Recent Advances in Biosensors Based on Hybridization Chain Reaction and Silver Nanoclusters
Abstract
Hybridization chain reaction (HCR) and DNA-templated silver nanoclusters (AgNCs) have emerged as powerful tools in biosensing. HCR enables cascade amplification through programmable DNA interactions, while DNA-AgNCs serve as transducing units with unique fluorogenic and electrochemical properties. Integrating these components into a hybrid sensor could significantly enhance sensing capabilities across various fields. Nonetheless, limited studies and the lack of systematic guidelines for HCR-AgNCs systems have hindered research progress, despite their potential. This review aims to address this gap by providing a comprehensive overview of HCR-AgNCs biosensors, facilitating further innovation in this field. The working principles, performance factors, and complementary features are discussed. Thereafter, reported HCR-AgNCs studies are assessed, emphasizing their distinct sensing mechanisms (e.g., fluorogenic, electrochemical), applications across various fields, and challenges in adopting the hybrid sensors. Drawing from the experience developing multiple HCR-AgNCs sensors, insights and guidelines for designing and developing HCR-AgNCs systems are provided for future researchers. Finally, prospective directions in HCR-AgNCs research, including multiplex assays and integration with emerging technologies, are explored to guide future advancements. The synergistic combination of HCR and AgNCs as a hybrid biosensor holds promise for addressing pressing challenges in healthcare, environmental monitoring, and beyond, paving the way for next-generation biosensing technologies.
1 Introduction
A DNA-based biosensor typically consists of synthetic DNA probes for target biorecognition and a transducer to generate a measurable signal (Scheme 1).[1] DNA-based biorecognition emphasizes Watson–Crick base-pairing between DNA probes and target analytes or between DNA aptamers and non-nucleotide targets. A transducer is an element (e.g., nanostructure, electrode, ion channel, photon, etc.) that converts the bio-recognition event into a measurable signal (e.g., electrochemical, optical, etc.), which is then analyzed and displayed accordingly.[2]
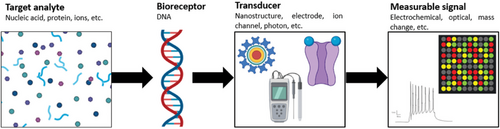
The versatility of DNA molecules facilitates the diverse application of DNA-based biosensors designed for a wide range of targets, including nucleic acids, proteins, metal ions, and small molecules.[3, 4] With the advancement of DNA nanotechnology, these biosensors can further integrate nanostructures like nanoparticles and nanoclusters for signal amplification.[5, 6] For example, gold nanoparticles (AuNPs) are extensively studied for their strong localized surface plasmon resonance (LSPR) response. Changes in the aggregation state of AuNPs lead to a visible optical blue or red shift, which is valuable in colorimetric biosensors and assesses DNA binding.[7] With recent research efforts driven toward point-of-care (POC) and point-of-need detection of biomarkers, DNA-based biosensors are expected to generate rapid results with good accuracy, precision, sensitivity, and selectivity over a wide detection range.[8]
Among the combinations of DNA bioreceptors and transducers, the hybrid system of hybridization chain reaction (HCR) and silver nanoclusters (AgNCs) holds a prospective diagnostic value with a remarkable signal enhancement. HCR is touted as a highly efficient signal amplification strategy that operates using only DNA hairpin probes in mild conditions. In other words, HCR eliminates the need for stringent reaction settings and thermosensitive molecules like enzymes, which are common in other amplification methods. This versatility has led to successful applications in various research areas, including food safety, environmental monitoring, and clinical diagnostics. On the other hand, AgNCs are nanostructures capable of emitting different types of signals, including fluorescence, electrochemical, or photothermal effects, depending on the reaction conditions. For example, integrating AgNCs with different DNA sequences or structures produces distinct signals reflective of the specific DNA conformation. AgNCs are known for their high biocompatibility, simple and cost-effective preparation, and good stability over time. Despite these intrinsic benefits, the development of the HCR-AgNCs hybrid system remains in its early stages, with less than 30 publications reported over the past decade. In addition, there is a lack of correlation and continuity between these studies, particularly in designing metastable DNA hairpin probes. This hinders the advancement and wider application of HCR-AgNCs in the diagnostics field.
This review aims to facilitate future research in designing and developing HCR-AgNCs biosensors. We will first briefly discuss the concepts of HCR and AgNCs, pinpointing congruent areas to foster the development of HCR-AgNCs. Subsequently, we will evaluate the reported HCR-AgNCs studies in detail, identifying the key components and challenges for a successful HCR-AgNCs system.
2 HCR Amplification
2.1 Principles of HCR Amplification
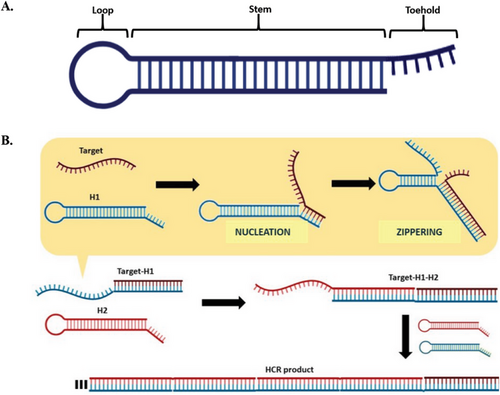
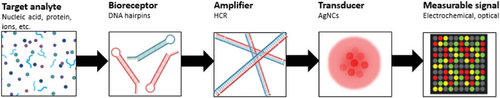
Hybridization chain reaction (HCR) is an amplification strategy that employs a set of DNA hairpins under isothermal conditions, without the need for enzymes.[9-13] A DNA hairpin is a single-stranded DNA (ssDNA) that adopts a hairpin shape, consisting of three regions: a short overhanging toehold (also known as sticky-end), a long duplex stem, and a loop with unpaired bases (Figure 1A).[14] The toehold plays a crucial role in regulating the kinetics of target recognition, hairpin opening, and subsequent hybridization events.[15-17] Meanwhile, the stem is responsible for maintaining the thermodynamic stability of the hairpin by kinetically trapping its potential energy in the loop region. The HCR amplification process requires at least two partially complementary DNA hairpins. When one hairpin opens, it triggers continuous cross-hybridization with the other hairpins. As such, this set of DNA hairpins should coexist metastably to avoid non-specific hybridization events that could lead to circuit leakage.[16, 17] The HCR amplification operates based on conventional DNA hybridization, initiated by a target sequence that unlocks a kinetic trap in the DNA hairpins (Figure 1B). The first step, called “nucleation,” involves complementary base-pairing between the target sequence and the toehold region of the first hairpin (i.e., H1). This step is a rate-limiting process reliant on the random diffusion of the target and H1 for contact formation. Thereafter, “zippering” occurs, where the target simultaneously hybridizes with the H1 and unfolds it along the stem toward the loop region. At this point, the zippering process becomes rate-limiting due to the separation of intra-strand base-pairs (bp) of the stem, as well as the formation of inter-strand bp between DNA hairpins.[18] This action exposes H1, leading to the opening of the other DNA hairpins in a similar pattern. This cascade of hybridization events continues until thermodynamic equilibrium is reached, resulting in long-nicked double-stranded (ds) DNAs. As is evident from its mechanism, HCR amplification is an automated process devoid of external components like enzymes and tedious reaction conditions such as thermal cycling.[19-21] Moreover, HCR amplification is defined by its linear amplification mechanism (i.e., increasing the amount or length of nucleic acid sequences in a stepwise or sequential manner) rather than through exponential replication (i.e., nucleic acid sequences replicating exponentially with each cycle of amplification, such as polymerase chain reaction [PCR]).
