Toward Green Battery Cells: Perspective on Materials and Technologies
Abstract
Research and development of advanced rechargeable battery technologies is dominated by application-specific targets, which predominantly focus on cost and performance targets, including high gravimetric energy, volumetric energy, and related power densities, while ensuring a high safety and long lifetime. The need for high-performance and low-cost batteries is driven by the growing market of electromobility, in order to fulfill key requirements, such as a sufficient driving range and fast charging ability, for achieving broad consumer acceptance. Currently, the lithium ion battery (LIB) is one of the state-of-the-art technologies able to meet most of these key requirements at a reasonable cost. In addition to performance and costs, the environmental impact, i.e., the sustainability of the battery and in particular of the battery cell over the whole life cycle—i.e., from raw material extraction and battery material production, to cell and battery pack production, battery utilization, and to possibilities for second life usage and recycling—does receive continuously increasing attention. Within this review, different approaches for the development of “greener” batteries are introduced with a view on the complete battery life cycle, while focusing on the LIB technology. Moreover, alternative battery technologies are critically evaluated regarding their sustainability aspects and competitiveness.
1 Introduction
Over the last decades, an increasing knowledge about environmental processes led to a growing awareness for manmade climate change and its precarious consequences for upcoming generations. Nowadays, one major cause for rising global temperatures is attributed to the greenhouse gas CO2 that is largely generated by the combustion of fossil energy carriers for energy and transportation purposes.[1] To curb the greenhouse gas emissions and stabilize the average global temperature, almost the whole global community more or less compulsorily committed to the Paris Agreement in 2015 to realize a general transition toward clean energy production and usage, including the utilization of renewable energy resources, e.g., solar or wind energy, as well as emission-free transportation. Both energy sources rely on suitable energy conversion and storage technologies, either due to the intermittent energy production of renewable resources or due to clean mobility needs. Electrochemical energy storage in form of rechargeable batteries represents the most efficient solution for various kinds of applications including mobile and stationary storage systems.[2] In 1991, the commercialization of the lithium ion battery (LIB) technology set a new milestone in the history of energy storage[3] and stimulated the continuous development of LIB materials to achieve higher energy contents, increased electrochemical stability, and lower cost.[4] Today, LIBs are not only implemented in portable consumer electronics (cell phones, laptops, tablets, etc.), but have also been emerged as the most suitable technology for all types of electric vehicles (hybrid, plug-in hybrid, or fully battery-powered), stationary energy storage, and other electrically powered devices like power tools or drones.[4, 5]. The success of the LIB technology and its continuous development can be easily ascertained over the compound annual growth rate (CAGR) of 24% between 2008 and 2018.[6] Currently, it represents the fastest growth rate within the battery market and promotes increasing investments into rechargeable batteries, also beyond LIBs, and their applications.
Depending on the application purpose, different key requirements for batteries apply. While, for instance, consumer electronic devices demand the largest-possible amount of energy in the most compact form and, therefore, need a battery with high volumetric energy density,[5, 7] stationary energy storage systems mainly require low installation and lifetime operational cost when used for the regulation of intermittent energy production.[8] Commonly, batteries are characterized by cost and several key performance indicators (KPIs), including specific energy (Wh kg−1) and energy density (Wh L−1), specific power (W kg−1), and power density (W L−1), safety, cycle and calendar life, and charging time (=fast charging ability). In turn, batteries can be designed and chosen according to the requirements needed for a certain application purpose. Considering these different KPIs, battery development in the recent decades mainly focused on performance improvements and cost reduction (Figure 1).
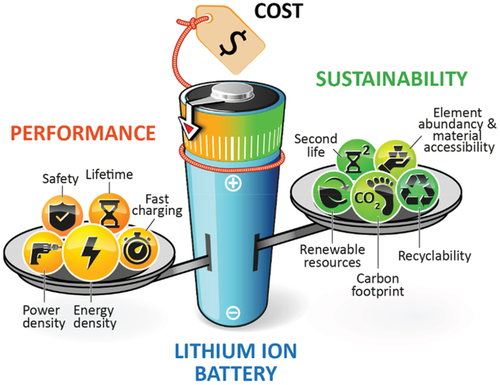
In the automotive sector, as one example, primary goals are to meet the highly demanding requirements for electric vehicle driving ranges of at least 500 km by a sufficient high energy density, to lower the charging times, and to decrease the battery pack cost <125 US$ kg−1, in order to achieve cost competitiveness with internal combustion engine vehicles.[5, 9] Besides, less attention has been paid so far on the environmental impact, i.e., on the sustainability of the battery.
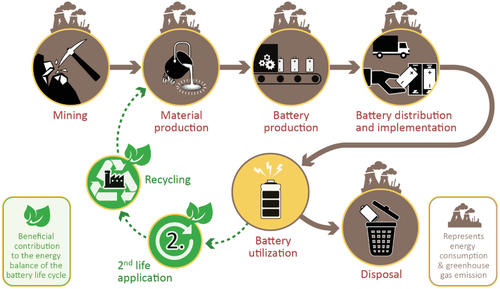
The above noted KPIs are mostly relevant for the usage of a battery cell, which represents, however, only one part of the whole battery life cycle. For a complete picture of the battery's environmental impact, one has to consider the entire battery life cycle. Besides utilization, it also includes raw material extraction and battery material production, cell and battery pack production, transportation, energy to charge batteries and regulate its condition, as well as possibilities for 2nd life usage, and cell disposal or recycling, as summarized in Figure 2.
Looking into the future, all markets based on the state-of-the-art (SOTA) LIB technology will have to face major challenges, as the LIB demand and production will strongly increase.[6] On the one hand, LIB material and cell production consume high amounts of energy, due to high-temperature furnace processes during material production (metal refinery, active material synthesis, etc.) and due to electrical energy-intensive processing steps during cell assembly (moisture control, electrode drying, etc.). Peters et al. showed with their recent review of several life cycle assessment datasets about LIB cells that the production of 1 Wh storage capacity requires a cumulative energy demand of 328 Wh and causes greenhouse gas emissions of 110 g CO2 equivalents on average.[10] On the other hand, the future supply situation of mandatory, especially critical battery raw materials is rather unknown.[11] The foreseen shortage of less abundant elements (e.g., cobalt (Co), nickel (Ni), and maybe also in the farther future, lithium (Li)) and limited mineral deposits of raw materials to only a few places in the world (e.g., natural graphite (NG), Co) impart certain battery materials with a strategic value.[11] Out of all cell constituents, Co is often considered as the most critical element regarding future cell production, as it is usually produced only as by-product of Ni and copper (Cu) mining. More than 50% of the worldwide produced Co origins from the Democratic Republic of the Congo, a country known for political tensions.[5, 11] From the US and European point of view, Co is classified as “critical” raw material for the European economy, along with NG that is mainly mined in China.[11, 12] In addition, raw material mining (e.g., Co, Ni, Cu, Al, Fe, and NG) is linked in some cases to heavy environmental pollution and social risks (e.g., child labor, corruption) due to minor or nonexisting labor regulations of the originating country.[13]
In order to obtain not only environmental benefits for the application of the final battery product, like emission-free transportation and higher efficiency of renewable energy resources, the whole battery life cycle (from “cradle to grave”) needs to become more sustainable to improve the overall energy balance including the carbon footprint and to enable advanced and independent production ways satisfying the growing energy storage market needs.
- a) sustainable material availability either due to a composition of highly abundant and easily accessible elements, or due to production possibilities based on renewable raw materials;
- b) significant benefit to the energy balance of the battery life cycle, e.g., prolonged battery cell usage in 2nd life applications, increased recycling ability, improved carbon footprint due to low-energy synthesis, and production processes.
The term “green” is often associated with less- or nontoxic materials. However, this subject is only selectively addressed, where necessary, as related content would exceed the scope and has been reported elsewhere.[14] Starting from the SOTA LIB technology, this review highlights recent proceedings of green alternatives for different parts of an LIB cell and for related technologies and processes. The major target is to evaluate the tradeoffs between environmental benefits and practical as well as economical limitations of the here presented green material and process approaches.
