Novel FRET-based Immunological Synapse Biosensor for the Prediction of Chimeric Antigen Receptor-T Cell Function
Abstract
Chimeric antigen receptor (CAR)-T cell therapy has revolutionized cancer treatment. CARs are activated at the immunological synapse (IS) when their single-chain variable fragment (scFv) domain engages with an antigen, allowing them to directly eliminate cancer cells. Here, an innovative IS biosensor based on fluorescence resonance energy transfer (FRET) for the real-time assessment of CAR-IS architecture and signaling competence is presented. Using this biosensor, scFv variants for mesothelin-targeting CARs and identified as a novel scFv with enhanced CAR-T cell functionality despite its lower affinity than the original screened. The original CAR promoted internalization and trogocytosis, disrupting stable IS formation and impairing functionality are further observed. These findings emphasize the importance of enhancing IS quality rather than maximizing scFv affinity for superior CAR-T cell responses. Therefore, the FRET-based IS biosensor is a powerful tool for predicting CAR-T cell function, enabling the efficient engineering of next-generation CARs with enhanced antitumor potency.
1 Introduction
Chimeric antigen receptor (CAR)-T cell therapy has brought a transformative paradigm shift in the field of oncology. When the single-chain variable fragment (scFv) domain of the CAR binds to tumor-associated antigens on the surface of cancer cells, CAR-T cells become activated and release lytic granule proteins, such as perforin and granzyme B, to directly kill the engaged cancer cells.[1] Consequently, effective engagement between the CAR and the tumor antigen is a prerequisite for a potent cytotoxic response in CAR-T cell therapy.
Immunological synapse (IS) constitutes a discrete supramolecular structure assembled at the interface between T cells and antigen-presenting cells.[2] This dynamic protein complex facilitates the segregation and T cell receptor (TCR) clusters along with associated signaling and structural molecules, thereby enabling the directed secretion of cytolytic effectors toward the engaged target cell. While the IS architecture formed between CAR-T cells and tumor cells exhibits distinct structural features compared to the canonical IS from TCR-peptide-MHC ligation,[3] accumulating evidence demonstrates a positive correlation between the qualitative parameters of the CAR IS and the therapeutic response of CAR-T cells.[4] Therefore, when designing and engineering new CAR constructs, it is necessary to comprehensively evaluate their capacity to assemble robust IS, as this represents a crucial determinant of therapeutic potency in CAR-T cell therapy.
The architecture and functionality of the CAR immunological synapse are largely dictated by the biophysical and biochemical characteristics of the scFv, as this domain initiates antigen recognition and synapse formation. Specifically, the binding affinity and avidity of the scFv toward the tumor-associated antigen profoundly affect the formation and dynamics of CAR microclusters during the IS assembly, which ultimately influences the functionality of CAR-T cells.[5] The ideal scFv exhibits an affinity sufficient to trigger a robust immune response while avoiding excessively stable interactions that could potentially lead to T cell exhaustion or undesirable off-target effects.[6] Thus, identifying scFv candidates with high IS quality is crucial for enhancing both the safety and efficacy of CAR-T cell therapy.
Conventional scFv screening approaches include bacterial display techniques to isolate high-affinity antibody clones.[7] The scFv properties are evaluated using surface plasmon resonance (SPR) or biolayer interferometry.[6, 8] However, these in vitro scFv screening methods primarily assess the binding affinity of scFv to purified target proteins but not their native conformation on the cell membrane. In addition, in vitro experimental conditions are different from complex cellular environments, for example, the presence of other membrane proteins proximal to the target antigen. Consequently, they often fail to predict the therapeutic outcomes of CAR-T cell therapy, underscoring the need for innovative approaches to optimize scFv affinity to maximize CAR functionality.
In this study, we introduce a novel IS biosensor based on fluorescence resonance energy transfer (FRET) that can assess the quality of the IS in live cells, thereby enabling the selection of CAR candidates. This FRET-based IS biosensor was designed to include a CAR library with scFv variants, cyan and yellow fluorescent proteins (CFP and YFP) separated by an optimized linker, and SH2 domains of zeta-chain-associated protein kinase-70 (ZAP-70) within the same molecule. Upon binding to target antigens on tumor cells, the immunoreceptor tyrosine-based activation motif (ITAM) domains of CAR in the biosensor are phosphorylated and subsequently bind to the ZAP-70-SH2 domains, leading to an increased FRET level between CFP and YFP. This novel IS biosensor represents a powerful tool to bridge the gap between conventional in vitro scFv characterization assays and the physiologically relevant cellular context, thus providing a more accurate prediction of CAR-T cell activation.
Utilizing the FRET-based IS biosensor, we screened the scFv library for CARs targeting mesothelin (MSLN), a promising antigen for solid tumors, including malignant mesothelioma, ovarian, pancreatic, and gastric cancers.[9] The IS biosensor platform allowed for an efficient and comprehensive evaluation of the qualitative parameters of IS for the library of MSLN-CAR constructs with distinct scFv variants. Surprisingly, our IS biosensor screening identified a moderate-affinity scFv that enhanced CAR-T cell functionality compared to an existing high-affinity scFv. We further discovered that the original high-affinity CAR promoted detrimental trogocytosis, while the new moderate-affinity scFv facilitated a more stable immunological synapse, contributing to improved effector functions. These findings highlight the importance of optimizing IS quality over simply maximizing scFv affinity for superior CAR-T cell therapeutic responses. Therefore, this innovative FRET-based IS biosensor, which enables the evaluation of initial CAR-T cell activation, represents a potent platform to guide the optimization of next-generation CAR constructs for potent therapeutic responses in CAR-T cell therapy.
2 Results
2.1 Construction of MSLN scFv Variant Library and Design of FRET-based IS Biosensor
The MSLN-specific CAR comprises scFv, hinge, and transmembrane domain from CD8, CD137 (4-1BB) domain, and CD3ζ signaling domain. The scFv domain of CAR directly binds to target antigens on the tumor cell surface,[10] and this interaction initiates CAR clustering and the recruitment of signaling and structural molecules, resulting in the IS assembly.[3] This leads to the ITAM phosphorylation in CD3ζ and the subsequent recruitment of ZAP-70 via its dual SH2 domains.[11] These initial events at IS trigger the downstream signaling pathways, including CD25, CD69, CD137, interleukin (IL)−2, interferon (IFN)-γ, and surface CD107a (LAMP-1). CAR-T cells then release lytic granule proteins, including perforin and granzyme B, thereby enabling the elimination of the targeted cancer cells.[1]
To screen optimal scFv that binds to MSLN and effectively initiates the CAR functions, we first generated a focused library of scFv (M5).[12] by randomizing the heavy chain complementarity-determining regions 3 (H-CDR3) using degenerate codons that introduce alanine (A) or glycine (G) (Figure 1A). The M5 scFv variants with randomized H-CDR3 were then cloned into the bacterial expression vector pMopac12.[13] Ninety-six clones were randomly selected for the analysis of their expression levels and MSLN-binding affinities (Figure 1A). A subsequent analysis ranked each antibody variant based on its expression level, MSLN binding activity, and the binding-to-expression ratio. Seven antibody variants (M5DVAY, M5GVAD, M5DGDY, M5DGDD, M5GVDD, M5DGAD, and M5GCAY) were chosen for further study (Figure 1B,C) due to their diverse binding-to-expression ratios. However, variants with insufficient expression levels were excluded to ensure the feasibility of subsequent studies.
