops danae (Meek & Worthen, 1865), which is rare in the formation (Babcock & Merriam 2000). Recent studies on xiphosurids preserved in Mazon Creek-type deposits have shed new light on these long-lived animals (Bicknell et al. 2018a; Haug & Rötzer 2018; Bicknell & Pates 2020; Haug & Haug 2020). Here we consider a specimen of E. danae from the Lawrence Formation and document the oldest known evidence of xiphosurid muscles.
Method
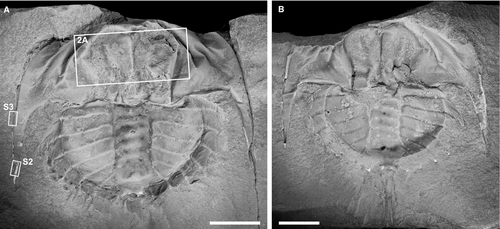
Euproops danae specimens in the Division of Invertebrate Paleontology collections of the Yale Peabody Museum (YPM IP), New Haven, CT, USA were examined. One partially complete specimen (YPM IP 35153; Fig. 1), previously considered by Babcock & Merriam (2000) and Haug & Rötzer (2018), shows evidence of prosomal musculature preserved in the vicinity of the cardiac lobe (Fig. 2). These structures are herein identified as bundles of muscle fibres associated with the walking legs. This specimen was photographed as a series of stacked images under normal LED light using a Canon EOS 5DS digital SLR camera fitted with a Canon MP-E 65 mm macro lens (for imaging the entire specimen), a Canon MP-E 65 mm 1–5× macro lens (for close ups of the muscles), and a Cognisys Stackshot 3X stacking system. Photos were stacked and stitched using Helicon Focus 7. Specimens were coated with ammonium chloride sublimate prior to photography. Muscle assignment follows Shultz (2001) and Briggs et al. (2005), the most recent summaries of extant and fossil xiphosurid musculature, respectively.
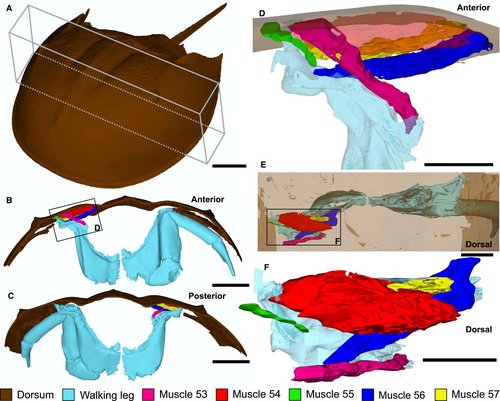
To corroborate the muscles identified in YPM IP 35153, neontological data from a modern xiphosurid were gathered. An adult female Limulus polyphemus (Linnaeus, 1758) specimen (214 mm long, excluding telson) was scanned using micro-computed tomography (µCT) to explore the dorsal morphology of the prosomal muscles. The studied L. polyphemus specimen is housed in the New England Natural History Arthropod collection (NENH-AR0001) at the University of New England, Armidale, NSW, Australia. Prior to scanning, NENH-AR0001 was immersed in 1% iodine-ethanol solution for 13 days. The specimen was then washed in 100% ethanol for 2 days (Gignac & Kley 2014; Bicknell et al. 2018b, c, 2021a). Staining increased muscle density and allowed extrinsic limb muscles (numbered following Manton 1964 and Shultz 2001) to be identified. NENH-AR0001 was scanned in a GE-Phoenix V|tome|xs µCT scanner with a 240 kV microfocus x-ray tube, at the University of New England, under optimized x-ray tube settings (150 kV, 200 μA, 200 ms) and using an isotropic voxel side length of 92 μm. Data were captured using Datos acquisition software version 2.2.1 (phoenix) and reconstruction software version 2.2.1. Scans were imported into Mimics version 23.0 (Materialise) and the most posterior set of walking legs (prosomal appendage set V), the prosoma above the appendages, and muscles 53–57 (numbered following Shultz 2001) were segmented using the Segmenting tool. Additional muscles and other internal features were removed with the Segmenting tool. Segmented muscles and exoskeletal components were converted to .STL files in Mimics and imported into Geomagic Studio (3D Systems). Segmented sections were smoothed in Geomagic Studio. Smoothed .STL files were exported from Geomagic Studio and a three-dimensional (3D) PDF of L. polyphemus musculature was generated using Terta4D (Adobe Systems) (Fig. S1).