2.2 Factors Affecting HCR Amplification
-
DNA sequence and structure: Canonical Watson–Crick base pairing involves adenine (A)⋅thymine (T) bp and cytosine (C)⋅guanine (G) bp that form two and three hydrogen bonds, respectively. Hairpins with more C⋅G bp in the stem are more stable and exhibit slower hybridization kinetics.[23] The DNA strand length also affects stability, with longer strands being more stable. Adversely, mismatched bases hinder DNA hybridization, with mismatches in the middle of the DNA causing greater inhibition than the ends.[24] The DNA structure further affects hybridization efficiency; structured DNAs (e.g., hairpins) are more stable and slower to hybridize than linear DNA. For instance, at high temperatures, both hairpin and linear DNA structures exhibit similar negative hybridization activation energies, but at low temperatures, DNA hairpins have higher hybridization activation energies compared to linear DNA structures.[25]
-
Buffer constituents: The DNA microenvironment significantly influences the hybridization process. The negatively charged phosphate backbone of DNA causes electrostatic repulsion that inhibits hybridization. To mitigate this, hybridization buffers typically include cations, such as sodium (Na+) and magnesium (Mg2+), which reduce electrostatic repulsion by shielding negative charges and promoting DNA hybridization at lower energy levels.[23, 26] Purwidyantri et al. demonstrated salts enhance DNA carrier mobility and reduce repulsion, favoring DNA duplex formation and hybridization, thereby enhancing sensor sensitivity.[27] Likewise, Chim et al. observed stronger fluorescent signals in DNA probes prepared with high salt concentrations, attributing this to reduced electrostatic repulsion and enhanced hybridization efficiency.[28] Divalent cations (e.g., Mg2+) have a more prominent effect over monovalent cations (e.g., Na+) due to stronger electrostatic and hydrogen bonding with the bases and phosphate backbone.[23] Špringer et al. found that buffers supplemented with Na+ and Mg2+ enhanced the stability of DNA duplexes, with Mg2+ being particularly more effective over Na+.[29] Conversely, redox indicators like methylene blue can inhibit DNA hybridization by interacting with guanine bases and competing with cations.[30]
-
pH: Optimal DNA hybridization occurs around pH 7.5, decreasing slightly under alkaline conditions (pH 8) and significantly at lower pH (e.g., pH 6). Within this range, nucleobases are neutral and hydrophobic, which facilitates better interaction and stability between DNA strands. At low pH, DNA becomes protonated and more hydrophilic, destabilizing DNA duplexes; while at high pH, the bases deprotonate, disrupting hydrogen bonds and reducing stability. Extreme pH can also damage DNA through depurination and deamination.[30, 31] Excess H+ at low pH increases electrostatic repulsion, compacting DNA and hindering hybridization. Conversely, OH− at high pH disrupts hydrogen bonds in DNA, leading to denaturation and reduced hybridization. Rashid et al. confirmed via response surface methodology that neutral pH is optimal for DNA duplex formation.[30] Ionic strength also stabilizes pH, with higher concentrations providing stronger buffering capacity.[27]
-
Temperature: The temperature should be optimized within a narrow range. At low temperatures, DNA hybridization is dependent on the rate of diffusion, making nucleation the rate-determining step. Increased temperature provides thermal energy to overcome electrostatic barriers, enhancing hybridization rates. However, excessively high temperatures destabilize DNA duplexes, especially with short ones, due to negative activation energy.[22] Markegard et al. found that low temperatures and high concentrations favor stable aggregates, whereas high temperatures destabilize bp interactions.[32] Rashid et al. also reported that elevated temperatures improve DNA flexibility and accessibility, thereby enhancing hybridization rates,[30] but optimal temperatures vary by system, ranging below 40 °C or above 60 °C.[33, 34] For hairpin structures, hybridization efficiency depends on whether nucleation or hairpin opening is the rate-limiting step, with temperature affecting the activation energy required for these steps.[25]
2.3 Designing a Functional HCR System
Taking the aforementioned factors that affect HCR performance into consideration, a functional HCR system can be systematically designed. Guidelines for constructing effective HCR hairpins are summarized in Table 1.[14, 35-38] Ideally, these hairpins should remain metastable and cross-hybridize effectively in the absence and presence of targets, respectively. The metastability of DNA hairpins can be controlled by regulating the size and sequences of the hairpins. For example, hairpins with long stems and short toeholds possess better metastability.[35] Moreover, it is advisable to avoid designing hairpins with large loops, as the unpaired bases in the loops could cross-interact and form undesired duplex structures called “kissed complexes.”[14] In the context of the hairpin sequence, Ang et al. found that a higher portion of C and G bases in the stem confers greater metastability than that in the toehold region.[35] Notably, the metastability of hairpins shares an inverse correlation with the efficiency of hairpin opening. For instance, a shorter stem length and a larger loop size could promote hairpin opening at the expense of its metastability. According to Green et al., the hairpins can be opened either via an external toehold or the loop region, with the former route facilitating faster hairpin opening than the latter.[14]
| Hairpin condition | Description | Refs. |
|---|---|---|
| Base composition | The C/G content at the toehold should be between 30% and 40%. | [35] |
| If the stem length is equal to the toehold length, the percentage of C⋅G bp in the stem must be above 60%. | [35] | |
| Segment | Opening the hairpin via external toehold is faster than loop-mediated opening. | [14] |
| A 2° toehold can destabilize a hairpin structure; it should be kept below three bases. | [37] | |
| Unbounded toehold sequences can lead to the formation of “tails,” which hinder subsequent DNA hybridization/ HCR. | [38] | |
| DNA hairpins often experience fraying at the ends of the stem region, potentially leading to premature opening. | [36] | |
| Size | The hairpin toehold should be less than 12 bases. | [35] |
| The stem length should be longer than the toehold length. | [35] | |
| The stem length should be longer than the loop size. | [14] | |
| Folding energy | The threshold ΔG for stable DNA hairpins is −16 kcal mol−1. | [36] |
As previously mentioned, unstable DNA hairpins can result in HCR system leakage and false-positive results, which are particularly detrimental in biosensing applications. To develop a leak-proof HCR design, Li et al. studied the thermodynamic properties of DNA hairpin structures, focusing on their free energies (ΔG) and behavior. In this study, ΔG of DNA bases was comprehensively calculated, with C⋅G and A⋅T bp possessing −1.5 and −0.8 kcal mol−1, respectively. It was concluded that a minimum free energy (MFE) of −16 kcal mol−1 is required to prevent HCR leakage.[36] Another factor that could contribute to HCR leakage is fraying, where the ends of DNA hairpin stems are prone to frequent transient localized opening events, typically confined to the two terminal bp. This occurs due to reduced structural constraints at the end of the helical stem structure.[39, 40] To minimize this effect, it is suggested to introduce mismatch bases in hairpin loops, minimizing sequence symmetry, and ending the fraying regions with strong C⋅G bp, to create higher energy barriers.[36, 41]
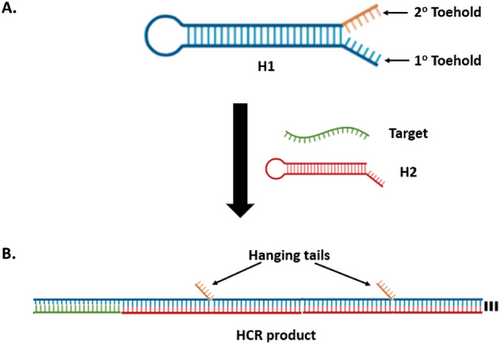
DNA hairpins can sometimes have a secondary (2°) toehold adjacent to the original primary (1°) toehold (Figure 2A). A recent study by Xing et al. found that 2° toeholds with more than three bases could destabilize the DNA hairpin structure, causing HCR system leakage.[37] Meanwhile, Yu et al. studied the effect of hanging “tails” on hybridized DNA structures. These hanging tails, characterized as unbounded sequences that appear as appendages from HCR products, typically originate from long, unreacted 1° or 2° toehold sequences (Figure 2B). The negative charges and swaying motion of these tails can destabilize a hybridized DNA complex and significantly slow down the nucleation rate between DNA strands. The repulsive force from these negative charges is speculated to be proportional to the length and number of tails in the hybridized structure.[38]
Designing DNA hairpins can be complicated and cumbersome due to the constraints mentioned earlier. This process would conventionally require screening numerous sequences and performing intensive calculations, resulting in only a few viable DNA probes. Pierce and co-workers developed an online platform called NUPACK (www.nupack.org) specifically for analyzing and designing nucleic acid structures for systems containing one or more interacting strands.[42] This software predicts DNA hybridization reactions based on the nearest neighbor model of base stacking. The thermodynamic calculation from the algorithm provides a relative prediction of the partition function, MFE structures, and melting temperatures (Tm) of DNA constructs. Tm represents the temperature at which 50% of dsDNA dissociates into ssDNA and is a measure of thermodynamic stability.[43, 44] NUPACK is an invaluable tool to visualize various DNA secondary structures, including hairpins and pseudoknots, thanks to its positive and negative design paradigms. The former optimizes the affinity between a sequence and a target structure for a desired outcome, whereas the latter optimizes the sequence specificity toward a target structure. NUPACK also provides the flexibility to adjust the concentration of interacting strands and modify the reaction conditions of the DNA hybridization process. However, the software has limitations regarding the number of strands it can analyze. It was reported that the web-based software could not compute seven or more interacting strands (n ≥ 7) due to the high computational cost and complexity.[45] Despite the existing gaps in the systematic design of functional HCR systems, recent studies have provided valuable insights into improving the efficiency of future HCR designs and systems.[14, 35-38, 42] These insights will be discussed in the following sections.
2.4 Application of HCR in Biosensing
Hybridization chain reaction (HCR) has found widespread application in biosensing and can be primarily categorized according to the target of detection: i) nucleotide target and ii) non-nucleotide target. An HCR system tailored according to a nucleotide target features a direct detection strategy defined by the interaction between the toehold sequence of HCR hairpins and nucleotide-based targets, such as DNA and RNA. HCR amplification proceeds directly, exhibiting a corresponding signal indicative of the target's presence. Conversely, a non-nucleotide target (e.g., protein, metal ions) requires an HCR strategy that employs different bioreceptors before HCR amplification, namely aptamers, and antibodies. Aptamers are synthetic nucleic acid sequences capable of binding to a specific non-nucleotide target. Upon binding, the aptamer undergoes conformational changes that initiate HCR amplification and subsequent signal transduction. Likewise, antibodies are functionalized to detect target molecules and initiate HCR amplification. The application of HCR with regard to the target of detection will be described in detail in the following subsections.
2.4.1 Nucleotide Target Detection
Nucleotide target detection (e.g., DNA and RNA) is the most common direct application for HCR amplification. The HCR system can be easily designed by translating the nucleic acid sequence onto the DNA hairpins. The mechanism involves the opening of DNA hairpins, typically starting from the toehold region, after complementary base-pairing with the target sequence. In one of the earlier studies, Pierce et al. developed the concept of HCR based on a 24-nt ssDNA sequence.[9] The HCR system features two basic hairpins that are fully complementary to the target sequence and each other. Upon HCR initiation, these hairpins form nicked double helices, analogous to alternating copolymers. It was also established that the average molecular weight of HCR products is inversely proportional to the target (initiator) concentration.[9]
Pierce and coworkers then adapted HCR amplification as an in situ hybridization method for multiplex detection of mRNA expressions in biological samples.[11] In such systems, RNA probes complementary to mRNA targets served as HCR initiators, and conventional DNA hairpins were substituted with RNA hairpins for improved stability. The system successfully imaged five target mRNAs simultaneously in zebrafish embryos, highlighting the robustness of HCR amplification for deep sample penetration, high signal-to-background ratio, and precise signal localization.[11] This in situ hybridization technique was then utilized by the group for imaging mRNA expressions in various biological models, including bacteria, fruit fly embryos, chicken embryos, and human tissue (Figure 3A).[46]
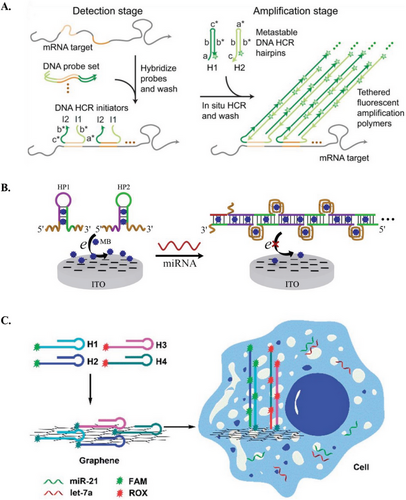
Other research groups have also employed the direct approach for the detection of microRNAs (miRs).[47] For instance, Hou et al. developed an HCR-based electrochemical biosensor for the detection of let-7 (Figure 3B).[48] The HCR system features DNA hairpins that form long duplex DNA chains with multiple G-quadruplexes upon successful HCR amplification. The electrochemical indicator, methylene blue (MB), was subsequently added, whereby the MB selectively intercalated into duplex DNAs, including DNA hairpin stems and the G-quadruplexes within the DNA chains. This intercalation caused a change in the electrochemical signal (i.e., turn-off signal), enabling selective and sensitive detection of let-7.[48] Moreover, HCR amplification has also been used for intracellular imaging of mRNAs and miRs.[49, 50] Li et al. developed a strategy for simultaneously imaging let-7a and miR-21 in living cells, utilizing HCR for signal amplification and graphene oxide (GO) as a carrier for the DNA hairpins.[51] The system features four DNA hairpins: with each pair associated with one miR target and labeled with different fluorescent dyes. All four DNA hairpins were adsorbed onto GO, which serves as a quencher. In living cells, the presence of the target miRs initiated HCR amplification, causing the DNA hairpins to detach them from GO and activate their corresponding fluorescent signals (Figure 3C).[51] These applications suggest that HCR can enable highly sensitive and simultaneous detection and imaging of multiple nucleotide biomarkers, thereby broadening its application across various fields, including disease diagnosis.