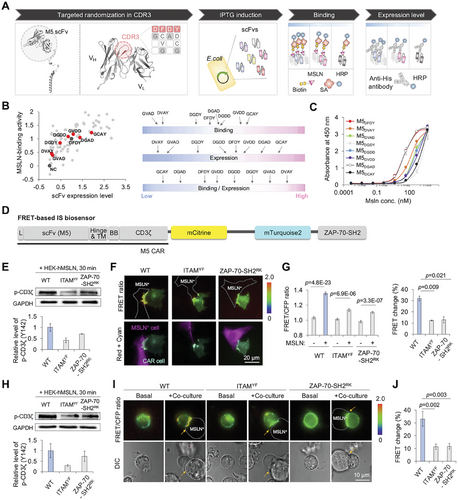
To evaluate the IS quality of the MSLN-CAR library, we designed a FRET-based IS biosensor that can visualize the CAR activation at IS. The biosensor was designed to consist of the MSLN-CAR (M5) followed by cyan and yellow fluorescent proteins (CFP and YFP), and dual SH2 domains from ZAP-70 (Figure 1D). Upon binding to MSLN via the scFv domain, the CD3ζ-ITAM domains are phosphorylated and the phosphorylated ITAMs (p-ITAMs) subsequently recruit ZAP-70, facilitating the CAR downstream signaling pathways. Thus, the FRET signals between p-ITAMs and ZAP-70-SH2 domains will increase due to the intramolecular interaction of these domains in the biosensor, visualizing the real-time CAR activation at the IS. For an efficient FRET pair, mCitrine and mTurquoise2 were chosen (Figure S1A, Supporting Information) and separated by a long linker to minimize the basal FRET levels before stimulation.
To validate this design of the FRET-based IS biosensor, mutant biosensors were generated: ITAMYF in which six-tyrosine (Y) residues in the ITAMs of the biosensor were substituted with phenylalanine (F), and ZAP-70-SH2RK which includes the arginine (R) to lysine (K) mutation in the ZAP-70-SH2 domains of the biosensor (Figure S1B, Supporting Information).[14] The treatment of a phosphatase inhibitor, pervanadate (PV), resulted in a significant FRET increase in the IS biosensor (wild type, WT) in HEK293A cells, but no FRET change was observed in mutant biosensors with ITAMYF or ZAP-70-SH2RK (Figure S1C–E, Supporting Information). The results confirmed that PV phosphorylated the ITAM tyrosine residues in the WT and ZAP-70-SH2RK mutant biosensors, but not in the ITAMYF mutant biosensor (Figure S1F, Supporting Information). These findings highlight that the interaction between p-ITAMs and ZAP-70-SH2 domains is critical for the FRET signal of the IS biosensor.
We next investigated the kinetics of ITAM phosphorylation in the IS biosensor during co-culture with MSLN-expressing HEK293A cells. Stimulation with these MSLN+ cells resulted in strong phosphorylation of CD3ζ-ITAMs in the IS biosensor, with a peak at 30 min (Figure S1G, Supporting Information). Indeed, imaging results showed that the FRET/CFP ratio of the IS biosensor increased locally in the contact region with MSLN+ cells (Figure S1H,I, Supporting Information). Therefore, the biosensor can visualize the initial process of CAR activation at IS upon antigen binding: the ITAM phosphorylation and subsequent ZAP-70 tyrosine kinase recruitment.
2.2 Optimization and Characterization of FRET-based IS Biosensor
The prototype IS biosensor showed a ≈13% FRET ratio change (from 1.13 to 1.28) upon binding to MSLN+ cells (Figure S1I, Supporting Information). To improve the dynamic range in FRET changes of the IS biosensor, we further optimized the linker regions in the biosensor: linker region 1 between mCitrine and mTurquoise2, and linker region 2 between mTurquoise2 and the ZAP-70-SH2 domains (Figure S2A, Supporting Information). In an attempt to separate the donor and acceptor FPs in a default state, we chose the ER/K linker as linker 1, which forms an α-helical structure laterally stabilized by salt bridges between the glutamate (E) and arginine (R)/lysine (K) residues.[15] We further examined the dynamic ranges of biosensors with other linkers, EAAAK.[16] and EV,[17] but they were not further improved (Figure S2B, Supporting Information). We next evaluated different lengths of the GSG repeat in the linker region 2, and the linker with 7 GSG repeats yielded the highest dynamic range of FRET changes in the IS biosensor (Figure S2C, Supporting Information). Consequently, the optimized IS biosensor, incorporating ER/K and GSG (×7) linkers, achieved a significantly improved dynamic range (32% FRET change).
We next characterized the optimized IS biosensor. Upon co-culturing with MSLN+ cells, the IS biosensor visualized the real-time activation of the MSLN-CAR at IS, as a FRET increase of over 30% was observed with a peak at 30 min (Figure S2D–F, Supporting Information). Wild-type and ZAP-70-SH2RK biosensors, but not the ITAMYF biosensor, showed the CD3ζ phosphorylation in HEK293A cells (Figure 1E). In addition, the WT biosensor exhibited a significantly higher FRET increase compared to the ITAMYF and ZAP-70-SH2RK mutant biosensors (Figure 1F,G), confirming the design of the biosensor. We observed a ≈10% FRET ratio change in the ITAMYF and ZAP-70-SH2RK mutant biosensors upon binding to MSLN+ cells (Figure 1F,G), unlike the PV treatment (Figures S1B–E and S2G–J, Supporting Information), which is likely due to the intermolecular FRET between clustered CARs.
Although Lck is absent in HEK293 cells, the ITAM phosphorylation observed in our experiments can be explained by the presence of another Src family kinase (SFK), Fyn. HEK293 cells have been shown to express Fyn,[18] which has been reported to mediate ITAM phosphorylation.[19] Interestingly, there are substrate selectivity differences between Lck and Fyn, with Fyn preferentially phosphorylating CD3ζ whereas Lck preferentially targets CD3ɛ.[19] Moreover, Fyn has been shown to phosphorylate ITAM domains within the CAR, effectively inducing CAR-mediated signaling.[19] Given that several studies have demonstrated that Fyn is expressed in HEK293 cells and mediates phosphorylation of CD3ζ, we believe that Fyn can mediate the phosphorylation of ITAMs for CAR activation in HEK293 cells.
Next, the FRET-based IS biosensor was introduced into Jurkat T cells using an electroporation system and cocultured with MSLN+ K562 cells. Upon binding to MSLN+ K562 cells, the CD3ζ phosphorylation of WT and ZAP-70-SH2RK biosensors was confirmed (Figure 1H). Consistent with the results in HEK293A, the IS biosensor in Jurkat T cells also showed a 30% increase in FRET ratio at contact regions, while mutant biosensors exhibited minimal non-specific changes (10%) (Figure 1I,J).