2 Green Materials and Concepts for Lithium Ion Batteries
The LIB has emerged to the most important electrochemical energy storage technology since its commercialization in 1991 by Sony.[4, 5] Market demand toward higher energy content and lower cost have primarily shaped the development of LIB cell materials and components within the physical, chemical, and technical limitations to more and more performance-optimized configurations. The current materials in SOTA LIB cells raise, however, issues regarding environmental and sustainable aspects (Figure 3), which might become showstoppers in the future, especially when considering a continuously growing demand for battery cells.
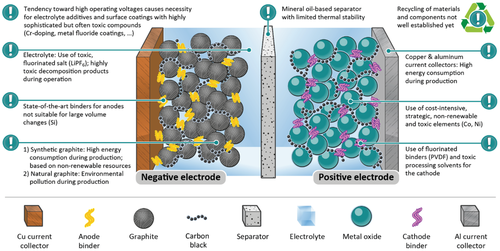
Main ecological challenges of the LIB are—among others—energy-intensive material production (current collectors, graphite, and metal oxides), fluorinated and toxic compounds (electrolyte, cathode binder, and metal oxides), costly electrode processing (nonaqueous solvents used for processing of cathodes), energy-intensive cell production, and low recycling rates, as illustrated in Figure 3. Against this background, we discuss in the following green material alternatives with focus on the different material classes (anodes, cathodes, electrolytes, and inactive components) and emphasize optimization approaches regarding the sustainability and the energy balance of SOTA LIBs.
2.1 Anode Materials
Carbonaceous materials and in particular graphitic carbons can be considered as the SOTA anode material in LIBs.[15] They have outstanding electrochemical characteristics, including a low and flat operating potential (close, but still sufficiently separated to the potential of Li metal), which in turn offers a high cell voltage, a relatively high specific capacity (372 mAh g−1), high durability in suitably composed carbonate-based electrolytes as well as low voltage hysteresis (=high voltage efficiency) and, therefore, high energy efficiency (=product of Coulombic efficiency and voltage efficiency).[16] In the last decades, the energy density of LIBs could also be continuously increased as a result of ongoing material improvements with respect to the anode, i.e., from coke-based anodes (soft carbon; 1st generation) to hard carbons and/or later on to graphite.[4, 17]
Graphitic carbons can be distinguished between artificial or synthetic graphites (SGs) and natural graphites (NGs). Even though graphite displays relatively low cost compared to SOTA cathode materials (e.g., layered transition metal oxides), there are huge cost differences between SGs (2018: ≈12–13 $ kg−1) and NGs (≈4–8 $ kg−1),[6] which results in an ongoing trend to replace SGs by NGs, as long as NG is still abundantly available. The cost differences are mainly due to the energy-intensive production of SGs and, further, due to the high abundance and high supply of NG, especially in China.[6] Currently, NG (≈35%) and SG (≈56%) display the major market shares of commercial LIB anode materials, while other materials only play a minor role (amorphous carbons (≈2%), lithium titanate (LTO, ≈3%), Si-based anode materials (≈3%)).[6] SGs display the highest utilization rate due to their applications in EV batteries, as they show outstandingly high levels of purity and a less fluctuating quality compared to NGs, which is important to fulfill the highly demanding lifetime requirements of advanced battery cells. Further, amorphous carbons and SGs are often used in a certain blend in order to optimize the power to energy ratio in the anode, which is of utmost importance to tailor thefast charging ability of the LIB cells incorporating those materials.[5]
SG-based materials can be differentiated between so-called “primary” and “secondary” type materials. The secondary type (“scrap”) is a byproduct of electrode manufacturing for the steel or aluminum (Al) industry.[18] The quality of this material strongly differs in dependence of the production batch and, thus, its basic properties (ash content, density, crystallite size, etc.). In contrast, primary SG is prepared for particular application and, thus, has well defined properties and high quality, suitable for defined battery applications.[18] The production of graphitic carbons mainly follows two different routes, i.e., either for SGs or NGs, as depicted in Figure 4. Each production process can be described by three major steps, including first, the pretreatment of selected carbon precursors for SGs (Figure 4a) or mining and treatment of the graphite ore for NGs (Figure 4b), second, either the graphitization procedure for SGs or further material processing for NGs, and third, the particle refinement step.[18, 19]
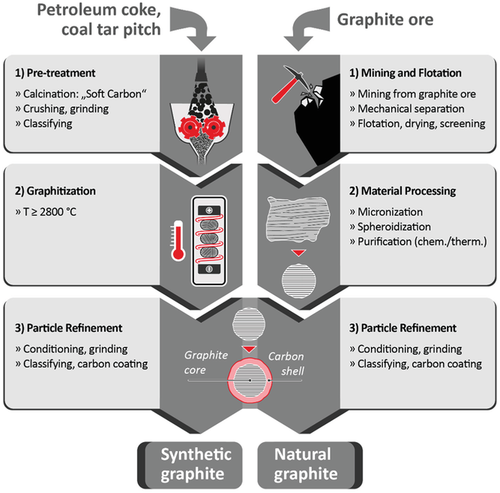
SGs are typically prepared from precursor materials such as petroleum coke, often mixed with coal-tar pitch, using a high temperature treatment (2500–3000 °C). In the first step, the precursor is transformed into an intermediate material by calcination at 800–1200 °C, i.e., into a soft carbon.[19] This material is further mechanically treated by crushing and grinding for adjustment of the particle size, shape and morphology, followed by classification of the particles (grading/sighting and weighing) according to their particle size.[19] The obtained soft carbon can be directly used for battery applications or is transformed into graphitic carbon. For the production of SG, the most important but also cost-intensive step (>25% of total production cost) is the graphitization at temperatures above 2800 °C. The whole graphitization procedure may take several days up to weeks including the cooling phases.[18, 19] In the final step, mechanical refinement of the graphite particles (grinding, classifying, carbon coating) is performed in order to optimize the final particle design with respect to its requirements (particle size, specific surface area, surface morphology, surface composition, etc.).[18, 19]
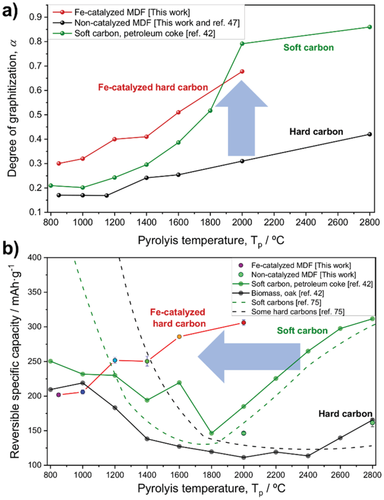
One prominent strategy to lower the graphitization temperature and, thus, high energy consumption and cost (≈10–12 kWh kg−1) is the use of certain metal additives (sometimes also called “catalysts,” though the additive—unlike a typical catalyst—is irreversibly converted into another species and remains incorporated in the active material), e.g., FeCl3 or Ni(NO3)2.[20] For example, Gomez-Martin et al. reported that the graphitization degree of biomass-derived carbons (MDF, medium-density fiberboard) can be strongly increased by addition of iron (Fe) catalysts (Figure 5a). In turn, a high reversible specific capacity can also be achieved at remarkably reduced pyrolysis temperature (Figure 5b). Overall, future comprehensive studies are mandatory to analyze the impact of different metal additives on the material properties and to evaluate the effect of additive impurities on the electrochemical performance in LIBs.