2.4.2 Non-Nucleotide Target Detection
Non-nucleotide targets (e.g., metabolites, metal ions, proteins, etc.) necessitate an aptamer serving as both the bioreceptor and the initiator for the HCR system. Typically, aptamers for these targets are derived from a library containing random sequences using a method called Systematic Evolution of Ligands by Exponential Enrichment (SELEX). The selected aptamer recognizes the analyte via intra-molecular interactions, before undergoing a conformational change to adopt a complex 3D structure.[52] The structural alteration triggers the opening of DNA hairpins from the HCR system and initiates the entire cascade of hybridization reactions. For example, Lu and coworkers developed a fluorogenic biosensor for platelet-derived growth factor-BB (PDGF-BB) detection by employing aptamer strands as initiators to trigger a cascade of hybridization events between two stable, quantum dot-labeled DNA hairpins. The resulting long concatemers then reacted with antibody-captured PDGF-BB on the well surface (Figure 4A).[53] Similarly, Po et al. utilized the aptamers to initiate HCR for detecting anterior gradient protein 2 homolog (AGR2). They designed two fluorophore-labeled hairpin probes with sticky tails. Without AGR2, the aptamer initiated HCR between the hairpins, forming a long nicked dsDNA duplex that could not adsorb onto gold nanoparticles (AuNPs), resulting in a strong fluorescence signal. In the presence of AGR2, the aptamer specifically recognized it, leaving the hairpins with sticky tails available to adsorb onto the AuNPs surface, bringing the fluorophores close to the AuNPs and quenching the fluorescence signal.[54] The same concept was applied in an electrochemical sensor for interferon (IFN)-γ biosensor, where the recognition probes containing the IFN-γ aptamer initially bound to the targets, and the unbound probes triggered HCR on the electrode. The electrochemical signal observed was inversely proportional to the concentration of IFN-γ present.[55] Another study by Feng et al. involved a three-hairpin system, one of which incorporated an aptameric structure for PDGF-BB. In the absence of PDGF-BB, the hairpins coexisted stably on the GO surface, resulting in weak fluorescence when SYBR Green I (SG) was added. However, in the presence of PDGF-BB, the aptamer recognized the target and triggered HCR, generating a long DNA chain that bound with SG. This produced a strong fluorescent signal when GO was added, indicating the concentration of PDGF-BB.[56] The aptameric approach can also be tailored for detecting other targets, such as metal ions. For example, a structure-switching probe (H0) was designed to recognize mercury ions (Hg2+) through the formation of a stable T–Hg2+–T complex, initiating HCR between two other hairpin probes. The resulting nicked double-helices were unable to protect AuNPs from salt-induced aggregation, leading to a color change from red to blue. Conversely, in the absence of Hg2+, all hairpin probes could stably coexist in solution, with their exposed sticky ends capable of stabilizing AuNPs and preventing them from aggregation (Figure 4B).[57]
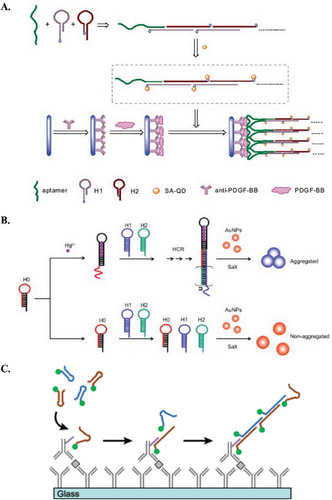
Apart from nucleic acid bioreceptors, non-nucleotide molecules have also been used in HCR biosensing applications. Antibodies are one of the most common options, because of their innate ability for analyte binding with high affinity and specificity. In most cases, when antibodies are coupled with HCR, the resultant immunosensors would be a sandwich-type model. Christopher et al. utilized an antibody-coated glass surface to capture chemokines and cytokines. They introduced secondary antibodies covalently linked with oligonucleotide-based initiators, initiating HCR with fluorescent-labeled hairpin oligomers (Figure 4C).[58] Comparably, Zhang et al. introduced HCR initiators along with secondary antibodies onto AuNPs to detect human IgG.[59] They employed immobilized capture antibodies on magnetic beads to facilitate the formation of sandwiched immunocomplexes in the presence of IgG. This setup allowed for signal amplification using hairpin probes labeled with ferrocene, resulting in an electrochemical signal. Likewise, Yuan et al. devised a multiplexing platform with an antibody-bound HCR complex to detect four different antigens (AFP, CEA, CA125, and PSA) within a single electrochemical scan.[60]
3 DNA-AgNCs
3.1 DNA-AgNCs as Biosensing Transducers
DNA-templated silver nanoclusters (DNA-AgNCs) are biosensing transducers characterized by their ultrasmall size, consisting of silver atoms closely aligned with the Fermi wavelength of electrons. These nanoclusters exhibit fluorogenic and electroactive properties, making them versatile tools in biosensing applications.[61] In general, DNA-AgNCs have several advantages over other types of dyes and materials, such as better solubility and photostability, as well as lower photobleaching and toxicity index.[62-64] AgNCs are highly sensitive to changes in their environment, making them suitable for detecting low concentrations of analytes. Moreover, their small size and efficient electron transfer properties enable rapid response time for quick detection and analysis, and they can be integrated into miniaturized devices for point-of-care testing, among others. AgNCs also exhibit a high quantum yield upon excitation, making them sensitive fluorophores. Additionally, their fluorogenic properties are reversible, i.e., allowing for both active activation (turn-on) and quenching (turn-off), which enables real-time monitoring applications.[65] Most importantly, DNA-AgNCs have tunable fluorescence emission from UV to near-infrared region, which can be regulated through the DNA sequence, AgNCs size, and buffer constituents.[66, 67] Based on current evidence, the fluorescence properties of AgNCs are better understood than their electrochemical aspects. Hence, this review will focus more on the fluorogenic aspect of DNA-AgNCs.
The formation of DNA-AgNCs emphasizes the affinity binding of silver ions (Ag+) to nucleobases, especially C bases. On a molecular level, Ag+ binds to the heterocyclic groups of purines and pyrimidines, with a particular preference for nitrogen atom 3 (N3) of C and T bases, and N7 of A and G bases.[68, 69] Apart from being a template for AgNCs formation, the DNA strand also stabilizes the synthesized AgNCs to better regulate their morphology and size.[68, 70] Over the years, extensive research around DNA-AgNCs, especially through the works of Gwinn et al., has led to a better understanding of their molecular and optical properties, such as the use of different DNA secondary structures,[71, 72] DNA sequences,[73, 74] and preparation methods.[75]
3.2 Preparation of DNA-AgNCs
DNA-AgNCs can be controllably prepared in situ via a simple mixture of silver salt (e.g., Ag+), reducing agent (e.g., NaBH4), and a DNA template, in a buffered solution (Scheme 2). This is followed by an incubation period for the nucleation and stabilization of the nanoclusters.[76]

The amount of Ag+ and NaBH4 relative to the DNA concentration can affect the fluorescence properties of DNA-AgNCs. Typically, the amount of silver salt is equivalent to that of the reducing agent. Neither reactant (i.e., Ag+ or NaBH4) in excess will affect the fluorescence analysis, so additional purification steps are unnecessary.[76-78] Besides that, a buffer with monovalent and divalent salts is required to facilitate AgNC formation by shielding the negative charges and stabilizing the DNA structure,[79] as described in Section 2.2.2.
The selection of a specific DNA template is a little more complex. In addition to being sequence-specific as aforementioned, the fluorescence properties of AgNCs also rely on the structure of the DNA template. The most common type of DNA used is linear ssDNA, known for its structural flexibility to accommodate Ag+. Typically, ssDNA templates contain between 12 and 30 bases, with longer sequences producing AgNCs with better stability and yield. In addition, longer strands are more prone to multi-base bonding, thereby forming larger AgNCs that emit fluorescence at longer wavelengths.[80, 81] Most bright AgNCs species originate from consecutive C bases. The simplest sequence is a homo-polyC sequence, which comprises only C bases. The resultant fluorescence can be tuned between green and red regions, by adjusting the number of C bases. A different type of sequence, termed “chemopalette,” incorporates other bases (A, T, and G) within C-rich segments. This produces different colored bright species of AgNCs.[82]
Another relevant structure known to host AgNCs is the DNA hairpin structure. AgNCs are highly susceptible to oxidation which can alter their fluorescence properties. A closed hairpin structure can improve the stability of AgNCs. The hairpin loop can host AgNCs with a homo-polyC loop (6C, 12C, etc.). This configuration is known to produce red fluorescence emission similar to an average homo-polyC ssDNA. The fluorescence properties can be regulated by the C and G base composition within the stem sequence.[83] However, the resultant fluorescence intensity significantly decreases when the DNA hairpin opens up.[82]
In accordance with the variety of available DNA templates, DNA-AgNCs have been innovatively amalgamated into different biosensing platforms.
3.3 DNA Templating Sequence and Structure
Various templating sequences have been thoroughly investigated for their role in DNA-AgNCs formation, encompassing a wide array of potential sequences, each capable of influencing the size, stability, and optical properties of the resulting nanoclusters. In this section, however, we will focus on the most common types of templating sequence and structure.
3.3.1 Homo-PolyC AgNCs Templating Sequences
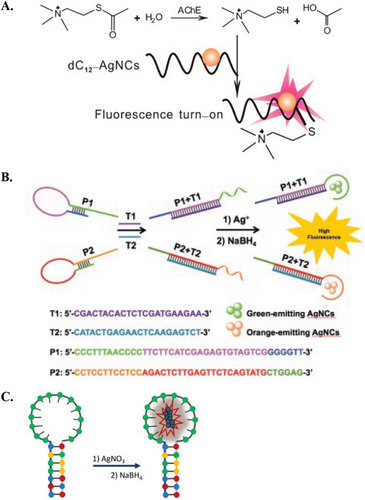
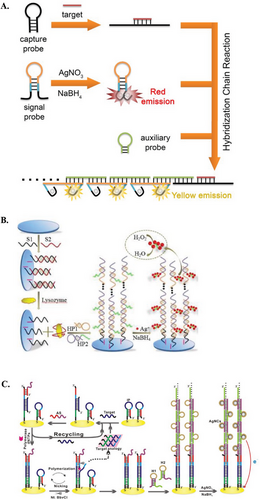
Homo-PolyC AgNCs templating sequence can be easily integrated for biosensing purposes, due to it being a well-studied sequence and having a predictable fluorescence response.[84-86] Thus, homo-polyC AgNCs biosensors have been used to measure the oxidative activity in cancer cells,[87] identify S1 nuclease activity,[88] detect miR,[84] and analyze antibiotic content.[89] For instance, Hosseini et al. reported a biosensor using DNA probes comprised of two functional regions, i.e., one for recognition of the p53 gene with ends hosting 12C scaffolds for AgNCs. They found that single-base mismatch sequences reduced the intensity of the red AgNCs fluorescence emission. This reduction was likely due to less rigid duplex structures formed after hybridization, which did not adequately protect AgNCs from quenching.[90] In another example, Zhang et al. utilized a 12 C sequence to develop a turn-on fluorescence assay for detecting acetylcholinesterase activity and screening acetylcholinesterase inhibitors (Figure 5A).[91] The catalytic hydrolysis of acetylthiocholine chloride produced thiocholine, which could form Ag–S bonds with the AgNCs, thereby increasing the fluorescence signal. In the presence of inhibitors, the hydrolysis reaction was inhibited, allowing for analysis of enzyme activity and inhibition.