2.3 Identification of a Novel scFv M5DVAY with Improved CAR Activity at IS
To identify scFv variants exhibiting enhanced CAR activity at IS, we replaced the scFv domain of the IS biosensor containing the original M5DFDY (hereafter, M5DFDY IS biosensor) with scFv variants (Figure 1A–C). Because the FRET signals of the biosensor would visualize the degree of CAR activation at IS, we can select scFv variants demonstrating enhanced CAR activation at IS (Figure 2A). When expressed in HEK293 cells, most of the variant IS biosensors exhibited membrane localization, while the biosensors with M5GVAD or M5DGDD exhibited poor membrane expression and no detectable FRET change upon stimulation with MSLN+ cells (Figure 2B,C). Among the variant biosensors, the IS biosensor with M5DVAY showed the most drastic change in FRET signals at IS when stimulated with MSLN+ cells (41.9%) (Figure 2B,C; Figure S3A, Supporting Information). We further evaluated M5DFDY, M5DVAY, and M5GVDD in Jurkat T cells, and similar trends in FRET signals of variant IS biosensors were confirmed (Figure 2D,E). The IS biosensor with M5DVAY showed ≈45% of FRET increase at IS, confirming the optimal scFv sequence for enhanced CAR activity at IS. The levels of an activation marker CD69 corresponded to the FRET signals (Figure 2F; Figure S3B, Supporting Information), indicating that the FRET signals of the IS biosensor accurately reflect the activation of CAR. Thus, we identified a variant M5DVAY that demonstrated superior CAR activation at IS using the FRET-based IS biosensor. Therefore, this FRET-based IS biosensor system can be applied to predict the potency of CAR candidates.
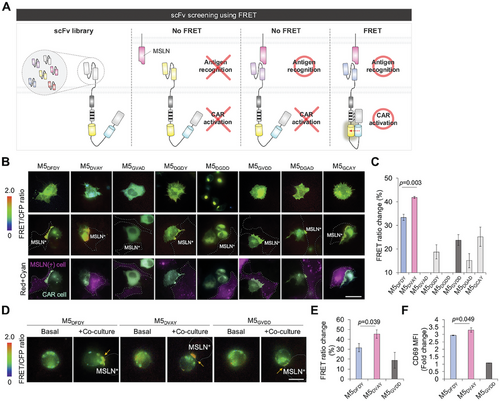
The ITAMYF mutation in the IS biosensors with the M5DVAY or M5GVDD showed a FRET change of ≈10% upon binding to MSLN+ cells (Figure S3C,D, Supporting Information), as we observed in the ITAMYF and ZAP-70-SH2RK mutant biosensors with M5DVAY (Figure 1G,J). These results suggest that cell-cell contact may cause this nonspecific intermolecular FRET between biosensors. Thus, we think the biosensor showing no more than 10% FRET change likely contains an scFv that is unable to efficiently induce ITAM phosphorylation upon target cell binding.
2.4 M5DVAY Exhibits a Reduced Affinity for MSLN Compared to M5DFDY
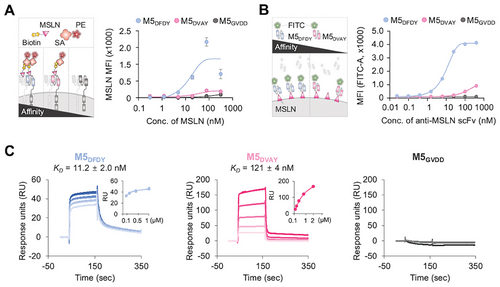
To evaluate the properties of the scFvs, we conducted binding affinity assays and surface plasmon resonance (SPR) analysis (Figure 3). Interestingly, the binding affinity of M5DVAY-CAR was lower than that of M5DFDY-CAR (Figure 3A). In addition, M5DVAY showed a lower binding affinity to MSLN-expressing Expi293 cells than M5DFDY (Figure 3B). SPR data confirmed that the affinity of M5DFDY for MSLN is 11.2 ± 2 nM, whereas that of M5DVAY is 121 ± 4 nM (Figure 3C). This suggests that high-affinity M5DFDY CAR can bind to MSLN even at low expression levels. Additionally, the dissociation kinetics of M5DFDY was much slower than M5DVAY, indicating that M5DFDY CAR binds to MSLN more strongly. We further confirmed the comparable thermostability of M5DFDY and M5DVAY (Figure S4, Supporting Information).
To explore the molecular basis of this difference in affinity, we utilized AlphaFold2[20] to build the structures of M5DFDY and M5DVAY (Figure S5A, Supporting Information) and applied them for molecular docking with MSLN using the Covalent Dock tool within the Schrödinger suite (Figure S5B, Supporting Information). The analysis revealed a notable divergence in the orientation of tryptophan residue (W97), a sequence immediately preceding DFDY or DVAY. According to our docking analysis, it appears that W97 is situated within the binding pocket to MSLN. Thus, the altered orientation of W97 may contribute to the change in the binding affinity of M5DVAY.
Thus, our FRET-based IS biosensor identified the promising scFv M5DVAY, which displays enhanced CAR activity at IS while its affinity and binding strength to MSLN are lower than those of the original scFv. Indeed, emerging results suggest that the CAR functionality may be not directly correlated with the scFv affinity,[6, 21] suggesting the limitation of conventional in vitro screening methods that identify scFv displaying high affinity to target antigens. Instead, our FRET-based IS biosensor can identify scFv that displays high CAR activation at IS, allowing for a more effective selection of potent CARs.
2.5 M5DVAY-CARs Rapidly form Robust Immunological Synapse
When the scFv domain of CAR recognizes target antigens, it has been suggested that the CARs are clustered at the IS to generate signaling platforms for downstream molecules.[22] The accumulation of the actin cytoskeleton at the immunological synapse is crucial for dynamic structural integrity in this cell-cell interface, allowing the intimate communication between CAR-T cells and target antigen-presenting cells. The actin cytoskeleton is also important throughout the CAR signaling cascade, including centripetal transportation of receptor microclusters, microtubule organizing center (MTOC) polarization, and release of lytic granules.[22, 23] Therefore, we next compared the clustering of M5DVAY- or M5DFDY-CARs as well as actin accumulation in Jurkat cells during co-culture with MSLN+ cells (Figure 4A,B). The quantified results of the CAR clustering at IS (Figure 4C).[24] indicated the stronger clustering of M5DVAY-CARs compared to M5DFDY-CARs (Figure 4D). In addition, we observed dramatic actin accumulation at IS in the M5DVAY-CAR-Jurkat T cells (Figure 4E). Consequently, M5DVAY-CARs exhibited faster kinetics of robust IS formation than M5DFDY-CARs (Figure 4F).