Comparing the musculature of extinct xiphosurids to L. polyphemus may introduce a degree of circularity, given that we are comparing fossil groups to a modern analogue. Also, dorsal compression of the muscle groups has affected the complete expression of these structures. However, we follow the most established means of identifying muscle groups in fossil xiphosurids (cf. Briggs et al. 2005). Furthermore, being able to rotate the L. polyphemus muscles in three dimensions enables us to consider the variation in dorsal expression of muscles, allowing for a more confident assignment.
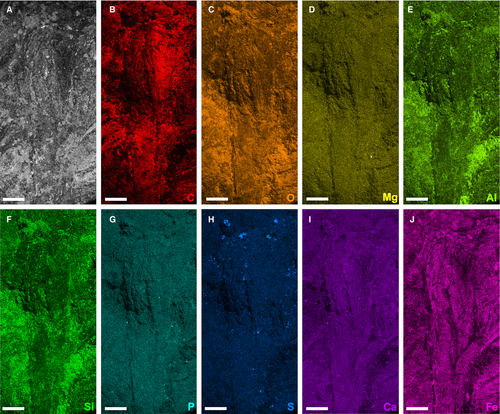
To examine the elemental composition and possible preservation mode of the musculature in the Euproops danae specimen YPM IP 35153, one of the largest muscle groups was examined using scanning electron microscopy (SEM) energy dispersive x-ray spectroscopy (EDS). Additionally, the distal portion of the left genal spine showing white material and an area including the left genal spine margin and adjacent matrix, were mapped to determine compositional differences between fossil and matrix (Fig. 1A and Figs S2, S3). The specimen was analysed uncoated under low-vacuum conditions in the JEOL JSM-6010LA scanning electron microscope at the University of New England. Element mapping of the muscle was conducted at a voltage of 20 kV for 8 h. Supplemental element mapping was conducted at a voltage of 16 kV for 4 h.
Results
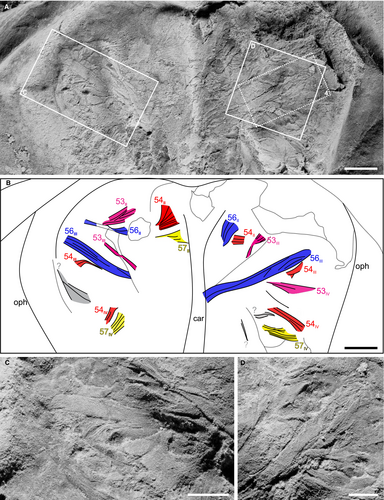
YPM IP 35153 consists of a part and counterpart preserved within a siderite concretion that represents approximately the 8th instar stage of Euproops danae (Haug & Rötzer 2018) (Fig. 1; Table 1). The prosoma is mostly complete, with a break along the anteriormost section. The thoracetron is completely preserved, and only the anteriormost section of the telson is present. Muscle tissue is identified by a fabric of fibres that are organized into 3D, bilaterally symmetrical, serially arranged bundles (Fig. 2), in a manner consistent with segmentation; the fibrous bundles are less compatible with alternative interpretations, such as degraded cuticle. Exceptional preservation allows identification of individual muscles within the prosomal area (Fig. 2A, B). Muscles with origins on the internal prosomal surface that probably inserted into prosomal appendages II–V (walking legs I–IV) are recognized and considered primarily as extrinsic leg muscles. This implies a considerable divergence in the insertion of limbs from anterior to posterior along the prosoma, but not greatly differing from that of Limulus polyphemus (Snodgrass 1952, fig. 7F). Muscle bundles mostly represent the origin sections, tapering to show sections of distal ends (Fig. 2C, D), and are morphologically comparable to the extrinsic muscles of L. polyphemus when viewed dorsally (Fig. 3). Muscles in E. danae are homologized with those of L. polyphemus (cf. Shultz 2001) based on correspondences in approximate position, fibre orientations, muscle shape, segmental repetition, and symmetry, following the approach of Briggs et al. (2005). The majority of muscles could be identified using these criteria; however, three muscle bundles could not be confidently assigned to a muscle group (Fig. 2B). The muscles are not aligned with the sagittal plane of the specimen, so are not considered axial muscles. As such, we identified four sets of extrinsic limb muscles, including the main promotor (muscle 53), abductor (muscles 54 and 57), and levator/adductor (muscle 56) muscles, following the functional interpretations of Manton (1964: muscles 25–29 therein). Given the somewhat limited expression of muscles, they probably collapsed upon themselves during preservation (Moore et al. 2011).
| YPM IP ID no. | PL | PW | CL | IW | TW | TL |
|---|---|---|---|---|---|---|
| 35153 | – | 47.51 | 15.96 | 22.93 | 30.36 | 18.38 |
- CL, cardiac lobe length; IW, width of the interophthalmic area; PL, prosoma length; PW, prosoma width; TL, thoracetron length; TW, thoracetron width.