3.3.2 Chemopalette AgNCs Templating Sequences
Chemopalette sequences feature a mixture of bases in between C-rich segments, and these subtle base substitutions can generate significant changes in the fluorescence species.[92] Thus far, various chemopalette sequences have been developed, ranging from 12 to 46 mer. However, there is a lack of correlation between these sequences, in terms of cluster size, emission properties, and Ag–Ag bonds.[82] Nonetheless, Gwinn et al. have determined several key considerations in fabricating DNA sequences that yield bright AgNCs species, including the number, type, and location of bases (particularly C and G bases).[71] In this regard, Zhang et al. applied two different chemopalette sequences for the multiplex detection of H1N1 and H5N1 (Figure 5B). These sequences comprise multiple consecutive C blocks separated by A and T bases.[93] In the absence of targets, the formation of AgNCs was prohibited by stem-loop structures. Upon hybridization with the respective targets, these structures opened, releasing the chemopalette sequences and resulting in green and orange fluorescence.
3.3.3 DNA-AgNCs Templating Hairpin Loop Sequences
DNA hairpin loops with homo-polyC sequences have been reported to host AgNCs that produce intense red fluorescence. Structural changes within the hairpin can change the fluorescence properties. For example, AgNCs hosted within a C13 loop resulted in a bright-red emission peak, and the intensity was significantly quenched when Hg2+ interacted with the DNA hairpin (Figure 5C).[94] In a different study, a 12C hairpin loop was used to detect miR-21 via a dual-emissive response. Upon opening the DNA hairpin, the red fluorescence emission of AgNCs within the hairpin loop was quenched, while a green fluorescence emission was generated from the opened structure.[95] In a different approach, one can induce fluorescence quenching using the enzymatic activity of Exonuclease III. Shen et al. and Xu et al. designed systems where the free toehold of hairpins binds to targets, e.g. Hg2+ and DNA. This binding triggered the stepwise removal of mononucleotides from blunt 3′-hydroxyl termini in the duplex region. The enzymatic action unfolded the hairpins, leading to a turn-off signal of AgNCs.[96, 97]
3.4 DNA-AgNCs in Biosensing Applications
DNA-AgNCs have found diverse applications in the field of biosensing. As aforementioned, DNA-AgNCs are more commonly studied for their fluorescence properties than their electrochemical properties. As such, their application in ECL and electrochemical analysis has been relatively limited. Nonetheless, AgNCs, with their good oxidation-reduction properties, hold promising applications in ECL and electrochemical analysis.[98, 99] For instance, Guo et al. developed an electrochemical immunoassay for the detection of the protein biomarker mucin 1 (MUC-1) and subsequently MCF-7 cancer cells, where MUC-1 is overexpressed.[100] The immunosensor was constructed using a sandwich protocol, by sequentially immobilizing anti-MUC-1 antibodies onto glassy carbon electrodes, followed by the targets and DNA strands containing the MUC-1 aptamer and C12 AgNCs-templating sequence. The electrochemical signal was measured after silver enhancement (i.e., reduction of Ag+ in AgNCs to Ag metal) via square wave voltammetry, achieving a detection limit of 0.5 nm and a high sensitivity of 50 cells mL−1 when measuring MCF-7 cancer cells.[100] Likewise, Hu et al. developed a highly sensitive label-free electrochemical sensor to detect terminal deoxynucleotidyl transferase (TdT) activity (Figure 6A).[101] TdT catalyzes the addition of deoxycytidine triphosphate to the end of the ssDNA, generating a C-rich DNA nanotail, which was subsequently used as the AgNCs templating sequence. The AgNCs formed can be adsorbed onto GO-modified electrodes and measured via H2O2 reduction for an electrochemical signal readout.[101]
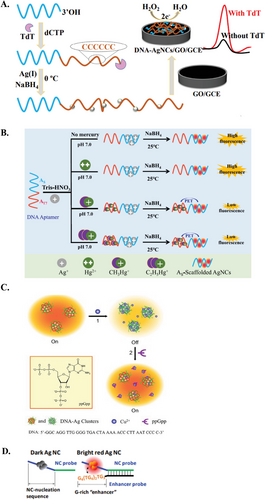
Conversely, DNA-AgNCs have found widespread application in fluorogenic sensors. Briefly, fluorogenic AgNC sensors generally revolve around either i) switch-off (intensity quenching)[102] or ii) switch-on (intensity enhancement)[95, 103] mechanisms. Switch-off AgNC sensors generally rely on the quenching effect of the target analyte,[104] whereby fluorescence intensity is inversely proportional to the amount of target analyte. For example, Huang et al. established a DNA-AgNCs fluorogenic switch for the detection of organic Hg2+ (e.g., CH3Hg+ or C2H5Hg+). In this system, organic Hg2+ binds to a corresponding aptamer, generating photoinduced electron transfer (PET) between the organic group and the as-formed AgNCs, effectively quenching the fluorescence of AgNCs (Figure 6B).[105] Likewise, the switch-off mechanism can also be induced by disrupting the AgNCs-templating sequence or templating sequence-enhancer pairs (as detailed in the next paragraph).[106, 107] In contrast, switch-on mechanisms feature a more direct approach, where a target analyte activates or enhances the fluorescence signal, creating a directly proportional correlation. For example, Zhang et al. fabricated a highly sensitive and selective turn-on sensor for the detection of ppGpp, a bacterial alarmone.[108] Briefly, Cu2+ acted as a mediator that quenched the fluorescence of DNA-AgNCs owing to its paramagnetic nature and its affinity for binding to the phosphate groups of DNA. During detection, Cu2+ readily dissociated from the AgNCs surface and bound to ppGpp via the electron-rich phosphate groups of ppGpp, thereby restoring the fluorescence of AgNCs (Figure 6C).[108] Similarly, Ye et al. developed an AgNCs-based sensor coupled with carbon nanoparticles for detecting HIV DNA.[109] The prepared ssDNA-AgNCs were adsorbed onto the carbon nanoparticles via π–π interaction, and the close proximity induces FRET, thereby quenching AgNCs fluorescence. The HIV DNA target hybridized with the ssDNAs, causing them to readily dissociate due to the weak adsorption with the carbon nanoparticles, thus restoring the fluorescence properties of AgNCs.[109]
Notably, a common design in many DNA-AgNCs sensors is the concept of proximity-dependent AgNCs. An example of this is nanocluster beacon (NCB) or in simpler terms, G-rich activation. This switch-on mechanism involves a non-fluorescent “dark” AgNCs templating sequence that is optically activated when brought into close proximity with a G-rich “enhancer” sequence. It is understood that both the physical interaction between the N7 atom of G and Ag+, as well as the electron transfer from G to AgNCs, lead to the reassembly of Ag+ on the combined enhancer-dark AgNCs templating sequence to form new clusters. As a result of Ag+ translocation and new AgNCs formation, the fluorescence from the templating sequence is activated and/or enhanced.[110, 111] NCBs were initially developed to circumvent the issue of photobleaching, allowing specific AgNCs fluorescence emission to be activated on demand.[111] That said, the fluorescence activation of NCBs is regulated by various factors, including sequence, length, and relative position of the enhancer and dark AgNCs templating strands. NCBs have been successfully integrated in AgNCs biosensors for sensitive and selective detection of various analytes (e.g., cocaine, and adenosine triphosphate [ATP]).[112, 113] In one of the early studies on NCBs, Yeh et al. reported a 500-fold fluorescence enhancement when a G-rich enhancer sequence was brought close to a pre-prepared non-emissive dark AgNCs (Figure 6D).[114] In contrast, Wen et al. developed a switch-off NCB assay to detect viral DNA. They first prepared AgNCs using C-rich sequences, with either end containing detection regions for target DNA recognition. The fluorescence of the AgNCs was enhanced using a G-rich strand. In the presence of target DNA, the G-rich strand was displaced, resulting in a decrease in the fluorescence intensity of the AgNCs.[115]
Recently, another variation of proximity-dependent AgNCs has led to the formation of “chameleon” AgNCs, or in simpler terms, color-switching AgNCs fluorescence emission. Instead of using conventional enhancer-dark AgNCs, this adaptation features two fluorescence-intensive AgNCs sequences (i.e., AgNCs templating sequence and emitter-pair) whose fluorescence properties change in a proximity-dependent manner.[116-119] For example, Xu et al. employed two 6C sequences that produce an orange/yellow fluorescence individually. In the presence of a target miR, these sequences were brought close together, resulting in a read fluorescence emission.[120] This has significantly expanded the biosensing capabilities of DNA-AgNCs, in terms of ultrasensitive detection, intracellular imaging, ratiometric detection, in vivo studies, and more.[121-130] A slightly different approach to chameleon AgNCs involves splitting a long AgNCs templating sequence into fragmented pairs. These fragmented pairs do not initiate any fluorescence response but only emit fluorescence when the pairs are brought near to each other. This concept was successfully implemented in several works, such as the detection of miR and DNA in human serum samples.[131-134]
4 HCR-AgNCs Biosensors
4.1 Principles of HCR-AgNCs Biosensors
As introduced earlier, HCR-based biosensors hold remarkable potential in the field of diagnostics but have limitations in design, operating conditions, and application. DNA-AgNCs can be integrated as signal transducers in HCR systems to overcome some of these limitations, and simultaneously enhance diagnostic potential in a hybrid HCR-AgNCs biosensor (Scheme 3). HCR-AgNCs biosensors measure the transduced AgNCs signal (fluorescence and/or electrochemical) as the DNA hairpins undergo structural changes during HCR amplification. Since DNA serves as the key element guiding both HCR and AgNCs, AgNCs can be introduced into an HCR system by simply adding an AgNCs templating sequence into the DNA hairpin probes. The isothermal mild reaction conditions for HCR amplification provide an ideal, stable, and compatible environment for AgNCs preparation because AgNCs are highly sensitive to their microenvironment (e.g., temperature, pH, ionic strength, etc.). Additionally, in the HCR-AgNCs biosensor, AgNCs are often incorporated after the HCR amplification stage. As a result, the formation of AgNCs on the specific AgNCs templating sequences does not affect the amplification efficiency or the stability of the HCR products.[69, 70] Thus, HCR-AgNCs biosensors can provide accurate, sensitive, and reproducible measurements during analyte detection.