NG originates from organic material deposits subjected to a combination of high temperatures and heavy pressure over a long period of time. Economically exploitable deposits possess a ratio of graphite up to 20%. NG is classified as “critical” raw material by the European Union and by the United States, as the worldwide largest deposits are located in China. Production of NG starts with mining of the graphite ores by rock drilling or application of explosives, while the graphite flakes can be liberated by a combination of successive steps of crushing, milling, screening, tabling, flotation, magnetic separation, electrostatic separation, and leaching (Figure 4b).[19] NG is further treated by sophisticated milling and classification procedures in order to receive round-shaped particles. These processing steps are typically referred to as “micronization” and “spheroidization.” To achieve purity levels ≥99.0%, further (wet-)chemical and/or thermal or thermochemical treatments are necessary.[19] For this purpose, the graphitic materials can be treated by acid leaching, i.e., by using hydrofluoric acid (HF), hydrochloric acid (HCl), oxidizing acids (H2SO4 or HNO3), or mixtures thereof. Acid leaching is reported to be the most effective way to remove silicate impurities. Furthermore, purification of NG to very high chemical purity can also be obtained by heat treatment above 2000 °C. Another thermochemical method, called “roasting”, which includes roasting (=thermal treatment in presence of an alkali reagent such as caustic soda at ≈250–1000 °C), water washing, and acid leaching, can also effectively eliminate silicate and sulfide impurities.[19] Even though this procedure is widely used as industrial technique (e.g., in China) to refine fine graphite, it is considered to cause huge environmental pollution, especially in wastewater. Therefore, alternative techniques that can produce high-grade (up to 99.99% C) graphite by energy-efficient and environmentally benign procedures, such as, e.g., microwave treatment, are currently studied.[19] The final step of NG production is related to particle refinement and includes in particular a carbon coating process.[21] Overall, production of NG in China is seen critical with respect to insufficient monitoring as well as missing measures to avoid high levels of environmental and air pollution.[22]
In order to achieve improved material sustainability for LIB application, there is a growing interest to develop synthetic carbonaceous anode materials by heat treatment of biomass precursors or industrial waste, according to the processing route in Figure 4a.[23] In recent years, various publications have reported on the preparation of carbons and their application as anodes in LIBs.[24] For material synthesis, not only different precursor materials have been used, but also different preparation conditions, such as different temperature ranges for carbonization and/or graphitization. These materials are of high interest due to their often favorable structures, the high abundance and low cost of the precursors, as well as the easy electrode processing using aqueous routes. A broad variety of biomass precursors has been studied so far, including, e.g., mushrooms,[24] apple waste,[25] sisal fibers,[24] coffee shells,[24] rice husks[24] and rice straw,[24] pinecone hull,[24] banana fibers or peels,[24, 26] cherry stones,[24] tea leaves,[24] coconut shells,[24] peanut shells,[24] shaddock peel (pomelo),[27] sterculia scaphigera,[24] cotton wool,[24] potato starch,[24] sweet potatoes,[24] microalgae,[24] algal blooms,[28] ox horn,[24] or even human hair.[24] Furthermore, there are numerous examples for utilization of waste products as precursors for carbon active material preparation including waste tire rubber,[24] plastic bag waste,[24] bamboo chopsticks,[24] or unburned carbon concentrates[24] as industrial waste. Major challenges for the application of these sustainable materials are related to the reproducibility of different material charges from renewable sources. Further, the industrial applicability of this approach is questionable, as very large material amounts are needed, considering the currently exponentially growing market for LIB raw materials.[6]
In terms of further energy density improvements by anode material measures, there is general agreement, that the most promising strategy will be a stepwise addition of silicon (Si) to the graphite-based anode material, i.e., in form of Si or SiOx (nano-) particles. However, the addition of Si is related to various challenges of Si materials limiting the LIB cell's cycle life, including the large volume changes, contact losses, and mechanical electrode degradation, instability of the solid electrolyte interphase (SEI), and losses of active Li during lithiation/delithiation, which has been comprehensively discussed in various research and review papers.[29] Currently, Si is only used in few commercial cells (e.g., from Panasonic), i.e., in form of SiOx, while typically small amounts of up to ≈8 wt% are applied.[5, 30] In terms of the manufacturing of Si-based materials, it has to be considered that even though certain precursor materials are highly abundant, such as SiO/SiO2 precursors, there is also a growing research interest to prepare Si from biomass (e.g., rice husk[24, 31]) or waste products (e.g., Si wafers[32]). Various research strategies are currently under discussion for enabling high amounts of Si (≥10 wt%) in LIB cells, while still offering a sufficient cycle life. Among various approaches, the most promising ones include the development of advanced silicon/graphite blends (i.e., offering a high tap density and low specific surface area), an advanced electrode formulation (e.g., by novel binder concepts; see Section 2.4), an improved electrolyte formulation for effective SEI formation as well as suitable pretreatment and/or prelithiation concepts.[33]
2.2 Cathode Materials
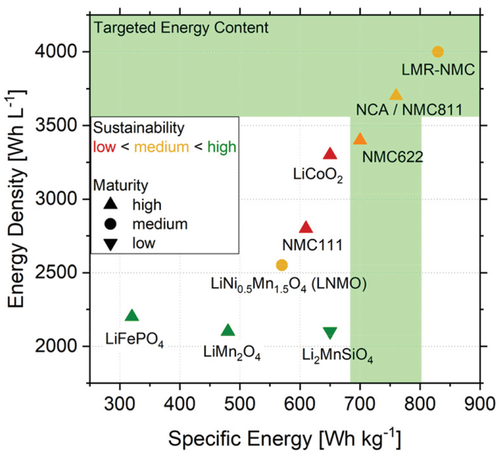
Layered metal oxides with the generic formula of LiMO2 and varying metal composition (with M = Ni, Mn, Co, Al, i.e., LiNi1−x–y–zMnxCoyAlzO2, commonly abbreviated as NMC or NCA) constitute the current SOTA cathode materials of most LIB cells. Most of their key material properties, such as practical specific capacity (140–210 mAh g−1), redox potential (≈3.7 V vs Li|Li+), material density (4.5–5.1 g cm−3), and rate capability, outperform other practicable cathode material candidates.[34] Li/Mn-rich layered oxide cathodes, i.e., Li1+xM1−xO2(with x = 0.15–0.20) and M = Ni, Mn, Co; abbreviated as LMR-NMC) offer very high initial practical capacities (200–300 mAh g−1),[35] however suffer from insufficient cycle life as well as lower rate capability, besides other minor shortcomings (lower redox potential, material density compared to NMC). An overview of various commercial and alternative cathode materials for application in LIBs, including the evaluation of their sustainability and technological maturity, is shown in Figure 6.
Critical raw materials of LIBs, such as Li, Co, and Ni, are nearly all linked to the cathode material, which also accounts for roughly one third the cost of NMC-based LIBs.[5] In addition, both Co and Ni are classified as carcinogenic, mutagenic, and toxic to reproduction (CMR).[36] Above all, Co is the main cost driver and further raises moral and environmental concerns, originating from partially questionable mining conditions in central Africa, allegedly involving child labor.[37] The insecure supply situation of Co is also reflected in pronounced price fluctuations.[11] While material producers, cell makers, and original equipment manufacturers (OEMs) for electronics and automotive applications are aware of ethical implications of Co mining, most companies take minimal to no action with regard to supply chain with due diligence in order to improve the “moral footprint” of their batteries.[37] A feasible approach for solving this problem could lie in the establishment of a sustainability label for batteries, comparable to existing labels for sustainable seafood or wood (e.g., the marine or forest stewardship council, MSC/FSC).