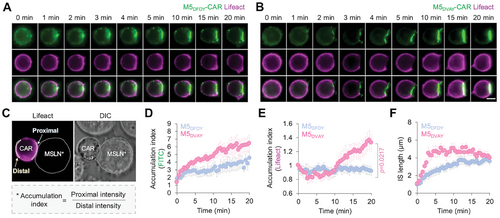
Actin accumulation started later than the CAR clustering at IS, implying that CAR clustering and initial signaling pathways are required for actin accumulation at IS to strengthen the cell-cell contact structures and CAR functionality. M5DFDY-CARs exhibited a strong affinity to MSLN but their hyper-stable binding may hinder the efficient clustering of CARs,[5] potentially preventing the accumulation of actin at the IS.
2.6 M5DVAY-CARs Form a Superior Quality of Immunological Synapse
To further investigate the molecular distribution within the IS, we employed 3D super-resolution structured illumination microscopy (SR-SIM). The results confirmed the superior clustering and robust phosphorylation of M5DVAY-CARs at IS (Figure 5A,B; Figure S7, Supporting Information). We also observed the actin accumulation at the IS of M5DVAY-CAR-Jurkat T cells. Turn-around Y image analysis revealed that actin accumulation is observed in the outer part of IS, aligning with the phosphorylated CAR clusters in the center (Figure 5A,B; Figure S6, Supporting Information). Collectively, these findings indicate that M5DVAY-CAR exhibits robust IS formation via actin accumulation as well as enhanced clustering and phosphorylation of CARs. This superior quality of immunological synapses in M5DVAY-CAR-T cells would contribute to their efficient CAR activation.
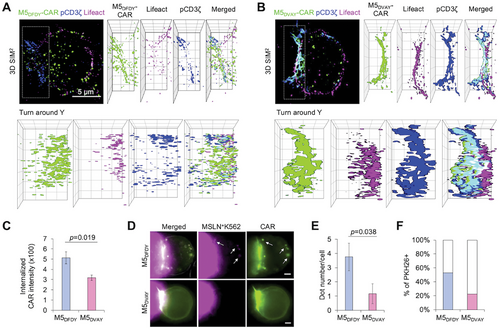
In contrast to the well-clustered M5DVAY-CARs at the IS, an increased population of internalized M5DFDY-CARs was observed within the cells (Figure 5A–C), consistent with previous studies suggesting that high-affinity antibodies are more rapidly internalized.[25] A recent study has shown that CD19-CARs with a high-affinity scFv FMC63 induce trogocytosis when co-cultured with cancer cells.[21] Trogocytosis involves the extraction of antigens from cancer cells, followed by its expression on the CAR-T cell membranes, thereby impairing CAR-T cell functionality. We thus investigated whether trogocytosis process could contribute to the internalized subsets of M5DFDY-CARs. After 4 h coculture with PKH26-labelled MSLN+ cells, we observed that internalized M5DFDY-CARs co-localized with the trogocytosed PKH26+ membranes (Figure 5D–F). These results suggest that the high affinity of M5DFDY-CAR may lead to its internalization, impeding effective IS formation and reducing CAR-T functionality (Figure S8, Supporting Information).
2.7 M5DVAY CAR-T Cells are More Active, Polyfunctional, and Cytotoxic Than M5DFDY CAR-T Cells
To evaluate the functionality of M5DVAY-CAR-T cells, we generated lentiviruses carrying CAR constructs and introduced them into human T cells. Subsequently, these CAR-T cells were co-cultured with MSLN-expressing K562 cells to evaluate the levels of activation markers and cytokines, as well as cytotoxicity (Figure 6A). Furthermore, we investigated the functionality of M5DFDY- and M5DVAY-CAR in response to low and high expression levels of MSLN. To modulate the MSLN expression in K562 cells, we modified the promoter of the MSLN-expressing vector by incorporating PGK promoter, resulting in lower MSLN expression levels than the original vector with EF-1α promoter (Figure S9, Supporting Information).
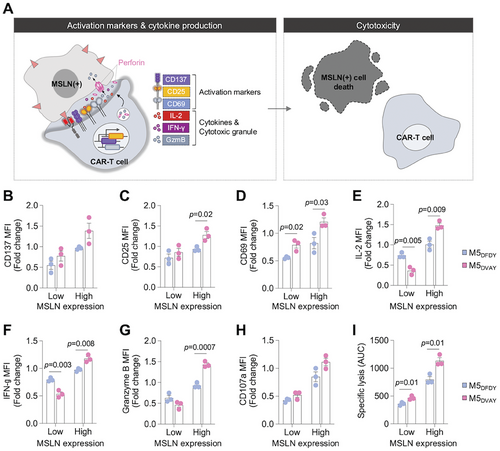
To compare the functionality of M5DFDY- and M5DVAY-CAR-T cells, we first evaluated the levels of activation markers (CD137, CD25, and CD69) after co-culture with MSLN-expressing K562 cells for 24 h. In response to the cells with high-MSLN expression, M5DVAY-CAR-T cells showed significantly higher levels of CD25 and CD69 than M5DFDY-CAR-T cells, with a similar trend observed in the expression level of CD137 (Figure 6B–D; Figure S10A, Supporting Information). Next, we assessed cytokine-production by M5DFDY- and M5DVAY-CAR-T cells following 6 h of incubation with MSLN-expressing K562 cells. In response to high levels of MSLN, M5DVAY-CAR-T cells produced significantly higher levels of IL-2 and IFN-γ than M5DFDY-CAR-T cells (Figure 6E,F; Figure S10B, Supporting Information), consistent with the results of activation markers (Figure 6B–D). Interestingly, when co-cultured with low MSLN-expressing K562 cells, M5DVAY-CAR-T cells produced lower levels of cytokines compared to M5DFDY-CAR-T cells (Figure 6E,F). These results align with a previous study suggesting that high-affinity CAR-T cells, compared to relatively low-affinity CAR-T cells, exhibit stronger cytokine production in response to tumor cells expressing low antigen levels.[26] In contrast, no statistically significant difference was observed in activation marker expression during co-culture with low-MSLN-expressing K562 cells.
To assess the impact of these differences in activation markers and cytokine production on cytotoxicity, we performed assays following co-culturing CAR-T cells with target cells for 8 h (Figure 6A). Consistent with the trends observed above (Figure 6B–F), M5DVAY-CAR-T cells showed elevated granzyme B production, degranulation, and robust cytolytic activity compared to M5DFDY-CAR-T cells (Figure 6G–I; Figure S10B,C, Supporting Information). Consequently, M5DVAY-CAR-T cells exhibited significantly improved killing efficacy, possibly due to their superior IS quality compared to the high-affinity M5DFDY-CAR-T cells. These findings emphasize the potential divergence between the scFv affinity for antigen and the CAR functionality, underscoring the significance of our IS biosensor system which can comprehensively evaluate CAR activation at antigen binding sites in live cells.