4.2 Optimization of HCR-AgNCs Biosensors
HCR-AgNCs biosensors function similarly to conventional HCR systems introduced in Section 2 but with the inclusion of a specific AgNCs templating sequence into the hairpin structure. These AgNCs-templating sequences typically contain C and G bases, and range between 6 and 18 bases in length, as discussed in Section 3. Consequently, the design of HCR-AgNCs hairpins defies most of the DNA hairpin metastability guidelines listed in Table 1.
To date, only a handful of HCR-AgNCs studies have been conducted since 2013.[135] These studies include both electrochemical- and fluorescence-based assays, focusing on the detection of various target analytes. The design of HCR-AgNCs hairpins is inherently more complex than typical DNA hairpins, due to the inclusion of AgNCs-templating sequences. Moreover, the limited number of HCR-AgNCs studies hinders the development of systematic guidelines for integrating AgNCs-templating sequences into DNA hairpins, making it challenging to balance hairpin metastability and HCR amplification efficiency. Table 2 summarizes the reported HCR-AgNCs studies, detailing the DNA hairpin structures, AgNCs templating sequences, and reaction conditions for both HCR amplification and AgNCs formation.
| Typea) | Target | AgNCs templating sequence and location | AgNCs preparation | HCR condition | DNA hairpin structure | Refs. |
|---|---|---|---|---|---|---|
| EL | P53 exon8 DNA |
Hairpin loop: CCC CCC CCC CCC |
Reactants: 0.5 µm H1/H2; 10 µL 100 µm Ag+; 2 µL 500 µm NaBH4 Incubation condition: 1 h, room temperature |
Buffer condition: 10 mm tris-HCl, 500 mm Na+, 1 mm Mg2+, pH 7.4 Reaction condition: 2.5 h, room temperature |
H1 and H2: 8-base toehold; 9-bp stem; 12-base loop | [136] |
| EL | Dam MTase |
ssDNA: CCC CCC CCC CCC |
Reactants: 2 µm H1/H2; 10 µL 200 µm Ag+; 8 µL 500 µm NaBH4 Incubation condition: 2 h |
Buffer condition: 20 mm tris-HCl, 140 mm Na+, 5 mm Mg2+, pH 7.4 Reaction condition: 70 min, 37 ° |
Non-hairpin ssDNA | [137] |
| FL | Cell surface glycans |
Hairpin toehold: CCC CCC CCC CCC |
Reactants: 10 mm Ag+; 2 mm NaBH4; DNA: Ag+: NaBH4 = 0.5: 10: 10 Incubation condition: 5 h, 4 ° |
Buffer condition: 20 mm phosphate buffer, 1 mm Mg2+, pH 7.4 Reaction condition: 2 h, 25 ° |
H1: 5-base 1° toehold; 16-base 2° toehold; 16-bp stem; 6-base loop H2: 11-base 1° toehold; 15-bp stem; 6-base loop |
[138] |
| FL | miR-17-5p |
Hairpin toehold: CCC CCT TAA TCC CCC |
Reactants: DNA: Ag+: NaBH4 = 1: 10: 10 Incubation condition: 3 h, in dark |
Buffer condition: 200 mm phosphate buffer, pH 7.4 Reaction condition: 4 h, room temperature |
H1: 12-bp stem; 29-base loop H2: 28-base 1° toehold; 21-base 2° toehold; 12-bp stem; 6-base loop H3: 12-bp stem; 12-base loop |
[139] |
| FL | Mucin 1 |
Hairpin toehold: CCC CCC CCC CCC |
Reactants: 1 µL 100 µm H1/H2; 10 µL 60 µm Ag+; 10 µL 60 µm NaBH4 Incubation condition: 1 h, in dark |
Buffer condition: 20 mm tris-HNO3, 50 mm K+, 10 mm Mg2+, 1 mm DTT, pH 7.9 Reaction condition: 1 h, 37 °C |
H1 and H2: 12-base 1° toehold; 12-base 2° toehold; 15-bp stem 12-base loop |
[140] |
| FL | miR-21 |
Hairpin stem: CCC ACC CAC CC |
Reactants: 10 µL 10 µm H2; 2.4 µL 0.5 mm Ag+; 2.4 µL 0.25 mm NaBH4 Incubation condition: 4 h |
Buffer condition: 10 mm HEPES, 200 mm Na+, pH 7.2 Reaction condition: 2 h, room temperature |
H1: 8-base toehold; 18-bp stem; 10-base loop H2: 6-base 1° toehold; 8-base 2° toehold; 24-bp stem; 8-base loop |
[141] |
| FL | miR-145 |
Hairpin loop: CCC CCC |
Reactants: 32.4 µL 100 µm DNA; 32.4 µL 1 mm Ag+; 32.4 µL 1 mm NaBH4 Incubation condition: 1 h, room temperature |
Buffer condition: 20 mm phosphate buffer, 1 mm Mg2+, pH 7 Reaction condition: 1 h, 37 °C Side note: 3 µm H1, 6 µm H2 |
H1 and H2: 6-base toehold; 17-bp stem; 6-base loop | [142] |
| EL; ECL | Thrombin |
Hairpin loop: C-rich (unspecified) |
Reactants: 1 µm H1/H2; 8 µL 200 µm Ag+; 5 µL 500 µm NaBH4 Incubation condition: 2 h, room temperature, in dark |
Buffer condition: 10 mm Tris-HCl, 1 mm EDTA, 12.5 mm Mg2+, pH 7.4 Reaction condition: 2 h, 37 °C |
Unspecified | [143] |
| FL | HIV DNA |
Hairpin toehold: CCC CCC CCC CCC |
Reactants: DNA: Ag+: NaBH4 = 0.1: 0.6: 0.6 Incubation condition: 2 h, room temperature, in dark |
Buffer condition: 10 mm phosphate buffer, 100 mm Na+, 5 mm Mg2+, pH 7.5 Reaction condition: 4 h, room temperature |
H1: 15-base 1° toehold; 12-base 2° toehold; 16-bp stem; 10-base loop H2: 10-base 1° toehold; 12-base 2° toehold; 16-bp stem; 15-base loop |
[144] |
| FL | let-7 | Hairpin loop:CCC CCC |
Reactants: DNA: Ag+: NaBH4 = 1: 10: 10 Incubation condition: 5 h, room temperature |
Buffer condition: 20 mm phosphate buffer, 1 mm Mg2+, pH 7 Reaction condition: 1 h, 37 °C Side note: 4 µm H1, 8 µm H2 |
H1 and H2: 6-base toehold; 16-bp stem; 6-base loop | [145] |
| EL | Lysozyme |
Hairpin toehold: CCC CCC CCC CCC |
Reactants: DNA: Ag+: NaBH4 = 1: 6: 6 Incubation condition: 1 h, 4 °C, in dark |
Buffer condition: 50 mm PBS, pH 7.4 Reaction condition: 10 h, 35 °C |
H1: 6-base toehold; 14-bp stem; 4-base loop H2: 12-base toehold; 14-bp stem; 4-base loop |
[146] |
| EL | miR-199a |
Hairpin loop: CCC CCC CCC CCC |
Reactants: 0.5 µm DNA; 10 µL 100 µm Ag+; 2 µL 500 µm NaBH4 Incubation condition: 2 h, room temperature, in dark |
Buffer condition: 10 mm tris-HCl, 500 mm Na+, 1 mm Mg2+, pH 7.4 Reaction condition: 2 h, room temperature |
H1: 8-base toehold; 9-bp stem; 12-base loop H2: 11-base 1° toehold; 3-base 2° toehold; 6-bp stem; 12-base loop |
[47] |
| FL | mRNA |
AgNCs templating sequence (external DNA template): CCC TTA ATC CCC Enhancer (hairpin toehold): GGG TGG GGT GGG GTG GGG |
Reactants: DNA: Ag+: NaBH4 = 15: 90: 90 Incubation condition: 18 h, room temperature, in dark |
Buffer condition: 50 mm phosphate buffer, 500 mm Na+, 50 mm Mg2+, pH 6.8 Reaction condition: 4 h, 37 °C |
H1: 6-base 1° toehold; 24-base 2° toehold; 18-bp stem; 6-base loop H2 and H4: 6-base toehold; 18-bp stem; 6-base loop H3: 34-base toehold; 19-bp stem; 4-base loop |
[147] |
| FL | DNA |
Hairpin stem: CCC CCC |
Reactants: DNA: Ag+: NaBH4 = 1: 3: 3 Incubation condition: 5 h |
Buffer condition: High-salt: 10 mm phosphate buffer, 200 mm Na+, 1 mm Mg2+, pH 7.0 Low-salt: 20 mm phosphate buffer, 1 mm Mg2+, pH 7.0 Reaction condition: Overnight incubation, room temperature |
High-salt: H1: 9-base toehold; 20-bp stem; 7-base loop H2: 9-base toehold; 21-bp stem; 6-base bulge; 5-base loop Low-salt: H1: 8-base toehold; 19-bp stem; 8-base loop H2: 8-base toehold; 21-bp stem; 6-base bulge; 4-base loop |
[148] |
| EL | Uranyl ion | Hairpin stem: Unspecified |
Reactants: 0.5 µm DNA; 5 µL 5 mm Ag+; 10 µL 12.5 mm NaBH4 Incubation condition: 50 min, in dark |
Buffer condition: Unspecified Reaction condition: 1.5 h, 35 ° |
H1 and H2: 6-base toehold; 14-bp stem; 6-base loop | [149] |
| FL | Telomerase miR |
Hairpin toehold: CCC CCT AAT TCC CCC |
Reactants: DNA: Ag+: NaBH4 = 1: 7: 7 Incubation condition: 3 h, 4 °, in dark |
Buffer condition: 20 mm tris-HCl, 1.5 mm Mg2+, 63 mm K+, 10 mm dNTPs, 0.05% Tween 20, 1 mm EGTA, pH 8.3 Reaction condition: 2 h, 37 ° |
H1: 6-base toehold; 8-bp stem; 14-base loop H2: 27-base toehold; 8-bp stem; 6-base loop |
[150] |
| EL | miR-155 |
AgNCs templating sequence (hairpin toehold): CCT CCT TCC TCC Enhancer (hairpin toehold): TTT TTT TTT TTT TTT |
Electrochemical deposition: 10 µL 1 mm Ag+; 1 h; 0 to −1.35V; 100 mV/s |
Buffer condition: 20 mm tris, 1.0 mm Mg2+, 1.0 mm Ca2+, 140 mm Na+, 5 mm K+, pH 7.4 Reaction condition: 2 h 20 min, room temperature |
H1: 13-base toehold; 13-bp stem; 9-base loop H2: 18-base toehold; 13-bp stem; 6-base loop H3: 6-base 1° toehold; 12-base 2° toehold; 14-bp stem; 4-base loop |
[98] |
| FL | Tyrosine-protein kinase-like 7 (PTK7) |
Hairpin toehold: TTCCCACCCACCCCGGCCCGTT |
Reactants: 20 µm DNA; 250 µm Ag+; 125 µm NaBH4; 20 mm ammonium acetate buffer Incubation condition: 18 h, room temperature, dark |
Buffer condition: Unspecified Reaction condition: 24 h, room temperature, dark |
H1: 35-base toehold; 16-bp stem; 10-base loop H2: 10-base 1° toehold; 25-base 2° toehold; 16-bp stem; 10-base loop |