Despite the criticality of Co, LiCoO2 (LCO), which purely relies on Co as the redox-active transition metal, is still widely used for small-sized batteries in portable consumer electronics (<50 Wh, e.g., smart phones, tablet computers). This is mostly due to its high material density of 5.1 g cm−3, good rate capability, and cycle life. Also, the absolute Co demand for a small-sized battery is relatively low, so that the benefits of a more compact battery often outweigh its higher cost. Despite their widespread utilization and high Co concentrations, most consumer cells are not being recycled to date.[38] The demand for LIBs with higher energy densities and the high cost of Co lead to continuously increasing Ni and decreasing Co contents in layered cathodes, i.e., from LiNi1/3Mn1/3Co1/3O2 (NMC-111) to currently LiNi0.8Mn0.1Co0.1O2 (NMC-811).[34] First cell manufacturers are currently introducing NMC-811 and NCA with Ni contents > 90% into their batteries, while NMC cathode formulations with Ni contents above 80% are currently (early year 2020) being investigated.[34] The high-Ni approach has so far lead to increasing energy contents at lower costs (in $ kWh−1),[5, 39] it concurrently lowers cycle life and safety of the cathode material though.[38] Among layered oxides, there is growing interest in the realization of low-Co[40]or Co-free[41] cathode formulations with high capacities based on LiNixMyO2 (x ≥ 0.8, M = Al, Mn, Mg, x + y = 1), revisiting efforts of Ni-rich/Co-free layered cathodes in the early 1990s.[42] Recent progress in that direction is promising and even puts the necessity of Co within high-Ni cathodes (>90% Ni) into question.[41] In order to improve the long-term cycling stability of layered cathodes materials, the synthesis of single crystal materials has been more intensively explored as of late. In comparison to the typically polycrystalline cathodes, which are synthesized via a coprecipitation route, single crystal cathode materials, which are typically prepared in a salt flux, consist of larger particles with lower specific surface area.[43] Likely due to the reduced area of the material/electrolyte-interphase, these single crystal compounds exhibit a significantly lower tendency for parasitic reactions, such as electrolyte oxidation or gas release and thus show a longer cycle life compared to the usual polycrystalline material. Despite their slightly lower capacities,[44] single crystal cathodes could play a role in long-life battery applications.
In the light of possible raw material scarcity, Co-free cathode chemistries such as the high-voltage spinel LiNi0.5Mn1.5O4 (LNMO) as well as certain Li/Mn-rich oxide compositions, e.g., Li1.2Ni0.2Mn0.6O2,[45] or disordered rock salt phases such as Li4Mn2O5,[46] gain attention as potential alternatives to current cathode materials. Element availability is especially important for larger-scale mobile applications such as electromobility (20–100 kWh) or grid storage, where considerable absolute amounts of raw materials in the kg-range are required for one battery pack. Up to now, the utilization of Co-free cathode materials has been hindered owing to insufficient stability of the battery electrolyte under high voltage conditions (LNMO) or poor capacity retention and rate capability of Li-excess materials, which typically involve an anionic redox activity contribution.[47]
Phospho-olivine-type cathode materials such as LiFePO4 (LFP) or LiFe1−xMnxPO4 (LFMP) with 0 ≤ x ≤ 1 are considered as ecofriendly since they consist solely of abundant elements.[48] The high structural stability of the polyanionic phosphate-network and the relatively low operational voltage window, which averts unwanted parasitic reactions of the battery electrolyte, allow long cycle and shelf life for LFP-based LIBs. However, they fail to meet the requirements of the automotive industry in terms of energy content at the material level (min. 680 Wh kg−1 and 3600 Wh L−1, see green bands in Figure 6)[34] and are thus preferably used in stationary energy storage systems as well as electric buses and other heavy duty automotive applications, such as trucks. Their long cycle life and low raw material costs, however, could lead to a low total cost of ownership (TCO) of LFMP-based batteries compared to other high-energy chemistries, especially since their currently high production cost is expected to decrease in the coming years.[6] The isostructural analogues LiMPO4 with M = Co, Ni, which involve Co or Ni as redox-active metal, promise higher energy densities due to their higher redox plateau at a similar capacity. However, this material class not only suffers from low ecofriendliness, but also limited practical feasibility due to their very low electronic conductivity and insufficient compatibility with current electrolytes.[48]
A diverse field of polyanionic frameworks beyond phosphates exists, which include borates, sulfates, and silicates and involves a variety of redox-active central atoms (e.g., V, Mn, Fe, Co, Ni, Mo).[34] The theoretical redox potential of the cathode is determined by both anion and cation chemistry. In some cases, the potential may be slightly increased by the introduction of electron withdrawing anions (e.g., OH− or F−), like in the case of fluorophosphates (PO4F)4− or fluorosulfates (SO4F)3−.[49] The attainable capacity of a cathode material is largely determined by the amount of extractable Li per formula mass (i.e., molecular weight). In comparison to layered transition metal oxides (LiMO2), the formula mass of polyanionic host structures is relatively high, which results in low theoretical capacities for compounds with only one Li+ ion. This disadvantage can be compensated for compounds with multiple Li+ ions per formula unit. Li metal silicates (Li2MSiO4, with M = Mn, Fe)[50] show for instance several favorable characteristics: they contain only low-cost, abundant, and ecofriendly elements and contain two Li ions per formula unit, which in turn result in high theoretical capacities of ≈330 mAh g−1,[34] of which more than half could already be experimentally realized.[50] However, Li metal silicates, like many polyanionic materials, exhibit very low electronic conductivities, which are commonly tackled by nanosized active particles coated with relatively high amounts of carbon. This approach, however, lowers the attainable energy density at the electrode level due to the lower packing density and lower ratio between active and inactive materials. Also, the insufficient transport properties for Li+ ions and poor structural stability and thus low capacity retention of Li2MSiO4 have thus far hindered its commercial use.[50, 51]
Nonceramic cathode chemistries such as organic cathode materials (see Section 4.2.5) constitute a broad research field with many candidate materials (e.g., benzoquinone or dilithium rhodizonate), which are free of critical transition metals. Due to the mostly low material density, low electronic conductivity, and relatively low redox potentials of organic cathodes, they are not likely to qualify for mobile, but rather for stationary applications, if sufficient cycling stability can be achieved.[52]
2.3 Liquid Electrolytes and Porous Separators
The electrolyte has the challenging role to transfer Li+ ions between the positive and the negative electrode of an LIB. During battery cell operation, the electrolyte is not only in direct contact with a highly reductive anode, a highly oxidative cathode, the separator, and other inactive materials; it must also ensure an efficient Li+ ion transport over a wide temperature range. The electrolyte therefore needs to fulfill several requirements including high ionic and low electronic conductivity, a wide electrochemical window, and a high chemical as well as thermal stability. The basis of the SOTA electrolyte in LIB cells consists nowadays, as since market introduction, of carbonate-based solvents and lithium hexafluorophosphate (LiPF6) as salt.[53] This electrolyte composition does not perfectly fulfill all of the partially contradicting requirements, but it exhibits a good compromise. A nonaqueous mixture of linear carbonates and ethylene carbonate (EC), as member of the cyclic carbonates, has proved to be reliable for LIB cells. On the one hand, EC offers a high relative dielectric permittivity necessary for good solubility of the Li salt and on the other hand, EC plays a major role in formation of an effective SEI, which forms on the anode and is a mandatory protection layer against further electrolyte decomposition. Due to the high melting point of EC (36.4 °C), the addition of linear carbonates is needed to obtain a liquid electrolyte with a high ion mobility.[54] From an environmental point of view, carbonates possess a low ecotoxicity, are available in large amounts, and the synthesis from renewable resources, e.g., from alcohols and urea, is possible.