3 Conclusion
The immunological synapse formed by CARs exhibits distinct structural differences compared to that formed by TCRs.[3] The TCR-IS has a highly organized structure with well-defined supramolecular activation clusters (SMACs): central (cSMAC), peripheral (pSMAC), and distal (dSMAC), where receptors, signaling molecules, and adhesion molecules are segregated into specific regions.[2] Additionally, TCR-IS features a cleared central region lacking F-actin and a peripheral adhesion ring in the pSMAC. This organized IS facilitates efficient and sustained signaling, allowing effective T-cell activation and immune responses to the engaged cells.[27] In contrast, the CAR-IS is less structured, with some cells forming cSMACs similar to TCRs, but many exhibiting scattered, disordered microclusters of CARs and signaling molecules.[28] The organized molecular segregation and structural elements in TCR-IS are not observed in CAR-induced IS.
Despite the less organized structure, the formation of IS between CAR-T cells and target cells is critical for effective CAR functionality.[1, 23, 29] The assembly of IS involves a spatial reorganization of CAR-antigen complexes, signaling molecules, and cytoskeletal proteins into distinct membrane subdomains at the cell-cell interface.[23] The mature IS serves as a central hub for sustained signal propagation and the polarized exocytosis of cytotoxic granules toward the engaged target cell, ultimately leading to its elimination.[22, 29] Thus, the IS quality is crucial for effective CAR-T cell activation. Indeed, it has been reported that the anti-tumor efficacy of CAR-T cells can be significantly enhanced by strategies that reinforce the structural integrity of the CAR-T cell synapse, such as disrupting N-glycan synthesis in tumor cells.[30] or incorporating a PDZ binding motif into the CAR construct.[31] Furthermore, in vitro evaluations of CAR and antigen clustering, lytic granule polarization, the distribution of signaling molecules, as well as F-actin accumulation within the IS, have been shown to positively correlate with CAR-T cell efficacy in vivo.[4, 32] Therefore, evaluating the qualitative features of the immunological synapse would provide an effective strategy to optimize CAR design for enhanced CAR-T cell functionality.
In this study, we developed an innovative FRET-based biosensor that allows the real-time assessment of the IS quality (Figure 1) and the identification of M5DVAY-CAR with enhanced CAR activation (Figure 2). Our results indicate a positive correlation between IS quality and CAR-T cell function. The enhanced activation of M5DVAY-CAR-T cells likely stems from the efficient formation of IS, as M5DVAY-CAR-T cells exhibited faster kinetics of stable IS assembly compared to M5DFDY-CAR-T cells (Figure 4). Utilizing the 3D super-resolution SIM technique, we further confirmed the high quality of IS between MSLN and M5DVAY-CAR, such as actin accumulation, CAR clustering, and CD3ζ phosphorylation (Figure 5). We then demonstrated that M5DVAY-CAR results in improved CAR functionality (Figure 6), confirming the FRET-based prediction of CAR-T cell activation (Figure 2). Given that in vivo CAR screening methods are expensive and time-consuming, our FRET-based IS biosensor, which measures CAR-T cell activation, can be applied for efficient CAR screening before in vivo study.
A variety of fluorescent biosensors have been previously developed for the investigation of complex T-cell signaling pathways, as we have recently reviewed.[33] For example, FRET-based ZapLck.[34] and Zap70.[35] biosensors were developed to monitor the real-time activities of Lck and ZAP-70-kinase in T cells or CAR-T cells. While Lck and ZAP-70 kinase are indeed key signaling molecules in CAR-T cell activation, their activity can be influenced by various stimuli beyond antigen recognition.[36] In contrast, our IS biosensor is designed to directly report antigen-induced CAR activation at the immunological synapse. By focusing on the CAR-antigen interaction site, it provides a more specific and direct measure of CAR activation. This makes our biosensor particularly effective in assessing the activation state of different CAR candidates.
Our results suggest a potential disadvantage of high-affinity scFv in CAR design. It has been previously shown that, while high-affinity CARs may initially exhibit stronger binding to target antigens, they could potentially result in hyper-stable but not dynamic contacts, thus hindering the efficient formation of CAR microclusters.[5] Indeed, our SPR experiments have shown a strong interaction and slow dissociation kinetics between MSLN and M5DFDY (Figure 3C). Excessively strong binding of high-affinity scFv to target antigens may also limit detachment from engaged tumor cells, thereby hindering the serial killing of tumor cells by CAR-T cells. Consequently, it is essential to design CAR domains using a screening system capable of assessing CAR functionality, instead of prioritizing scFv with high affinity.
Furthermore, CARs with high-affinity scFv may exhibit increased internalization upon binding to target cells. This could limit the formation of sufficient CAR clusters at the IS and interfere with downstream signaling molecules. This aligns with previous studies showing that high-affinity antibodies are more susceptible to internalization than low-affinity antibodies.[25] The internalized CARs can be degraded through the lysosomal pathway,[37] and reduced CAR expression at the plasma membrane ultimately affects their long-term killing efficacy for tumor cells. In addition, current results highlight a potential downside of high-affinity CARs in promoting trogocytosis, where CAR-T cells engulf parts of the target cell membrane alongside the antigen.[21, 38] This disrupts the proper formation of immunological synapses, hindering the function of CAR-T cells.[38, 39] Therefore, the balanced scFv affinity is crucial for successful CAR-T cell therapy, as it mitigates the risks of internalization and trogocytosis while ensuring effective target antigen engagement.
In summary, our study presents a novel FRET-based CAR functional screening system, pioneering a live-cell approach for identifying next-generation CAR-T cells. This FRET-based system sensitively measures the IS quality of CAR, which can predict the CAR-T cell activation and facilitate the efficient screening of highly effective cell-based immunotherapies. This finding challenges the traditional perception of “higher affinity, better CAR” and highlights the potential drawbacks of overly strong binding. The success of this MSLN-CAR demonstrates the immense potential of our system for treating MSLN-expressing solid tumors. This paves the way for personalized and effective cancer therapeutics, marking a pivotal step toward the future of precision medicine.
4 Experimental Section
Construction of the FRET-based IS Biosensor and Its Mutants
Plasmids were constructed using standard molecular biology techniques, including polymerase chain reaction (PCR) and In-Fusion cloning (Clontech). The prototype IS biosensor was generated by fusing M5 scFv-derived CAR with mCitrine, a long ER/K linker, mTurquoise2, and dual ZAP-70-SH2 domains. ITAMYF or ZAP-70-SH2RK mutant biosensors were constructed by introducing mutations for specific amino acids via overlap-extension PCR. The scFv library for the IS biosensor was constructed by replacing the scFv region of the biosensor with scFv variants using a two-fragment In-Fusion procedure.
To visualize CAR localization and clustering, M5DFDY-CAR-YFP, and M5DVAY-CAR-YFP were constructed by removing mTurquoise2 and the ZAP-70-SH2 domains from the IS biosensor vector. The mCardinal-Lifeact-7 (#54 663) was obtained from Addgene. The pRk5 vector was used for plasmid expression in mammalian cells, and the pcDH vector was used for virus production. All cloning constructs and PCR products were confirmed by DNA sequencing.