[151] |
| FL | miR-155 | Hairpin toehold: ACC CAC CCA |
Reactants: DNA:Ag+:NaBH4 = 1:14:14 (µm) Incubation condition: 1.5 h, in dark |
Buffer condition: 20 mm tris-acetate, 2 mm Mg(NO3)2 Reaction condition: 2 h, 32 °C |
H1: 6-base 1° toehold; 12-base 2° toehold; 19-bp stem; 4-base loop H2: 6-base toehold; 18-bp stem; 6-base stem |
[133] |
| FL | miR-144-3p | AgNCs templating sequence (hairpin toehold): CAC CGC T – TTT TGC CTT TTG GGG ACG GAT A |
Reactants: DNA:Ag+:NaBH4 = 1: 12: 6 (µm) Incubation condition: 1 h, in dark |
Buffer condition: 10 mm tris-acetate, 1 mm Mg(NO3)2, 100 mm NaNO3, pH 8 Reaction condition: 2 h, 35 ° |
H1: 2-base 1° toehold; 6-base 2° toehold; 14-bp stem; 7-base loop H2: 7-base 1° toehold; 22-base 2° toehold; 17-bp stem; 4-base loop H3: 6-base toehold; 16 bp stem; 7-base loop |
[131] |
| FL | DNA (miR-214) |
AgNCs templating sequence (external DNA template – P1): CCC CTA ATT CCC Enhancer (external DNA template – P2): CCC CCC CCC CCC |
Reactants: DNA:Ag+:NaBH4 = 1: 15.4: 15.4 (µm) Incubation condition: 3 h, dark |
Buffer condition: 20 mm tris-acetate, 2 mm Mg(NO3)2, pH 8.0 Reaction condition: 1.4 h, 32 ° |
H1: 6-base 1° toehold; 6-base 2° toehold; 17-bp stem; 4-base loop H2: 6-base toehold; 16-bp stem; 6-base loop |
[132] |
| ECL | ATP; quinine |
Hairpin toeholds: CCC CCC CCC CCC CCC |
Reactants: 2 µm H1 and H2; 10 µL of 100 µm Ag+; 10 µL of 100 µm NaBH4 Incubation condition: 2 h, in dark |
Buffer condition: 20 mm tris-HCl, 10 mm MgCl2, 1 mm EDTA, 300 mm NaCl, pH 7.8 Reaction condition: 2 h, 37 °C |
H1: 21-base 1° toehold; 7-base 2° toehold; 17-bp stem; 7-base loop H2: 7-base 1° toehold; 22-base 2° toehold; 16-bp stem; 8-base loop |
[152] |
| FL | DYS14 gene DNA | AgNCs templating sequence (external DNA template): CCC CCC CCC CCC |
Reactants: 5 µL of DNA; 3 µL of 1 mm Ag+; 3 µL of 1 mm; DNA:Ag+:NaBH4 = 1:6:6; 20 mm PB; 1 mm Mg2+; pH 7 Incubation condition: Ice bath 30 min; 4 h |
Buffer condition: 50 mm Na2HPO4−, 0.4 M NaCl, pH 7.5 Reaction condition: 1 h, 25 ° |
H1: 10-base toehold; 10-bp stem; 11-base loop H2: 11-base toehold; 18-bp stem; 10-base loop |
[153] |
- a) Abbreviations: ECL: Electrochemiluminescence; EL: Electrochemical; FL: Fluorescence.
4.2.1 DNA Hairpin Design
Given the lack of specific guidelines for HCR-AgNCs hairpin design, several key factors must be considered to create a functional HCR-AgNCs system. Many reported HCR-AgNCs hairpins contain AgNCs-templating sequences either at the toehold or loop segment. For the former, the AgNCs templating sequence can be adopted as an extension of the 1° toehold,[150] or as an independent 2° toehold.[138] As a result, the HCR products would contain tails for AgNCs formation. However, tails on dsDNA might impede the hybridization efficiency of incoming DNA sequences, as aforementioned.[38] Alternately, C-rich hairpin loops can accommodate AgNCs, as discussed earlier in Section 3.4.3. The resultant signal is normally quenched in fluorescence-based sensors,[145] and enhanced in electrochemical sensors via HCR amplification.[136]
Apart from that, the initiation of HCR amplification should be optimized, whether through toehold- or loop-mediated hairpin openings. The former mechanism is preferred in most HCR-AgNCs biosensors[146] due to its more established and straightforward design, as well as reaction time that is faster by two magnitudes compared to the loop-mediated mechanism.[139] For instance, the HCR-AgNCs biosensor developed by Jiang et al. featured a loop-mediated opening mechanism, where the toehold segments were fully utilized for AgNCs templating sequences. This design enabled a highly sensitive ratiometric response for miR-17-5p detection but with a relatively long HCR incubation period of 4 h.[139]
Certain HCR-AgNCs studies have utilized more than two DNA hairpins for target detection, such as the four-hairpin HCR-AgNCs system developed by Liu et al., which is discussed in further detail later.[147] In some cases, additional DNA is utilized to initiate HCR amplification. These may take the form of capture probes that link a target analyte to the main HCR system via an additional hybridization stage with partial or complete base-pairing. For example, Jiang et al. presented an HCR-AgNCs system with a capture probe in the form of a hairpin. Upon target recognition at the DNA hairpin loop, the hairpin opened, exposing the HCR initiator (initially concealed within the closed hairpin) for HCR initiation (Figure 7A).[139] Alternatively, the HCR initiator DNA strand can be assistive without direct contact with the target analyte. For example, Chen et al. developed an electrochemical HCR-AgNCs system using an electrode modified with a duplex consisting of a lysozyme aptamer and a thiolated partially complementary ssDNA strand. Upon binding with lysozyme, the duplex was dissociated, leaving the thiolated strand on the surface of the electrode to trigger the subsequent HCR process (Figure 7B).[146] In another study, Chen et al. utilized an assistive strand as an initiator for HCR amplification. The electrochemical setup involved a closed DNA hairpin immobilized onto the electrode, which opened upon target binding. The assistive strand is then bound to the opened hairpin to initiate HCR amplification, thereby linking the AgNCs transducing unit to the electrode (Figure 7C).[136]
4.2.2 Other Factors Affecting HCR-AgNCs Performance
In addition to the DNA hairpin structure, the performance of HCR-AgNCs can be further regulated by adjusting reaction conditions. Reported HCR-AgNCs biosensors operate under selected isothermal conditions, typically within a temperature range of 20–25 °C (room temperature) and 37 °C.[150, 98] This narrow range provides the DNA hairpins with sufficient energy to overcome the energy barrier and initiate HCR amplification, without destabilizing the dsDNA product. The DNA hairpins are incubated for a fixed period, ranging from 1 h to overnight incubation.[142, 148] The duration of incubation is a subjective measure, as it is relative to the hairpin design and mechanism of the respective HCR-AgNCs biosensors.
The microenvironment for HCR amplification is compatible with AgNCs formation. Reported HCR-AgNCs biosensors use different buffer bases (such as Tris, phosphate, etc.), but maintain a relatively similar pH (neutral to slightly alkaline) and commonly add Na+ and/or Mg2+ salts.[47, 142] Nevertheless, the concentration of both the buffer base and metal salts varied across studies, leading to variation in fluorescence properties even for the same AgNCs-templating sequence. This was shown in a study by Orbach et al., where a high-salt buffer (containing both Mg2+ and Na+) produced yellow fluorescence emission, while a low-salt buffer (containing only Mg2+) produced red fluorescence emission from a common 6C DNA hairpin loop.[148]
Most reported HCR-AgNCs studies utilize equimolar concentrations of DNA hairpins. However, there are a few exceptions where the amount of H2 is higher than that of H1, without any justification for the concentration differences.[142, 145] Another condition to optimize is the concentration of DNA hairpin containing the AgNCs-templating sequence relative to the concentration of Ag+ and NaBH4 during AgNCs preparation. This is because even with the same AgNCs-templating sequences, deviation in the AgNCs preparation conditions can generate significant differences in fluorescence.[138, 140, 144]
In most HCR-AgNCs studies, AgNCs were prepared after HCR amplification for signal transduction.[141, 145] This approach aims to prevent undesired Ag+ binding to consecutive C and G bases, except for those in the AgNCs-templating sequences, as the undesired binding could block the bases required for DNA hybridization and impede HCR amplification efficiency.
4.3 Application of HCR-AgNCs Biosensors
In this section, several reported HCR-AgNCs biosensors will be reviewed, with respect to the location of AgNCs templating sequences and the innovative biosensing mechanisms.