[55] Regarding safety and recycling aspects, however, carbonate-based electrolytes are, like other organic solvents, volatile and flammable resulting in a limited thermal stability, hazard risks in abuse cases, and a low recyclability. Recent developments are going into the direction of fluorinated solvents and additives, which will bring in better performance at the expense of environmental friendliness.[56] Another research strategy is based on EC-free electrolytes, which are able to operate with high-voltage cathode materials (see Section 2.2), to develop novel high-energy battery technologies. Although EC is considered as a crucial component for the formation of a stable SEI, fluorinated additives such as fluoroethylene carbonate show promising results in EC-free electrolyte formulations in terms of cycling performance and energy efficiency.[57] Since most EC-free electrolyte mixtures contain either fluorinated additives, organosulfur compounds (such as sulfolane) or rely on the use of costly Li salts, EC-free electrolytes are expected to have a larger environmental impact compared to EC-based electrolytes when used in LIBs and further still struggle with inferior conductivities.[58] Room temperature ionic liquids (RTILs) are considered as alternative for organic solvent based electrolytes as they exhibit high thermal stability, low flammability, low volatility, and a wide electrochemical stability window (ESW).[59] While former attributes can enhance the battery cell safety, a few ILs with an ESW up to 6 V might be a suitable choice as electrolyte solvents for high-voltage cell chemistries. Due to their low volatility, ILs are very often (but not generally) classified as “green solvents”[60] and might be better recyclable compared to carbonates leading to an improved electrolyte recovery rate of spent battery cells.[61] Several studies about the ecotoxicity of ILs, however, highlight major environmental concerns of certain IL combinations, e.g., 1-butyl-1-methylpyrrolidinium bis(trifluoromethanesulfonyl)imide (PYR1,4TFSI).[62] The toxic effect and other properties of an IL strongly depend on the chemical nature of the actually used cation and anion, so that generalizations about ILs are often misleading and even biodegradable combinations are possible, although so far not for battery applications.[63]
The other major electrolyte component is the Li salt. Similar to electrolyte solvents, the Li salt faces a quite long list of requirements for use in LIB cells. Among the commercially available Li salts, lithium hexafluorophosphate (LiPF6) represents the SOTA Li salt because it offers a combination of well-balanced properties with concomitant compromises and restrictions. While LiPF6 shows a good solubility, conductivity, ion mobility, and cycling performance as well as a sufficient electrochemical stability for current cathode materials (<4.5 V vs Li|Li+), the drawbacks of LiPF6 are its high toxicity, low thermal and chemical stability, and that it easily hydrolyzes with trace amounts of water.[64] The latter properties lead to the formation of the highly active and toxic hydrogen fluoride (HF), which can further react with other electrolyte components to form different highly toxic compounds,[65] for instance alkyl fluorophosphonates, with increasing age of the LIB cell.[54, 66] Although HF formation of LiPF6 is considered as one of the major drawbacks, it is crucial for the formation of a fluorine containing passivation layer on the Al current collector that protects the Al surface against anodic dissolution,[67] underlining the contradicting requirements for Li salts. Not only because of safety aspects, but also to increase the recyclability and to get rid of the high cost LiPF6, efforts were made to find a suitable fluorine-free substitute for the PF6− anion. While LiClO4 reacts highly oxidative and unsafe at elevated temperatures and was therefore ruled out by the industry since 1970s,[54] other classes of Li salts such as the nitrile-based lithium 4,5-dicyano-1,2,3-triazolate (LiDCTA)[68] and the quite popular boron-based lithium bis(oxalato)borate (LiBOB)[69] were evaluated in LIB cells. Fluorine-free (“green”) Li salts are, however, in most cases not stable against oxidation on cathode electrode materials at potentials >4.2 V versus Li|Li+ and therefore, along with other drawbacks, not suitable for SOTA high-energy LIB cells.[53] Since LiBOB is compatible with LiFePO4, it is still interesting for sustainable Fe-based and completely fluorine-free LIB cell chemistries and might be considered for stationary and other low energy density application areas.[70]
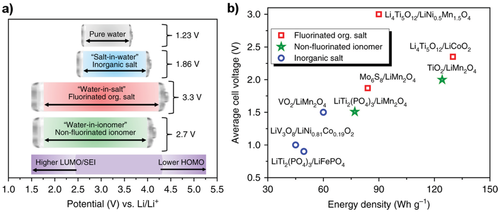
Recent works on water-based electrolytes present opportunities regarding safety, toxicity, and recyclability for LIB cells. The challenge to expand the narrow electrochemical window of water (1.23 V) to a practicable operation range sufficient for high-voltage positive (NMC, LCO, LNMO) and moderately negative (LTO, Mo6S8) electrode materials can be achieved through the reduction of noncoordinating (“free”) H2O molecules.[71] Therefore, the amount of water needs to be reduced to obtain highly concentrated, so-called “water-in-salt,”[72] electrolytes. The free water content can be also reduced using room temperature Li salt hydrate melts.[73] All these electrolyte approaches contain water as a green solvent, but the high amounts of Li salts makes them currently less desirable from an economic and ecological perspective. A new nontoxic and more sustainable LIB aqueous electrolyte concept comprises fluorine-free ionomers with a low salt concentration. He et al. developed recently a so-called “water-in-ionomer” gel electrolyte based on lithium polyacrylic acid (LiPAA) and water that offers a relatively wide ESW of 2.7 V and can keep pace with performances of highly concentrated “water-in-salt” LIB aqueous electrolytes containing fluorinated salts (Figure 7).[74] This novel material concept can open a path for fluorine-free aqueous electrolytes, which can significantly improve carbon footprint of battery cells.
The use of liquid electrolytes makes a separator essential for a safe and well-performing LIB cells. Commercially available microporous mono- or multilayer membranes made of low-cost, oil-derived polyolefines (e.g., polyethylene, polypropylene) are generally used as SOTA separators in LIB cells,[75] often together with a ceramic component for enhancing the safety.[76] Sustainable concepts for Li ion separators focus on polymer matrices consisting of biocompatible and ecofriendly materials from renewable resources. Cellulose, the most abundant naturally occurring biopolymer,[77] takes a leading position in the field of biogenic separator materials. Fibers of cellulose can enhance the mechanical strength, the electrolyte wettability, and the shape retention of the separator at elevated temperatures when used as main component of nonwoven composite separators (Figure 8).[78]
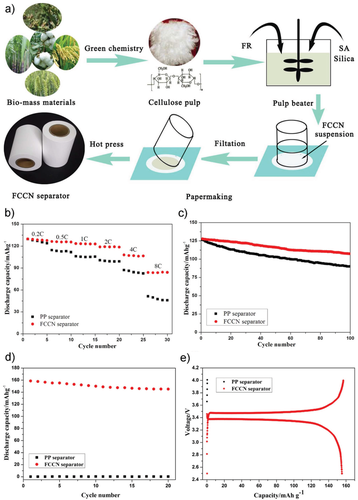
Further, cellulose-based separators can be produced via a cheap and environmentally friendly water-based papermaking processes.[79] Nanosized fibers of nanofibrillated cellulose are usable as green additive to control the pore size distribution and the wettability of composite separators.[80] Additionally, the cellulose biopolymer backbone is easily modifiable and derivatives like methyl cellulose represent an interesting, water-processable material class for ecological gel–polymer membrane separators.[81] Beside of cellulose, also other biopolymers exhibit interesting properties for battery separators. Nanofiber membranes made of chitin, the second most abundant natural polymer, show superior results in both, Li and sodium (Na) metal cells, compared to polypropylene-based separators especially at elevated temperatures.[82] Silk fibroin can be prepared to membrane films, sponges, and nonwoven membranes through electrospinning, which were compared as separator materials in LiFePO4 cells.[83] Finally, nonwoven separators based on alginate were proven to be a superior choice for high-voltage (LNMO) LIB cells because alginate offers a strong polymer backbone, a good electrochemical stability, and well-coordinating carboxyl groups.[84]
While options for greener liquid electrolytes might be limited due to the high and sometimes contradicting requirements, separators for LIB cells consisting of optimized, highly abundant, water-processable biopolymers can pave the way to more sustainable battery cells. The next evolution of more sustainable and safer electrolytes compared to the SOTA electrolytes could be achieved by combining the advantages of biogenic polymer matrices with immobilized Li salts resulting in solid polymer electrolytes, discussed in detail in Section 3.1.