Element maps of an area that includes a large individual levator/adductor muscle (muscle 56III) and other adjacent muscles (Fig. 2B) show a pervasive enrichment of carbon, oxygen and iron (Fig. 4B, C, J), representing the predominantly sideritic (FeCO3) composition of the fossil and its host concretion (see also Fig. S3). White patches enriched in aluminium and silicon (e.g. Fig. 4E, F and Fig. S2E, F) most likely represent the aluminosilicate clay mineral kaolinite. Kaolinite is known to fill voids in Mazon Creek-type fossil concretions as a late diagenetic precipitate, often replicating both soft and hard-part anatomy (Cotroneo et al. 2016; Clements et al. 2019; Bicknell et al. 2021b). These kaolinite infills are particularly evident on some parts of the exoskeleton, such as the genal spines on the prosoma and the marginal spines on the opisthosoma (Fig. 1).
Discussion
The musculature of modern horseshoe crabs has been used to model how the limbs of extinct euchelicerates (Fisher 1975, 1977, 1979; Selden 1981), trilobites (Bicknell et al. 2021a), and other artiopodans (Bicknell et al. 2018c) would have functioned. As such, documentation of xiphosurid muscles is of interest to a variety of researchers. To date, the preserved musculature of fossil xiphosurids is limited to four Mesozoic limulid taxa: Yunnanolimulus luopingensis Zhang et al., 2009 from the Middle Triassic (Anisian) Guanling Formation, China (Hu et al. 2017); Ostenolimulus latus Lamsdell et al., 2021 from the Lower Jurassic (Sinemurian) Moltrasio Limestone (Lamsdell et al. 2021); Mesolimulus walchi (Desmarest, 1822) from Upper Jurassic (Kimmeridgian) Solnhofen and Nusplingen limestones, Germany (Briggs & Wilby 1996; Briggs et al. 2005); and Tachypleus syriacus (Woodward, 1879) from the Upper Cretaceous (Cenomanian) Haqel and Hadjoula Konservat-Lagerstätten lithographic limestones, Lebanon (Bicknell et al. 2019a). The illustration of musculature from a non-limulid taxon represents novel anatomical information for understanding the evolutionary history of horseshoe crabs (Bicknell et al. 2018a). Indeed, the muscle groups identified in E. danae could be homologized with those observed in modern taxa. These muscle data augment the very limited information on belinurid appendages (Racheboeuf et al. 2002; Haug & Rötzer 2018; Bicknell et al. 2019a). Despite considerable morphological disparity of the dorsal exoskeleton among Carboniferous taxa (Lustri et al. 2021), this provides evidence of conserved aspects of horseshoe crab myoanatomy and extends the fossil record of xiphosurid musculature back by 60 myr (Hu et al. 2017; Bicknell & Pates 2019, 2020; Bicknell et al. 2019b). This is especially striking given the phylogenetic and morphological disparity between limulids and belinurids (Briggs et al. 2005), and allows the identified muscles to be inferred in the last common ancestor of these two main clades of Xiphosurida (Lamsdell 2016, 2020).
Muscles are also preserved in the synziphosurine Camanchia grovensis Moore et al., 2011 from the Silurian (Wenlock) Scotch Grove Formation Konservat-Lagerstätte. Formerly classified as horseshoe crabs, synziphosurines are a grade of stem-group euchelicerates (Lamsdell 2016). The majority of the muscles known from C. grovensis are longitudinal opisthosomal muscles and are therefore not comparable to those documented here (Moore et al. 2011). However, the prosomal muscles radially arranged about the prosomal midline of C. grovensis have broadly similar orientations to those of E. danae and the Mesozoic taxa noted above. This suggests that walking leg muscles in Silurian (and perhaps other) synziphosurines are similar to more derived horseshoe crabs.