Zhang and co-workers integrated 12C AgNCs-templating sequences onto the toeholds of both DNA hairpins for HIV DNA detection, with the assistance of GO. The liberated HCR product generated a strong fluorescent signal, which was otherwise quenched when the HCR hairpins remained in their configuration and attached to the GO surface (Figure 8A).[144] A similar GO-assisted design was reported by Jiang et al., who constructed an HCR-AgNCs fluorescence logic gate using chemopalette sequences of AgNCs (-CCC CCT AAT TCC CCC-) at the toehold of one of the DNA hairpins to detect telomerase miRs.[150] In a recent study, Wu et al. developed an HCR-AgNCs system for cellular recognition, based on a common chemopalette sequence (-TTC CCA CCC ACC CCG GCC CGT T-) integrated onto two different DNA hairpin toeholds.[151] Unlike other HCR-AgNCs studies, AgNCs are prepared first on the DNA hairpins before HCR amplification with an aptameric sequence of cell surface receptors as the initiator. The system produces fluorogenic probes that are used to tag cancer cells for visualization, and the group reported 20-fold sensitivity enhancement for the HCR-AgNCs system compared to individual aptamer tethered AgNCs.[151]
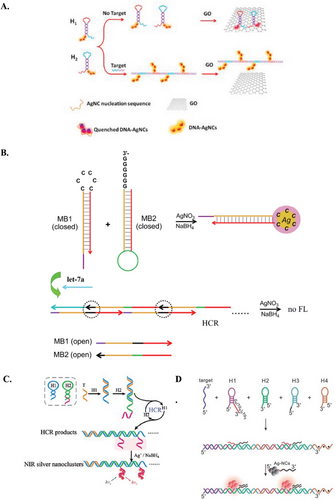
HCR-AgNCs biosensors with hairpin loops containing AgNCs-templating sequences are also common in both electrochemical- and fluorescence-based biosensors.[136] Fluorogenic AgNCs-templating loops focus on fluorescence quenching as the DNA hairpin loop sequence actively participates in HCR amplification. For example, Qiu et al. designed a biosensor to detect let-7 miR using a 6C loop to host AgNCs. When HCR occurred in the presence of let-7, the loop opened and hybridized with a 6G sticky end of another hairpin, subsequently quenching the fluorescence of AgNCs (Figure 8B). Interestingly, Qiu et al. also found that the stem length could affect the fluorescent of the AgNCs capped in the hairpin loop, with shorter hairpin stem length.[145] A similar design was adopted by Mansourian et al. to detect plasma miR-145 in human serum samples.[142]
A rather rare design reported by Orbach et al. incorporated a C-rich AgNCs-templating sequence into the stem of a DNA hairpin.[148] Upon HCR amplification, the hairpins opened and formed DNA chains. The C-rich sequence then reconfigured into a 6 C bulge along the DNA chains, which activated specific fluorescence emission, notably resembling the fluorescence properties of 6 C hairpin loops.[154, 155] Meanwhile, Zhang et al. embedded a chemopalette sequence (-CCC ACC CAC CC-) within the stem region of a DNA hairpin. Upon opening of the DNA hairpin during HCR amplification in the presence of target miR-21, the chemopalette sequence was released, enabling the subsequent formation of near-IR emitting AgNCs (Figure 8C).[141] However, the low number of stem-based HCR-AgNCs studies can be attributed to the challenge and complexity of maintaining good DNA hairpin metastability. Since hairpin stems are primarily responsible for stability, any variations (bulge, unbounded sequences, etc.) along the sequence can disrupt the ΔG from base-pairing and stacking, thereby destabilizing the hairpin and dsDNA structure.
There have been two reported HCR-AgNCs biosensors that emphasized proximity-dependent AgNCs designs. Liu et al. demonstrated a traditional NCB design using four DNA hairpins to detect mRNA targets. The AgNCs- templating strand and enhancer sequence were located on two different DNA hairpins. Upon HCR amplification, these sequences were brought into close proximity, activating intense red fluorescence (Figure 8D).[147] Jiang et al. reported a ratiometric fluorescence HCR-AgNCs biosensor by placing chemopalette sequences of AgNCs (-CCC CCT TAA TCC CCC-) as the terminal toeholds (1° and 2°) of one DNA hairpin species. When these AgNCs templating sequences are in close proximity, they collectively emit a red fluorescence. In the presence of target miR-17-5p, HCR was triggered, using this DNA hairpin for hybridization and leaving the two terminal nucleating sequences appended at a distance. This change resulted in a fluorescence emission shirt to yellow, enabling ratiometric detection of miR-17-5p.[139] The concept of proximity-dependent AgNCs is an underexplored area due to the difficulty in designing stable DNA hairpins for HCR amplification. Along with an AgNCs templating sequence, an enhancer sequence, such as G-rich or emitter-pair, etc., is required, which is also long and laden with C and G bases. Furthermore, it is crucial to corroborate the mechanism of bringing the two separate strands together post-HCR amplification to activate the signal.
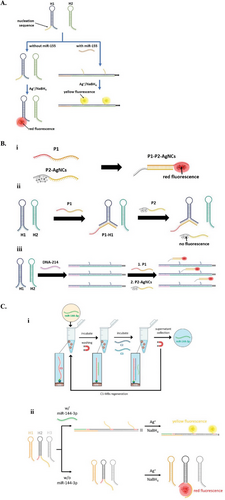
We have developed three different HCR-AgNCs biosensors for the detection of miRs and DNA. The first HCR-AgNCs biosensor was designed for detecting miR-155, incorporating a short AgNCs-templating sequence as the 2° toehold of one of the hairpins. In the presence of miR-155, HCR was triggered, forming DNA chains with hanging tails. Notably, AgNCs preparation resulted in red fluorescence in the absence of miR-155 and yellow fluorescence in its presence. The red-emitting AgNCs formed in the hairpin structure (based on the interaction between 1° and 2° toeholds), while the yellow-emitting AgNCs were caged by the hanging tails on the HCR chains. This design enables ratiometric measurement of miR-155, with a LOD of 1.58 fM in serum samples (Figure 9A).[133] In another biosensor, we introduced DNA-AgNCs sequences as independent ssDNA strands, labeled P2, rather than integrating them into hairpins, as most studies do. Alongside these ssDNA AgNCs probes, we also introduced assistive probes complementary to P2, named P1, to facilitate an end-to-end transfer of dark AgNCs to the C-rich region of P1, subsequently activating the red emission of the AgNCs. Notably, P1 was partially complementary to an HCR hairpin, thus influencing the cluster transfer and the formation of red-emitting AgNCs in the absence of a target. Conversely, when HCR was triggered and the hairpins unfolded, P1 hybridized with P2-AgNCs, leading to bright red emission. The cluster transfer approach not only prevented AgNCs from influencing HCR performance, but also produced AgNCs in a more predictable manner (Figure 9B).[132]
Given the linear nature of HCR amplification, several HCR-AgNCs biosensors have been supplemented with additional components to improve their overall performance. A common approach is introducing enzymatic activity into HCR-AgNCs designs, such as DNA polymerase and nicking endonuclease. These enzymes add another amplification cycle alongside HCR amplification, primarily to recycle targets and increase the sensitivity of the biosensor. For instance, Chen et al. developed an HCR-AgNCs biosensor featuring a secondary autocatalytic strand displacement amplification for DNA target recycling and achieved sub-femtomolar LOD.[136] Similar strategies involving different types of enzymes have also been reported in other HCR-AgNCs studies to detect Dam Mtase,[137] mucin 1,[140] and miR-199a.[47] Nonetheless, using enzymes complicates the sensing platform by adding more components to an already sensitive HCR-AgNCs system, and requires additional steps to remove the enzymes (e.g., heat inactivation or washing).
External components, namely GO, have been capitalized in multiple HCR-AgNCs studies to minimize background fluorescence from unreacted hairpins.[140, 144, 150] GO has been known to be an effective nano-quencher for AgNCs fluorescence due to long-range resonance energy transfer. DNA hairpins can reportedly adsorb onto GO at their toeholds and loops via π–π stacking.[47] Upon recognizing a target via hairpin toehold, the DNA hairpins can easily detach from GO due to the weak binding interaction, allowing HCR amplification and subsequent AgNCs formation. While this approach is appealing, the reaction condition must be carefully regulated because high-salt conditions and certain components in biological samples may coagulate GO, thereby disrupting the performance of HCR-AgNCs biosensors.[156, 157]
Additionally, we incorporated a magnetic module into an HCR-AgNCs system to circumvent interfering components in complex solutions, such as blood that might affect the detection accuracy of AgNCs. Starting with the separation of target miR-144 from a complex serum solution using magnetic beads coated with capture probes, we then introduced two assistive probes to displace the miR-144 from the beads. The displaced targets were subsequently subjected to the HCR-AgNCs system that enabled ratiometric detection of the miR-144, with a LOD of 4.88 fM (Figure 9C).[131]
4.4 Current Limitations and Future Direction of Designing HCR-AgNCs Biosensors
The development and application of HCR-AgNCs systems face several challenges, particularly in the DNA hairpin design. This section first evaluates the limitations of the HCR-AgNCs system, followed by our insights into designing DNA hairpins for effective HCR-AgNCs implementation, and potential future directions to advance the field.
4.4.1 Limitations of HCR Amplification and DNA-AgNCs Transducers
Generally, HCR-AgNCs systems face challenges during both the design and validation stages, which can limit their application. While the thermodynamic properties of HCR amplification can be accurately predicted from the DNA sequence, evaluating their kinetics is more inconsistent and labor-intensive. The kinetic studies often require trial-and-error optimization experiments using costly functionalized DNA oligonucleotides (e.g., fluorophore and nanopore), which limits the practicality of screening specific sequences for HCR amplification.[158-160] This experience-based design process, coupled with the complexity of kinetic studies and time-consuming thermodynamic analysis, underscores the need for ideal software to streamline the design of effective HCR amplification systems.
HCR amplification operates under ambient conditions that must be carefully defined to balance between the stability of DNA hairpins and the efficiency of HCR. This is particularly challenging, especially in biological samples that contain interfering molecules that can degrade the DNA hairpins and alter HCR performance.[161] Since HCR is a continuous process, it produces elongated dsDNA concatemers of different sizes and might lead to variability in tests for the same target. If specific HCR products are required for further analysis, they can be isolated and extracted using gel electrophoresis. However, this process is tedious, time-consuming, costly, and carries the risk of errors.
Furthermore, since HCR focuses on linear amplification, this could limit the sensitivity and application of HCR biosensors. A suggestion to overcome this would be the implementation of branched HCR systems, that would exponentially amplify measurable signals.[162] Even so, branched HCR would require more complex DNA designs and more DNA hairpins in the system, which would complicate the entire HCR mechanics.
Another common practice in HCR biosensors is the usage of different fluorophore combinations and/or fluorophore-quencher combinations. Albeit their partial application, these fluorophores have their respective internal charges that can interact locally with each other and the DNA template. As a result, these differential interactions can offset the overall hybridization kinetics.[62, 163]
In terms of DNA-AgNCs, the main fundamental challenge lies in incorporating AgNCs-templating sequences into DNA hairpins. DNA-AgNCs normally feature C-rich sequences (as presented in Table 2) and incorporating these sequences into an HCR system contradicts the aforementioned guidelines (Table 1). Moreover, these templating sequences are typically composed of more than six bases, with shorter sequences comprising higher percentages of C bases compared to longer sequences.[82] The location of AgNCs-templating sequences is also limited to the toehold or loop of DNA hairpins, except in a few reported studies. For example, Orbach et al. incorporated a C-rich bulge in the stem of the DNA hairpin,[148] but this approach may be difficult to replicate as the hairpins are thermodynamically unstable based on NUPACK analysis. Besides that, AgNCs preparation is generally limited to post-HCR amplification, except in the aforementioned cluster transfer study.[132] This limitation exists because AgNCs formed prior to HCR can bind to DNA bases, thereby affecting HCR amplification efficiency.