2.4 Inactive Materials
Beside of the active anode and cathode materials, classical LIB composite electrodes consist of polymer binder, conducting additive(s), and current collector. These compounds are commonly categorized as “inactive” electrode materials since they do not contribute to the energy storage capacity, although they fulfill diverse crucial tasks within the battery cell. Depending on the cell format, the active materials and the application field, the share of inactive materials may vary, but it represents in all cases an important factor for the overall energy density and cost as well as the carbon footprint of the LIB cell.
Polymeric binders of LIB electrodes have to ensure a sufficient cohesion between the active material particles, the conductive additive, and the current collector. Furthermore, they should be electrochemically and thermally stable, not hinder ion or electron mobility, not dissolve or swell in the liquid electrolyte. Lastly, the binder polymers need to be soluble/dispersible in an organic or aqueous processing solvent and lead to an optimized viscosity of the resulting electrode “slurry” or “paste”. Two major driving forces for binder innovations are i) the increase of the electrode long-term performance, which is determined by binder distribution[85] and binder chemistry, and ii) the reduction of processing cost. While commercial graphite-based LIB anodes are prepared with carboxymethyl cellulose (CMC), a bio-derived polymer, and styrene–butadiene rubber (SBR) in an aqueous and therefore quite resource-conserving production routine today, conventional high-energy cathodes (e.g., Ni-rich NMC) still rely in most cases on a polyvinylidene difluoride (PVdF) binder processed with N-methyl-2-pyrrolidone (NMP), a toxic and volatile organic solvent that needs to be recovered.[86] Wood et al. calculated for an LIB model cell that an aqueous electrode processing is 26 times less expensive than the conventional NMP-based electrode production mostly due to the cost savings on the omission of the energy-intensive solvent recovery process step.[87] Although a changeover to a water-based cathode production is desirable from an ecological and economic perspective, several obstacles have to overcome. Severe issues are that the binder has to withstand high oxidation potentials and that conventional NMC cathode material easily hydrolyzes resulting in Li+ leaching and an increased pH value of the aqueous processing solution, which leads to the corrosion of the Al current collector. Loeffler et al. showed that for the latter problem the addition of heat-curable polyurethanes (PU) to a CMC-based binder can greatly reduce the Al corrosion because PU encapsulates the positive active material particles and suppresses their hydrolyzation in water.[88] Guar gum, a member of the natural polysaccharides, tightly covers the active particle surface and enhances the cycling performance significantly, when used as binder for Li-rich materials.[89] Further, alginate biopolymer[90] and cellulose[91] have been used as binder for cathodes as well. Except for LiFePO4, where considerable research work was made to develop water-soluble and mostly naturally derived binders,[92] the scientific efforts for other cathode binder systems are less intensive since positive active materials usually do not exhibit large shape changes during cycling.[93]
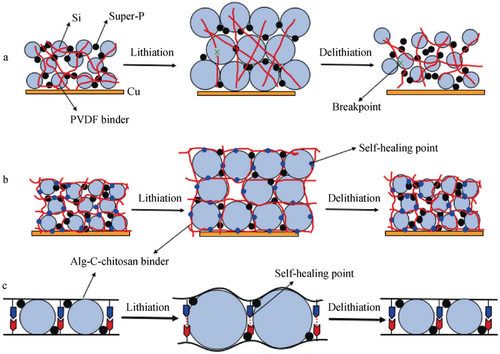
The development of binders for the anode shows, however, a quite different trend. Since the anode production early adopted an aqueous process with SBR and CMC as binder,[94] a majority of research activities on binders concentrate on Si as next generation high-energy anode material. Si exhibits huge volume changes which result into a rapid fade of the cell capacity upon continuous cycling due to various degradation mechanisms such as the iterated formation of an unstable SEI, the pulverization of the Si particles, and the disintegration of the whole composite electrode. The latter issues are addressed as key objective of the recent binder investigations since the binder maintains the structural integrity of the composite electrode. CMC was the first naturally derived polymer leading to an improved cycle life of Si anodes.[93] Spectroscopic studies suggest that a strong, physical and chemical interaction of the CMC polymers with the SiO2 surface layer plays a major role for the excellent binding properties of CMC.[95] The understanding of the CMC binder effects paved the way for an exploration of water-soluble polymers with carboxyl-, hydroxyl-, amine-, and other functional groups. Especially natural and bio-derived polymers are in focus of the current binder research because they offer the desired functional moieties and are often producible with low cost from renewable resources.[96] For LIB anodes, binders based on alginate,[97] cellulose,[98] chitosan,[99] lignin,[100] starch,[101] poly-γ-glutamate,[102] protein-,[103] or even DNA-derivatives[104] have been investigated and reviewed in more detail elsewhere.[93, 105] Out of the various approaches to design more sustainable anode binders, we want to highlight two binder systems that offer encouraging concepts to tackle the major challenge of the Si volume changes. Inspired by the natural wetness-resistant adhesion properties of mussels, Ryou et al. developed a new alginate-based binder equipped with catechol moieties, which can anchor strongly on the surface of Si particle even in liquid environments. In combination with the high rigidity of the alginate polymer backbone, a markedly improved capacity retention of Si-based anodes was achieved.[106] Wu et al.[107] were able to transfer the self-healing concept, first described for Si anodes by Wang et al.,[108] to a low-cost, water-soluble binder system incorporating alginate and carboxymethyl chitosan. While the PVdF binder system is not able to withstand the volume expansion of the Si particles during lithiation leading to a disintegration of the polymer network (Figure 9a), the alginate–chitosan binder can reestablish the original electrode structure after Si delithiation (Figure 9b). The self-healing effect is based on the electrostatic interactions between carboxylate (COO−) groups of alginate and protonated amine (NH3+) groups of carboxymethyl chitosan that restore the polymer scaffold structure after the volume expansion of the Si particles (Figure 9c).
Established conductive additives in commercial LIB electrodes are pristine carbon blacks synthesized by partial combustion or pyrolysis of hydrocarbon precursors from fossil resources.[109] Their main task is to establish an electronically conductive and porous network between all active particles and the current collector for high electronic conductivity and good electrolyte uptake.[110] A recent study about polysaccharide-derived mesoporous carbons shows that new conductive additives cannot only be produced from renewable resources but also lead to an improved cell performance at high charging rates due to optimized electrolyte wetting properties.[111] A concept to accommodate the large volume change of Si is to use nanostructured carbons as conductive matrix material, which, however, often involves expensive starting materials and elaborated synthesis procedures. Yao et al. developed a simple process to turn natural nanostructured chitin-protein fibers of crab shells into hollow carbon nanofibers suitable for Si and sulfur (S) active materials and hence opened a more sustainable production route for nanosized carbon structures.[112] Ongoing development on the green synthesis of graphene nanosheets[113] makes this material an interesting candidate as LIB conductive additive. Even with a lower fraction of graphene additive compared to conventional carbon additives, Su et al. demonstrated improved charge and discharge performances of graphene-based LiFePO4 cathodes.[114] Further research on conductive additives base on carbon nanotubes (CNT),[115] carbon nanofibers (CNF),[116] and nanosized graphite particles.[117]
A further big step toward more sustainable LIB electrodes could be a suitable replacement or even better the omission of the metal current collectors. While a Cu foil provides mechanical support and enables the current flow to the negative composite electrode of commercial LIBs, an Al foil fulfills these tasks on the positive side. The production of Cu and especially Al is, however, linked to high energy consumption and causes large amounts of CO2 emissions.[118] Both Cu and Al may also suffer from current collector dissolution under certain operation conditions and with certain cell chemistries.[119] Based on green-synthesized 3D structured nano carbons (graphene, nanotubes, nanofibers, etc.),[120] freestanding current collector-free LIB electrodes could make a tremendous beneficial contribution to the overall energy balance of the LIB cell.[121] Other flexible electrode substrates like conducting polymers, papers, or textiles might be also interesting light and green substitutes to metal current collectors, albeit all metal-free options still suffer from a low mechanical strength and have reached just early stages of development.[122]
While green materials for high-energy active materials might seem limited, our brief overview on alternative options regarding inactive components of an LIB cell points at enormous opportunities for more sustainable inactive cell materials.