Cell Culture and Transfection
HEK293 cells (Korean cell line bank, No.21573) and HEK293A cells (Invitrogen, #R70507) were maintained in Dulbecco's modified Eagle medium (DMEM, Gibco) supplemented with 10% fetal bovine serum (FBS, Hyclone), 1% penicillin/streptomycin (Corning) and 100 µM MEM non-essential amino acids solution (Gibco). Jurkat T cells (Korean cell line bank, No.40152) were maintained in Roswell Park Memorial Institute (RPMI) 1640 medium (Hyclone, #A10491) containing 10% FBS, 1% penicillin/streptomycin (Corning), and 1xGlutaMAX (Gibco). K562 and K562-MSLN cells were cultured in RPMI1640 medium (Hyclone, #SH30027.01) supplemented with 10% FBS, and 1% penicillin/streptomycin (Corning). All cell lines were cultured in a humidified incubator at 37 °C and 5% CO2.
For transient expression of plasmids in adherent cells, transfection was performed using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's protocol. To transfect Jurkat T cells with plasmids, cells were prepared at appropriate densities and subjected to electroporation using the NeonR Transfection system (Invitrogen). Following electroporation, the cells were resuspended in complete media, and transfection efficiency was evaluated after 24 h by measuring GFP or CAR expression levels using a CytoFLEX instrument (Beckman Coulter).
Western Blotting
To assess CD3ζ phosphorylation at the protein level, cells expressing the WT, ITAMYF, or ZAP-70-SH2RK mutant biosensors were harvested following treatment with 100 µM pervanadate (PV) or co-culture with MSLN-expressing cells at the indicated times. Subsequently, 20 µg of protein was separated by SDS-PAGE and transferred onto a membrane. The membrane was then probed with a rabbit antiphospho CD3ζ antibody (Tyr142, 1:1000 dilution, Cell Signaling Technology, Danvers, MA, USA, #67 748). Equal protein loading was ensured using an anti-GAPDH antibody (1:1000 dilution, Santa Cruz Biotechnology, #sc-47778). Membranes were developed using an enhanced chemiluminescence (ECL) solution, and images were captured with a luminescent image analyzer, Amersham ImageQuant 800 (Cytiva). Band intensities on the blot were quantified using Image Lab 5.2.1 software (Bio-Rad).
Live Cell Imaging and FRET Image Acquisition
For all imaging experiments, cover-glass-bottom dishes (SPL Life Sciences) were prepared by coating with either 10 µg mL−1 of fibronectin at 37 °C for 2 h (for HEK293A or HEK293 cells) or 0.1% poly-L-lysine at room temperature (RT) for 1 h (for Jurkat T cells). Cells transfected with each construct were then cultured on the coated cover-glass-bottom dishes for a minimum of 2 h prior to the imaging experiment. Live-cell imaging was performed in a controlled chamber (Live Cell Instruments) maintained at 37 °C with 95% air, 5% CO2, and. Images were collected by a Nikon Ti-E inverted microscope and a cooled charge-coupled device camera using NIS software (Nikon).
For FRET imaging of the IS biosensor, a 438/24 nm excitation filter, a 458 nm dichroic mirror, and two emission filters (438/24 nm for ECFP and 542/27 nm for FRET) controlled by a filter changer were used. The fluorescence intensity of non-transfected cells was quantified as background signals and subtracted from the fluorescent signals on transfected cells. The pixel-by-pixel ratio images of FRET/ECFP were calculated based on the background-subtracted fluorescence intensity images of FRET and ECFP by the NIS program to allow the quantification and statistical analysis of FRET responses. To quantify FRET ratios in cocultured cell images, regions of interest (ROIs) were manually selected at the IS region where the CAR-expressing cell contacts the target cell.
Images of M5DFDY-CAR-YFP and M5DVAY-CAR-YFP were acquired using a 482/40 nm excitation filter, a 506 nm dichroic mirror, and a 536/40 nm emission filter. Lifeact-mCardinal was imaged using a 531/40 nm excitation filter, a 562 nm dichroic mirror, and a 593/52 nm emission filter. Fluorescence intensity images were generated by subtracting background signals using the NIS program, enabling quantification and statistical analysis. To assess actin dynamics, the accumulation index was determined by designating the cell-cell contact area (immunological synapse, proximal) and the CAR-T cell region opposite the IS (distal) as ROIs, with the intensity of the proximal ROI divided by that of the distal ROI.
To quantify CAR internalization via trogocytosis, the membranes of MSLN+ K562 cells were labeled with PKH26 dye according to the manufacturer's protocol (Sigma Aldrich). Subsequently, MSLN+ K562 cells were co-cultured with M5DFDY-CAR-YFP cells or M5DVAY-CAR-YFP cells at an effector-to-target ratio of 1:1 for 4 h and then observed under a microscope.
Construction and Characterization of scFv Library
To generate the scFv library, diversity was introduced by randomizing residues D114, F115, D116, and Y117 of the anti-MSLN M5 in the H-CDR3 region using degenerate codons. The resultant scFv genes with randomized H-CDR3 were cloned into the pMopac12 vector via Gibson assembly (New England Biolabs) and transformed into E. coli Jude 1 competent cells by electroporation. Following transformation, the cells were recovered in LB broth, plated on LB agar with chloramphenicol, and incubated overnight at 37 °C. Randomization was confirmed by sequencing ten colonies using PCR.
For scFv characterization, 96 clones were randomly picked and cultured in 96-deep-well plates with TB medium containing chloramphenicol (TBcm) overnight at 37 °C. The cultures were transferred to a new plate and fresh TBcm was added per well. After 3 h, scFv expression was induced with 0.5 mM isopropyl β-D-thiogalactopyranoside (IPTG) at 25 °C for 16 h. The supernatants were harvested after centrifugation at 4000 rpm for 20 min and tested for scFv production via ELISA on a 96-well EIA plate, using horseradish peroxidase (HRP)–conjugated anti-His antibodies at a 1:8000 dilution. After blocking and washing steps, 3,3′,5,5′-Tetramethylbenzidine (TMB) substrate was added for 15 min. The reaction was stopped with 2 M H2SO4, and absorbance was read at 450 nm.
Preparation of Recombinant Proteins
The recombinant extracellular domain (ECD) of MSLN was produced by cloning the relevant gene segments into the mammalian expression vector pcDNA3.4 via Gibson assembly. Expression was carried out in Expi293 cells[13] (Thermo Fisher Scientific), and the His-tagged ECD was purified using Ni-NTA agarose resin (Thermo Fisher Scientific) affinity chromatography.[40] The eluted samples underwent buffer exchange and were analyzed for purity by SDS-PAGE gel analysis. For additional analysis, the recombinant MSLN was biotinylated using the EZ-Link Sulfo-NHS-Biotinylation Kit (Thermo Fisher Scientific).