Evidence of muscles in siderite concretion fossils has rarely been presented, despite a lengthy period of study on such deposits (e.g. Mazon Creek Konservat-Lagerstätte) that extends back to the mid-1800s (Meek & Worthen 1860; Worthen 1868; Clements et al. 2019). To date, the only examples are putative muscle blocks in the enigmatic ‘Tully monster’ (Tullimonstrum gregarium Richardson, 1966), possible muscle bands in the priapulid Priapulites konecniorum Schram, 1973 (two taxa from the Mazon Creek Konservat-Lagerstätte) and the trigonotarbid arachnid Anthracosiro woodwardi Pocock, 1903 from the ?Upper Coal Measures (Pennsylvanian, Moscovian), England, UK (Petrunkevitch 1949; Foster 1979; Schram 1979; McCoy et al. 2016; Sallan et al. 2017). The specimen of E. danae documented here thus strengthens the case for the preservation of musculature within siderite concretions. This raises the question of how the musculature has been preserved. Element mapping shows that the muscles are not enriched in elements such as calcium, phosphorus, or sulphur (Fig. 4G–I), suggesting that they may not have been initially replicated by minerals such as apatite or pyrite. Authigenic mineralization of apatite in particular is often implicated in the replication of certain labile tissues (e.g. muscle and digestive systems) during the early stages of fossilization (Briggs 2003). This is especially true of euarthropods from Cambrian Konservat-Lagerstätten (Butterfield 2002; Vannier et al. 2014; Paterson et al. 2016; Lerosey-Aubril et al. 2018; Harper et al. 2019), as well as the musculature of the limulid Mesolimulus walchi from the Jurassic of Germany (Briggs & Wilby 1996; Briggs et al. 2005), among other examples. In contrast, it is plausible that the muscles of E. danae from the Lawrence Formation were instead moulded by the rapid formation of siderite around and within the individual prior to tissue decay, as suggested for the preservation of the central nervous system in a specimen of E. danae from the Mazon Creek Konservat-Lagerstätte (Bicknell et al. 2021b). The cuticle encasing the muscles, in addition to dissolved Fe2+ in the ferruginous porewater that enabled siderite precipitation, may have helped to slow the decay process and facilitate rapid moulding of these soft tissues (Schweitzer et al. 2014; Anderson 2020; Saleh et al. 2020). This is supported by geochemical evidence indicating that siderite quickly forms around organisms during the earliest stages of fossilization (Cotroneo et al. 2016; Clements et al. 2019).
Conclusion
We re-examined a unique Euproops danae specimen from the Carboniferous Lawrence Formation of Kansas and present new evidence for prosomal musculature. We compare these muscles to those of the extant American horseshoe crab, Limulus polyphemus, and propose that they represent extrinsic walking leg muscles. In doing so, we demonstrate the oldest evidence for xiphosurid musculature, extending the record of muscles in horseshoe crab fossils back to the late Palaeozoic. This discovery also shows that Mazon Creek-type Konservat-Lagerstätten still hold great potential for providing important new information on the soft-tissue anatomy of Carboniferous animals preserved in siderite concretions. We conclude that despite the substantial variation in the dorsal morphology of xiphosurids across their evolutionary history, the musculature involved in fundamental aspects of locomotion (e.g. promotion, levation and adduction/abduction of the legs) is conserved.
Acknowledgements
We thank Susan Butts (Yale Peabody Museum) for kindly facilitating access to the examined specimen and Malcolm Lambert for aid with SEM-EDS. We also thank Jason Dunlop and an anonymous reviewer for their comments that improved the text and highlighted areas that could be further explored. This research was supported by funding from an Australian Research Council Discovery Project grant (DP200102005 to JRP, GDE), a UNE Postdoctoral Research Fellowship (to RDCB), and a Charles Schuchert and Carl O. Dunbar Grants-in-Aid award (to RDCB).
Author contributions
Conceptualization, RDCB; methodology, RDCB and JRP; investigation, RDCB, GDE, JNT and JRP; resources, RDCB; writing (original draft), RDCB, GDE, JNT and JRP; writing (review and editing), RDCB, GDE, JNT and JRP; figure creation, RDCB and JRP; funding acquisition, RDCB, GDE and JRP.
Open Research
Data archiving statement
Data for this study are available in the MorphoSource digital repository: https://doi.org/10.17602/M2/M363293 (CT image series); https://doi.org/10.17602/M2/M362376 (STL).