4.4.2 Insights into Designing HCR-AgNCs Biosensors
-
Location of AgNCs-templating sequence: As displayed in Table 2, there is a lack of correlation between the reported AgNCs-templating sequences and their positions. The same templating sequence can yield different outcomes under varying reaction conditions and mechanisms. For example, both Qiu et al. and Mansourian et al. used 6C loops for miR detection, but their sensors exhibited different fluorescence emissions (590 vs 633 nm) under relatively similar experimental conditions.[142, 145] This deviation warrants further research to elucidate how DNA hairpin structures (e.g., stem sequence and size) impact AgNCs fluorescence properties. It is crucial to identify optimal AgNCs-templating sequences and optimize the conditions for HCR and AgNCs preparation.
Our recent studies on HCR-AgNCs systems[150, 98, 151] have adopted different HCR mechanisms and AgNCs templating sequences, albeit utilizing similar HCR conditions (e.g., buffer composition and temperature) and AgNCs preparation (e.g., temperature and dark incubation).[131-133] We predominantly used non-loop-based AgNCs templating sequences, such as toehold-based and external AgNCs templating sequences,[150, 98, 151] and found that their fluorescence properties matched those reported in the literature from which the AgNCs-templating sequences were derived. For example, AgNCs-templating and enhancer sequences used for detecting DNA-214 (i.e., DNA model of miR-214) showed a fluorescence emission of 611 nm, similar to the 610 nm adapted from Teng et al.[132, 125] Similarly, detection for miR-155 using a toehold-based AgNCs sequence yielded an emission wavelength of 561 nm, close to the 564 nm adapted from Lee et al.[133, 164] Likewise, for the detection of miR-144, we obtained a yellow fluorescent emission at 564 nm, consistent with the 565 nm reported in previous literature.[131, 165, 124, 126, 166]
- 2) DNA hairpin design: An effective HCR-AgNCs system requires a complex DNA hairpin structure that elicits changes in the fluorescence properties in response to the structural transition from hairpins to dsDNA chains during HCR amplification. Several approaches, such as using multiple or different-sized DNA hairpins, have been proposed (Table 2). These HCR-AgNCs systems often deviate from the suggested design guidelines for DNA hairpins (Table 1) due to the lengthy C-rich AgNCs-templating sequences.
For our functional HCR-AgNCs systems,[131-133] we adopted part of the guidelines and followed specific computationally design steps (Figure 10). Briefly, we incorporated the target analyte or aptameric sequence into the stem and the AgNCs-templating sequence into the toehold of the DNA hairpins. This was followed by essential modifications, including minimization of the hairpin loop and adding 1–2 bps right before the loop. The former ensures that the loop size is smaller than the stem length and contains a minimum of four bases. Adding complementary bps before the loop is crucial to prevent the premature opening of the DNA hairpins, as they tend to fray at the ends of the stem, which might prematurely open the DNA hairpins.[28] We then complemented the toehold sequences with the corresponding hairpin loops. Finally, we computationally evaluated the DNA hairpins for their thermodynamics and kinetics, as well as the feasibility and potential system leakage of the HCR system. The thermodynamics of DNA hairpins were based on the threshold ΔG of stable DNA hairpins, reported to be −16 kcal mol−1.[28]
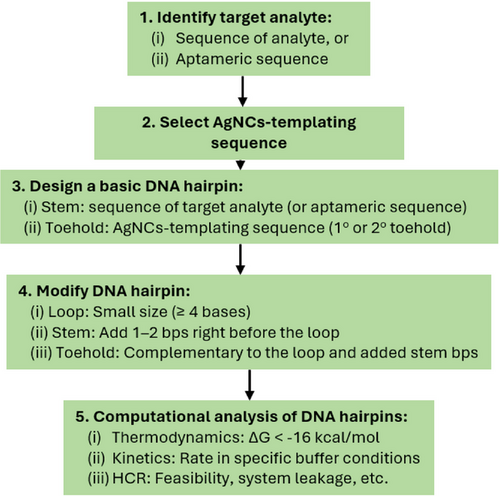
From our design workflow, we propose that the sequence for designing DNA hairpins with good metastability should prioritize the stem, then the loop, and finally the toehold. In other words, the loop's size and sequence play a more prominent role in determining the metastability of DNA hairpins than the toehold. Despite the formation of tails corresponding to unbounded toehold sequences (i.e., the AgNCs-templating sequences),[30] we observed that the performance of the HCR-AgNCs system was optimal.[131-133] Nonetheless, the proposed workflow adopted for these HCR-AgNCs systems[131-133] warrants further validation (and improvements, if needed) to prospectively establish a systematic guideline for designing HCR-AgNCs systems.
4.4.3 Future Direction
Despite the development of HCR-AgNCs systems for various sensing applications (Table 2), these systems face limitations in their HCR components, as previously discussed in Section 4.4.1. This warrants further research to improve HCR performance. Unlike linear HCR amplification, branched HCR amplification exponentially amplifies signals by crosslinking DNA hairpins to form a DNA network.[50, 162, 167-172] This method uses more than two DNA hairpins, each with two terminal toeholds to enable branching. As such, translating the AgNCs-templating sequence into a branched HCR-AgNCs system is formidable. While a loop-based AgNCs-templating sequence may be feasible for branched HCR amplification, introducing AgNCs in the stem region, where they can intercalate between the dsDNA strands, is also possible.[173-180] However, this requires careful design to ensure the stability and feasibility of both HCR amplification and AgNCs fluorescence. Alternatively, AgNCs can be introduced to the HCR component in an isolated and cluster-transfer manner. We found that such an approach not only could prevent AgNCs from influencing HCR performance but also produce AgNCs more predictably.[147]
Another area worth investigating for improving HCR-AgNCs performance is the AgNCs aspect of the system. Generally, AgNCs have their limitations, such as susceptibility to oxidation and photobleaching, leading to signal loss and decreased assay reliability over time. Developing strategies to enhance the stability of AgNCs under physiological conditions, such as surface modifications or encapsulation with protective coatings, can facilitate their application.[181-185] In the context of HCR-AgNCs sensors, the AgNCs-templating sequence plays a more crucial role. Table 2 presents the different AgNCs-templating sequences utilized in HCR-AgNCs biosensors, including C-rich ssDNA, C-rich hairpin loops, and chemopalette sequences, as well as the concept of proximity-dependent AgNCs (i.e., NCBs and chameleon AgNCs). Alternative AgNCs-templating sequences that have yet to be tested in HCR-AgNCs biosensors include intercalation within dsDNA structure (gaps, abasic, mismatch sites, etc.)[174-180] and secondary structure scaffolds like G-quadruplex.[186, 187] Despite its prominence, adding specific sites into dsDNA and DNA hairpins results in a complex and tedious design process. A missing bp can disrupt the energy balance of DNA hairpins, and there is a lack of follow-up studies on dsDNA-AgNCs.[188, 189] Previous studies show that G-quadruplex can form from G-rich sequences located as split pairs at the terminal toeholds of DNA sequences or in a large loop.[190-196] Designing DNA hairpins to accommodate these structures is challenging, as split pairs lead to hanging tails that extrude from HCR products and cause steric effects, while the large loop introduces mismatch sequences that may hinder HCR amplification.[174-180, 186, 187] These factors must be considered to maintain the efficiency of HCR amplification and DNA hairpin stability. Alternatively, principles like NCBs and chameleon AgNCs can be applied differently. For instance, Liu et al. used a four-hairpin system, and Jiang et al. employed loop-mediated DNA hairpin opening with two AgNCs templating sequences.[139, 147]
It should also be noted that HCR-AgNCs systems are still in the early developmental stage, owing to the pending issues discussed earlier. Once these matters are resolved, research efforts could focus on integrating HCR-AgNCs systems into POC devices, such as miniaturized paper-based sensors and microfluidics. This integration would require miniaturization, automation, and robust instrumentation to create a compact, user-friendly platform suitable for clinical applications. In this regard, several factors need to be considered, including the cost-effectiveness and scalability of HCR-AgNCs systems in POC devices. Optimizing production processes, sourcing affordable materials, and minimizing assay reagent costs are essential for cost-effective deployment. Moreover, HCR-AgNCs systems could be integrated into smartphone-based detection platforms for real-time data analysis and remote monitoring, enhancing the accessibility and functionality of HCR-AgNCs sensors.[197, 198] By capitalizing on the computational power and connectivity of smartphones, researchers can enable rapid and cost-effective diagnostic capabilities even in resource-constrained settings.
5 Conclusion
HCR-AgNCs sensors represent a promising platform for the sensitive, selective, and versatile detection of biomolecules, with significant implications for clinical diagnostics. This review has summarized the concepts of HCR and AgNCs, highlighting recent advancements, challenges, and future directions in the development and application of HCR-AgNCs biosensors. Despite their immense potential, several challenges remain for the widespread adoption of HCR-AgNCs biosensors in clinical settings, particularly the design of a functional HCR-AgNCs system. We have provided our insights on designing HCR-AgNCs sensors that we believe would streamline the overall process. Notwithstanding, strategies, such as probe optimization, signal amplification, device miniaturization, biocompatibility assessments, and rigorous validation studies are crucial for translating functional HCR-AgNCs biosensors from the lab to real-world applications.
In summation, future collaborative efforts and technological advancements will be pivotal in realizing the full potential of HCR-AgNCs biosensors as powerful tools for clinical diagnostics, with the potential to transform healthcare delivery through their sensitivity, specificity, and versatility.
Acknowledgements
BioRender (biorender.com) was used to create Schemes 1, 2, 3 and Figures 1 and 2. This work was supported by the Royal Society of Chemistry (RSC) Research Fund grant (under the project code: R23-0045517198) and L'Oréal-UNESCO FWIS 2021 (Malaysia).
Conflict of Interest
The authors declare no conflict of interest.
Author Contributions
All authors conceptualized. Z.W.W. investigated. S.Y.N. supervised. Z.W.W. wrote the original draft. All authors reviewed and edited the writing.
Biographies

Zheng Wei Wong holds a Ph.D. in Pharmacy from the University of Nottingham and a bachelor's degree in pharmaceutical chemistry. His research focused on developing DNA nanobiosensors, and his expertise lies in the design and application of advanced biosensing technologies, particularly those utilizing DNA-based systems for high-sensitivity detection. He is currently a journal editor, allowing him to contribute to the dissemination of cutting-edge research and support the academic community in pushing boundaries in biosensor technologies.

New Siu Yee is an Associate Professor at the School of Pharmacy, University of Nottingham Malaysia. She earned her PhD from the Department of Chemistry at the National University of Singapore, after which she served as a research scientist at the Institute of Materials Research and Engineering, A*STAR, Singapore. Her primary research focuses on the development of nanosensors for biomedical applications, particularly in biotemplated-metal nanoclusters and metal nanoparticles. Currently, her group is concentrating on designing computationally aided smart DNA nanosensors, which hold significant potential for diverse target applications.