The genes for anti-MSLN scFv were cloned into the bacterial expression vector pMopac12 using Gibson assembly cloning. These anti-MSLN scFvs were expressed in E. coli BL21 (DE3) cells using previously established protocols.[41] E. coli cultures were grown in TBcm at 37 °C until reaching an optical density (O.D. 600 nm) of 0.6−0.8. Following IPTG induction for protein expression, cells were incubated for an additional 16 h at 25 °C and then harvested. The cells were resuspended in a Tris-sucrose solution (pH 7.5; 100 mM Tris, 0.75 m sucrose), and lysozyme (20 mg mL−1) was added. After a 20 min incubation at 4 °C, 1 mM EDTA (pH 8.0) was introduced, and the mixture was incubated for another 20 min at 4 °C with shaking. Cells were then pelleted by centrifugation, and the supernatant containing the periplasmic fraction was collected and filtered through a 0.22-µm syringe filter. The His-tagged recombinant scFv proteins were then purified using the methods described above.
Enzyme-linked Immunosorbent Assay (ELISA)
To evaluate MSLN binding of anti-MSLN scFvs, 1 µg of each scFv variant protein was added into a 96-well immunoplate (Thermo Fisher Scientific) and incubated overnight at 4 °C. After blocking with phosphate-buffered saline (PBS) containing 3% bovine serum albumin (BSA) for 1 h at RT, the plate was washed three times with PBS containing Tween-20 (PBS-T). Serially diluted biotinylated MSLN was then added and incubated at RT for 1 h. After 3 washes with PBS-T, 50 µL of HRP–conjugated streptavidin (diluted 1: 15000 in PBS) was added and incubated at RT for 1 h. Following another wash with PBS-T, the plate was treated with 50 µL of TMB for 15 min, followed by 50 µL of 2 M H2SO4 to neutralize. The absorbance at 450 nm was measured using a microplate reader.
Surface Plasmon Resonance (SPR)
The scFv binding kinetics were evaluated using surface plasmon resonance (SPR) with the BIAcore T200 instrument. For analysis, the matured form of MSLN was immobilized on CM5 biosensor chips by amine coupling, as recommended by the manufacturer (Cytiva, USA). HBS-EP buffer (Cytiva, BR100669) was used for binding experiments. Serially diluted scFv variants were injected at a flow rate of 30 µL mi−1n for 120 sec with a dissociation time of 5 min. After each run, the chip was regenerated by injecting 5 mM NaOH and 0.5 M arginine (pH 8.0) sequentially for 30 s each. Equilibrium dissociation constants (KD) were determined by fitting the data to a 1:1 Langmuir model using BIAevaluation 3.2 software (Cytiva). The calculated KD values were averaged over three separate experiments (n = 3 for each scFv variant).
MSLN Binding Assay of M5 Variants CAR-expressing Cells
The M5 variant CAR constructs were transfected into Expi293 cells using previously established methods.[42] After 48 h, the cells expressing CAR were collected, and 5 × 104 cells were resuspended in 100 µL of PBSF (1% FBS in PBS) per well in a 96-well round-bottom plate. The cells were then centrifuged at 300×g for 3 min. Following centrifugation, the supernatant was discarded, and the cell pellets were washed with fresh PBSF. Subsequently, various concentrations of biotinylated MSLN ECD were added to the cells and incubated at 4 °C for 1 h. Following this incubation, the cell pellets were washed again with fresh PBSF and then incubated with SA–PE (diluted 1:400) at 4 °C for 15 min. Finally, after an additional washing step with fresh PBSF, the cells were prepared for analysis via flow cytometry.
Cell Binding Assay of M5 Variants
The MSLN+ K562 and MSLN− K562 cells (5 × 104 cells) were resuspended in PBSF per well in a 96-well round-bottom plate and centrifuged at 300 ×g for 3 min. The cell pellets were washed with fresh PBSF. Recombinant anti-MSLN scFvs (M5DFDY, M5DVAY, M5GVDD) were added at various concentrations and incubated at 4 °C. After 1 h, the cell pellets were washed with fresh PBSF and then mixed with anti-HIS-FITC (1:400) at 4 °C. Following a 15-min incubation, the cell pellets were washed again with fresh PBSF and subjected to analysis using flow cytometry.
In Silico Structure Analysis
To determine the 3D structure of each scFv, AlphaFold2 within the Google ColabFold notebooks was utilized.[20] Five models were generated for each scFv, and one sample was chosen from the model demonstrating the highest predicted Local Distance Difference Test (pLDDT) and predicted TM-score (pTM). Comparative structural analysis was then conducted using PyMol software. For molecular docking of MSLN with the scFv, a Covalent Dock within the Schrödinger suite was used for small molecular docking. In contrast, protein-protein docking was performed using Maestro 13.6 software with antibody mode masking non-CDR regions. The docked pose exhibited the strongest binding affinity while maintaining default settings for all other parameters in the docking software was prioritized.
Analysis of CAR Activation in Jurkat T Cells
CAR-transduced Jurkat T cells were co-cultured with MLSN+/− K562 cells in a 3:1 ratio in a 24-well plate and incubated for 4 h. Following the incubation, cells were harvested and washed twice with PBS at 1,350 rpm for 3 min. Subsequently, cells were stained with a viability dye, eFluor 506 (1:2500, BioLegend), and a PE-Cyanine7 (Cy7)–anti-human CD69 (1:2500, BioLegend) antibody for 20 min at 4 °C. After staining, cells were washed twice in PBS. The level of CD69 expression was determined using a CytoFLEX instrument (Beckman Coulter). The presented data illustrates the fold changes in mean fluorescence intensity (MFI) ± standard error of the mean (SEM) based on more than three independent experiments.
Super-resolution Structured Illumination Microscopy (SR-SIM)
For super-resolution structured illumination microscopy (SR-SIM) imaging, cells were fixed with 4% paraformaldehyde for 10 min and permeabilized with 0.1% Triton X-100 for 5 min. Next, the cells were incubated with PBS containing 1% BSA for 1 h, followed by overnight incubation at 4 °C with rabbit anti-phospho-CD3ζ antibody (Tyr142, 1:1000 dilution, Cell Signaling Technology, #67 748). After three washes with PBS, the cells were incubated with Alexa Fluor 405–conjugated goat anti-rabbit antibody (diluted 1:200) for 2 h. Following three washes with PBS for 10 min each, the stained cells were observed under an Elyra 7 microscope (Zeiss) with a 60×/1.46 objective. Images were acquired using 405, 488, and 561 nm lasers. The 3D SIM images were obtained every 0.2 µm along the z-axis and then aligned (3D) and reconstructed using the Zeiss Zen 3.0 SR (black) program. Image reconstruction was performed using dual iterative SIM (SIM2), a nonlinear iterative reconstruction algorithm developed by Zeiss.