2.5 Electrode Processing
The production of an LIB can be divided into five main sequences: i) electrode production, ii) electrolyte formulation, iii) cell production, iv) cell conditioning, and v) assembly of the cells. The LIB production process is thoroughly covered elsewhere.[86] Here, we will focus on the first part, electrode production, which is strongly affected by the properties of the applied battery materials and has significant potential for optimization with regard to environmental aspects. Electrode processing includes four major steps: i) mixing of the electrode slurry/paste, ii) coating, iii) drying, and iv) calendaring. While the preparation of commercial LIB anodes already takes place using a water-based electrode dispersion today (see Section 2.4), the biggest ecological challenges emerge from the cathode production route involving the toxic process solvent N-methyl-2-pyrrolidone (NMP).[123] The changeover from an NMP-based to an aqueous electrode production process could not only reduce the energy consumption tremendously due to a simplified process without the otherwise mandatory solvent recovery procedure for NMP. The switch to an aqueous processing route could also lead to cost reduction of over 90%, as shown by a cost study of Wood et. al.[87] Therefore, high efforts are made to solve the challenges of an aqueous cathode production, which are on one hand attributed to side reactions of the moisture sensitive high-energy cathode materials (e.g., Ni-rich materials) such as proton-Li+ exchange, thus delithiation of the active material,[124] and pH increase of the aqueous dispersion leading to a dissolution of the Al current collector.[125] On the other hand, the high capillary forces that arise when a water-based dispersion is used for the production of a composite electrode result in the formation of cracks when the electrode thickness becomes too large.[126] While the former is tackled by the addition of an acid (e.g., phosphoric acid) to lower the pH value of the dispersion,[127] the latter challenge of crack formation could be solved with the addition of small isopropyl alcohol quantities to the electrode slurry.[128] A recent study shows that the aforementioned obstacles of moisture sensitive cathode materials (e.g., Ni-rich NMCs) seem to be less severe for the aqueous processing of industry-related, larger pouch cells, which offer almost the same performance compared to NMP-processed cells.[129] An optimized slurry formulation including a suitable binder or binder combination as well as fast processing with minimized water content and contact time between the active materials and water will presumably lead to the aqueous production of Ni-rich cathode materials on an industrial scale.[126]
LiFePO4-based cathodes can be manufactured with a water-based preparation method on a large scale,[130] do not contain critical metals (Co, Ni) and thus represent a potentially vastly environmentally friendlier option, when lower energy densities are acceptable for the specific application.
Ecological and cost optimization of the electrode coating process is directly connected with the drying step. The focus of recent R&D efforts lies on the decrease of energy consumption and cost for drying. Reducing the solvent content of the dispersion or a replacement of the coating process of a wet slurry could distinctly reduce or even avoid the necessary drying time of the electrodes. An interesting approach is the replacement of the typically used slot die coating process by a continuous extruder technique to operate electrode dispersions with high solid content, which could reduce the electrode drying time enormously.[131] Further new approaches are based on novel solvent-free ways to apply the composite electrode coating with completely different coating techniques, e.g., dry powder spray or electron beam methods,[132] to avoid any usage of process solvents and energy-intensive drying steps. However, since the above mentioned methods are just at early research stages, much engineering development effort is heading toward the implementation of alternative, efficient, low-energy drying techniques into the LIB cell production process. In conventional LIB electrode production lines, convective dryers are used with different lengths depending on the electrode production speed and other factors. To shorten the dryer length and thus reducing the energy consumption and production cost, other physical drying methods such as infrared (IR), microwave,[133] or laser techniques[134] are currently under development. While the application of microwave and laser methods are still in a lab experimental status, IR drying shows the most advanced progress and has been utilized for fast heating sections at pilot scale and first industrial coating lines usually in combination with convective drying.[86]
The energy consumption to establish a moisture free atmosphere that is necessary for cell assembly due to the moisture-sensitive LIB electrode and electrolyte materials remains a major ecological and economic problem that is hardly to circumvent. A study of Ahmed et al. shows that the energy demand for the dry room is directly correlated with the mass of air flowing through the dry room.[135] Therefore, smaller dry room space with automated cell manufacturing could make a significant contribution to a lower overall energy consumption of the LIB cell production.
In summary, a greener LIB electrode production can be reached by avoiding toxic processing solvents and in particular, by reducing the overall energy consumption. Even though making all processing steps more efficient and less energy-intensive is the most important goal, using renewable energy resources, as announced by some LIB cell producers, i.e., Tesla Giga factory[136] or Northvolt,[137] could complete the picture of a more sustainable battery cell production.
3 Lithium Metal Cells
Compared to even advanced lithium ion chemistries, an important step toward further enhancement of specific energy is offered by the use of Li metal anodes.[4, 138] Metallic Li was studied intensively for rechargeable batteries, but was moved out of focus because of critical drawbacks.[3, 139] Li metal suffers from severe safety issues mostly related to highly reactive Li deposits (so-called “high surface area Li (HSAL)” or “dendritic Li”), which form during cycling.[140] Nevertheless, when combined with a transition metal cathode (from an LIB), resulting in a lithium metal battery (LMB), an increase in energy content of 40–50% was predicted to be achievable.[7, 141] This promise led to a new revival of the LMB research. In this review, Li metal is assessed with regard to its controversial position in more environmentally friendly and sustainable energy storage systems. In modern SOTA LIB cells (18650-type, NCA || graphite; values can be found in Table S1 in the Supporting Information) a share of ≈3 wt% are Li (as Li+ ions) as part of the cathode and the electrolyte salt compared to the other elements. In comparison, an LMB cell containing solely Li metal as anode active material (100% excess of Li compared to the active Li content in the cathode material), would comprise a total Li share ≈7 wt% (see Table S1 in the Supporting Information for details). Cell technologies with a Li metal anode will therefore further increase the necessity of higher Li mining rates.[142] The big leap to high-energy contents, nevertheless, makes the LMB an interesting candidate for mobile applications, if the safety concerns can be overcome.[143]
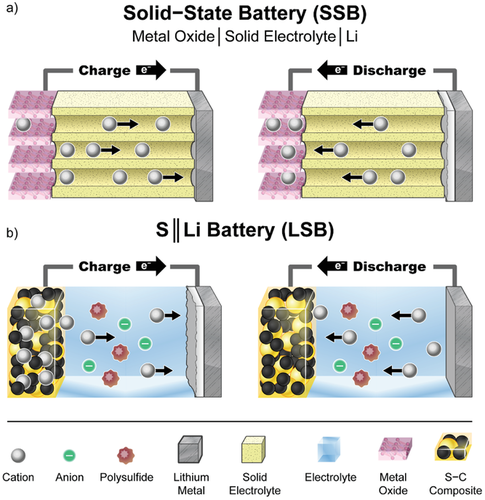
We constrain this review to the, in our view, most advanced Li metal technologies today which are depicted in Figure 10: a) solid-state battery (SSB) based on polymeric or ceramic electrolytes, and b) S || Li battery (LSB) systems. Solid electrolytes are expected to be an enabler for LMBs to be commercialized since solid-state electrolytes are possessing increased safety features compared to regular liquid electrolytes.[144] During charge, Li+ ions from the cathode are transferred through the solid electrolyte, most favorably a single-cation-conductor, and electrodeposited on a Li metal anode (or a current collector) (Figure 10a). This process is reversed during discharge and Li+ ions are intercalated/inserted back into the cathode material. In contrary, an LSB is assembled in the charged state when elemental S is used at the cathode (Figure 10b). Li is electrochemically oxidized at the anode while S is simultaneously reduced. During this discharge, not only Li2S, but in addition so-called Li-polysulfides are formed, which are partially soluble in the electrolyte.[145] Today, both cell technologies have already entered certain market fields on a small scale, either for electric vehicles (LFP || Li, Bolloré Group[146]) or in form of start-ups (S || Li, OXiS Energy;[147] “semisolid Li metal cell”, SolidEnergy Systems[148]).