Lentivirus Production and Transduction Into Human T Cells
To prepare for transfection, Lenti-X 293T cells (8 × 106) were seeded on 10-cm cell culture dishes (Corning) with DMEM supplemented with 10% FBS and 1% penicillin/streptomycin (all reagents from Thermo Fisher Scientific;, i.e., complete media) and incubated at 37 °C and 5% CO2 for 24 h. When Lenti-X 293T cells were at 90% confluency, lentiviral vectors were transfected using Lipofectamine 3000 according to the manufacturers’ protocol (Thermo Fisher Scientific). The lentiviral plasmids used in this study were as follows: envelope plasmid pMD2.G and packaging plasmids pMDLg/pRRE and pRSV-Rev, all of which were purchased from Addgene. In brief, 25 µg of a lentiviral packaging plasmid mix (at a ratio of 2:1:1:1, CAR-T transfer vector:pMD2.G:pMDLg/pRRE:pRSV-Rev) was used. After 6 h, the medium was replaced with complete media. Cell supernatant containing lentivirus was harvested at 24 and 48 h post-transfection and filtered through a 0.45-µm polyether sulfone syringe filter to remove debris. The lentivirus was then concentrated up to 100 times by centrifugation at 12700 ×g for 120 min at 4 °C. The physical particle of lentivirus was titrated using a p24 ELISA kit (OriGene) according to the manufacturer's instructions.
The Institutional Review Board of Seoul National University Hospital approved the study (IRB No. H-2009-160-1160), and written informed consent was obtained from all participants, in accordance with the Declaration of Helsinki. Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized peripheral whole blood using a Ficoll–Histopaque gradient (1.077 g mL−1: GE Healthcare Life Sciences). PBMCs were stored in liquid nitrogen until analysis in a freezing medium comprising 50% FBS, 10% dimethyl sulfoxide (DMSO), and 40% RPMI-1640 (Thermo Fisher Scientific). After thawing, PBMCs were pre-incubated at 37 °C for 3 h and then cultured on a plate with 10 µg mL−1 of anti-human CD3 antibody (clone OKT3; Thermo Fisher Scientific) and recombinant human IL-2 (200 IU mL−1; Peprotech) in AIM-V media (Thermo Fisher Scientific) for 48 h at 37 °C, 5% CO2 atmosphere condition. After T-cell activation, cells were transduced with a multiplicity of infection (MOI) 7 of lentivirus and incubated for 48 h. Lentiviral transduced cells were further cultured with IL-2 (200 IU mL−1) twice a week for 10 to 11 days before subsequent analysis.
Analyzing Activation Status of CAR-T Cells
To produce target cells expressing low MSLN, the pLVX-MSLN OX-cFLAG-IRES-Pgk plasmid was constructed. First, the Pgk promoter using a template (Addgene, #79 123) was amplified. Next, both the insert and vector with Mfe I and Xba I (New England Biolabs) and replaced the EF-1 alpha (EF-1α) promoter in the pLVX-MSLN OX-cFLAG-IRES-Puro plasmid with the newly digested Pgk promoter were digested. To ensure the accuracy of the cloned plasmid, its sequence using Sanger sequencing was verified.
To determine the expression levels of activation markers, CAR-T cells were co-cultured with K562 target cells expressing MSLN at different levels at a 1:2 effector-to-target ratio in the presence of Brilliant Ultra Violet 805 (BUV805)–anti-human CD4 antibody (clone RPA-T4) and fluorescein isothiocyanate (FITC)–anti-human CD107a antibody (clone H4A3) for 24 h. After antigenic stimulation, surface antigens were stained with BUV496–anti-human CD8 (clone RPA-T8), BUV395–anti-human CD137 (clone 4B4-1), Brilliant Violet 711 (BV711)–anti-human CD25 (clone ACT35), and PE–anti-human CD69 (clone FN50) antibodies (all from BD Biosciences, except BioLegend's anti-human CD25 antibody).
To assess the cytokine production capacity of CAR-T cells, the same co-culture system with BUV805–anti-human CD4 antibody for 6 h, followed by treatment with BD Golgistop (monensin, BD Biosciences) and BD GolgiPlug (brefeldin A, BD Biosciences) for the final 4 h was employed. Next, cells were stained with BUV496–anti-human CD8 antibody to identify the CAR-T cells. To allow for intracellular cytokine detection, cells were then fixed and permeabilized. Finally, the cells were stained with PE-Cy7–anti-human IFN-γ (clone B27; BD Biosciences) and BV711–anti-human IL-2 (clone MQ1-17H12; BD Biosciences) antibodies in Perm/Wash buffer combined with Brilliant Stain Buffer (BD Biosciences). Stained cells were acquired using a BD LSRFortessa flow cytometer (BD Biosciences) and analyzed with FlowJo 10.8.1 software (Tree Star, Ashland, OR, USA).
Cytotoxicity Assays of CAR-T Cells
To label the target cells, carboxyfluorescein diacetate succinimidyl ester (CFSE, 5 µM; Thermo Fisher Scientific) for 10 min at RT in the dark was used. After labeling, the cells were washed twice with complete media. 2.5 × 104 CFSE-labeled target cells with CAR-T cells, in various ratios for 8 h at 37 °C were then cocultured. To exclude dead and apoptotic cells, the cells with 7-amino-actinomycin D (7-AAD, BD Biosciences) before analyzing them using flow cytometry were stained. The percentages of dead target cells positive for both CFSE and 7-AAD after subtracting the percentage of spontaneous death in target cells were calculated. Two controls, one in which only target cells were spontaneously lysed without effector cells, and one in which complete lysis was induced using 100 µL of 0.1% Tween-20 (maximum lysis) were also included. To calculate the percent lysis of target cells, the formula: [(% experimental lysis – % spontaneous lysis)/(% maximum lysis – % spontaneous lysis)] × 100 was used. Finally, the cytotoxic efficiency of CAR-T cells using the area under the curve (AUC) in GraphPad Prism 9 (GraphPad Software Inc., La Jolla, CA, USA) was calculated.
Statistical Analysis
All data were presented as mean ± SEM or SD as indicated in the legend of each figure. Data were based on at least three independent experiments. The sample size was displayed in the legend. Statistical differences between groups were assessed using a two-tailed Student's t-test or one-way ANOVA, followed by post hoc Tukey's multiple comparisons test (GraphPad Prism 9). Statistical significance was defined as p < 0.05.
Acknowledgements
This work was supported by the Samsung Research Funding & Incubation Center of Samsung Electronics under Project Number SRFC-TC2003-02 and the National Research Foundation of Korea (NRF) (RS-2023-00227950 and RS-2024-00338426).
Conflict of Interest
The authors declare no conflict of interest. J.S., M.J., H.N.L., H.Y., C-H.L., J.J., H-R.K., and S.L. are inventors on a provisional patent application.
Author Contributions
J.S., H-R.K., C-H.L., and M.J. conceived the study. J.S., M.J., and H.N.L designed the FRET-based CAR biosensor. H.N.L constructed and validated CAR biosensors, performed all FRET imaging and super-resolution imaging, and prepared the first draft of the manuscript. H.Y. performed the flow cytometric experiment of CAR-Jurkat T cells. C-H.L, J.J., and J.H. designed, and generated scFv library, and characterized the selected scFv such as binding affinity and thermostability. H-R.K., S.L., Y-W.K., and H.M.S. constructed Pgk promoter, generated human CAR-T cells, and analyzed the performance of CAR-T cells using flow cytometry. J.S., H-R.K., C-H.L., M.J., and H.N.L. wrote the manuscript, and all authors provided feedback on the manuscript.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.