3.1 Solid-State Batteries
The concept of SSB (Figure 10a), i.e., replacing the porous separator filled with liquid electrolyte by a solid electrolyte layer serving as both separator and electrolyte, is well known. Iodine || Li metal battery cells with this concept were already used in the 1970s.[149] In general, solid electrolytes (SEs) have several advantages compared to their liquid counterparts, mostly concerning safety, due to their reduced flammability and the prevention of solvent evaporation or leakage.[4, 144] Nevertheless, some of them, e.g., sulfide glasses, can react with water under evolution of poisonous gases.[150] For certain chemistries with high ionic conductivity, one major advantage of SE could be to facilitate decreased charging times and higher power capabilities compared to standard liquid organic carbonate-based electrolyte systems.[151] Furthermore, a negative influence of crosstalk between the electrodes happening in liquid electrolytes[152] would be circumvented and an improved cycle life could be achieved.[144] In S || Li cells, a detrimental cross talk is the so-called “polysulfide shuttle effect,” which can be prevented by using an SE as solid barrier between the electrodes.[153] SEs are expected also to prevent the growth of HSAL during electrodeposition/-dissolution, one major challenge in LMBs.[144] The Bolloré group realized the commercial application of LMBs with an LFP || Li set-up and polyethylene oxide (PEO) as solid polymer electrolyte (SPE) for electric vehicles used in car sharing services.[154] A general disadvantage of SPEs like PEO is the necessity of high operating temperatures (>60 °C) to achieve practically usable ionic conductivities.[155] This leads to a constant energy consumption (from the battery energy) to keep the battery at elevated operation temperature. This constant loss of stored energy negatively affects the energy efficiency and thus the environmental footprint of this cell type. Further, this cell system still requires a Li salt and a three-fold excess of Li metal at the moment.[155]
SEs can be classified into two main groups, polymeric (SPE), and the ceramic SEs. Apart from PEO, many different SPEs have been developed, however, challenging in most cases is the ionic conductivity. Batteries with SPEs can therefore only achieve low charge and discharge rates or have to be cycled at elevated temperatures.[144] Major research interests are focused on the utilization of polymers from renewable resources, which could replace polymers produced from fossil fuels.[156] Many SPEs are soaked with organic solvents as plasticizer (gel polymer electrolyte, GPE) to increase the ionic conductivity.[156, 157] This not only leads to a reduced mechanical stability, but the added liquid solvent introduces similar problems to the battery cell as described in Section 2.3. The same applies for the Li salt, although a larger variation of salts can be used for SPEs or GPEs compared to their liquid counterparts since Al dissolution is reduced in these systems. The addition of a Li salt can be completely avoided by anchoring anionic functional groups to the polymer backbone leading to an increased Li+ ion transference number.[158] These advanced polymer structures are usually linked to higher synthesis efforts resulting in higher carbon footprints. Still, low ionic conductivities also of these polymers hinder the practical usability in battery cells.[158]
Although most polymers are produced from fossil fuels nowadays, a production from renewable sources is possible in many cases, starting, e.g., from bioethanol. Aromatic compounds can be synthesized with specialized catalysts starting from alcohols such as methanol.[159] It should be noted, however, that many synthesis steps and large amounts of energy are necessary to obtain purified monomer products. Only ≈3% of the global fossil fuel production is going into the fabrication of chemicals at the moment, while the rest is mainly used for energy and transportation.[160] The essential question here is to ponder how much more energy is needed for producing a polymer from renewable resources compared to fossil resources and whether it is beneficial for the environmental impact. PEO represents for example a polymer, where the obstacles for a transition toward a synthesis with renewable resources are quite low. In this case, the monomer is produced via oxidation of ethylene.[161] The latter is mainly produced from fossil fuels at the moment, however, it can be easily obtained from renewable resources, e.g., sugar cane, and production lines are already working.[160] Nevertheless, the production of sugar cane is connected to intensive agriculture and the ethylene processing itself is energy-intensive, so that even in this simple case, the impact on the environment is not clear.[162] Recycling of SPEs is possible, but it is connected with high costs and a presumably low quality of the recycled material. Most SPEs will presumably be thermally recycled, i.e., energy is harvested via combustion of waste materials.[163] Expensive Li salts could be regained before combustion through solvent extraction.[163]
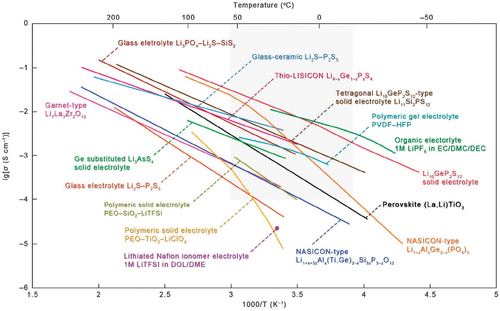
Up to now, several different material classes of ceramic SEs are known (Figure 11). The most common representatives can be categorized into two main groups, on the one hand oxides and phosphates including perovskites (e.g., Li3.3La0.56TiO3, LLTO), NASICON (e.g., Li1.3Al0.3Ti1.7(PO4)3, LATP), LISICON (e.g., Li14Zn(GeO4)4), garnet-type materials (e.g., Li7La3Zr2O12, LLZO), and on the other hand sulfides, e.g., the argyrodites (Li5PS6X, X = Cl, Br, I) or the Li2S–P2S5 glass electrolytes. Although quite impressive ionic conductivities can be achieved at moderate temperatures, ceramic SEs exhibit still several drawbacks. For example, many of the ceramic electrolytes react when in direct contact with Li metal,[158] moisture, or CO2.[165] Further, most oxidic SEs are rigid and exhibit a high Young's modulus so they cannot buffer well volume changes in the cell, which are especially high with Li metal anodes.[166] Multiple volume changes can lead to contact loss and reduced ionic conductivities at the interfaces between the particles. Finally, the production and the processing of oxide ceramics involves several high temperature steps including the cell assembly, which raise the total energy consumption and are challenging for the chemical stability of the other components.[158]
A parameter, often not considered when discussing SEs, is their density. When assuming the standardized cell format of an 18650 cell with a transition metal cathode, the electrolyte takes up between 10 and 35 vol% of the cell (derived from calculations in Table S1 in the Supporting Information). The SE does not only have to serve as separator but also has to be mixed into the cathode or at least coated on top of the particles.[4] Therefore, its density is an important factor with respect to the specific energy, the material abundance, and the processing cost of the whole cell. Liquid carbonate-based electrolytes have a density between 1.1 and 1.5 g cm−3, depending on the concentration of the Li salt. SPEs like PEO have a similar density and can therefore be a suitable replacement with regard to the specific energy of the cell. However, when Li metal is used as an anode, a solid barrier has to be implemented between the electrodes to avoid cell shortcuts by HSAL (dendrites).[167] Since SPEs and especially GPEs are not as mechanical stable as oxide ceramics, a sufficient thickness of this protection layer is necessary. The density of oxidic SEs on the other hand is often higher. A commercial realization in high-energy battery cells is very challenging for some ceramic materials, e.g., LLTO or LLZO, which have a density of ≈5.0 g cm−3. Beside of that, these two ceramic SEs consist further of high lanthanum amounts of at least 3.5 g per 18650 cell (calculated with an optimistic value of 10 vol% of electrolyte). The production of lanthanum requires difficult and energy-intensive processing steps, including hydrofluoric acid treatment, and it is present in the earth's crust in a quantity similar to Co, but more dispersed.[168]
In contrast, LATP offers a reasonable ionic conductivity and does not contain critical elements. The density of LATP (≈2.9 g cm−3)[169] is lower than most other ceramics, but still higher than sulfide ceramics or polymers. It can be synthesized by mechanical milling and heat treatment above 700 °C from TiO2, Li2CO3, (NH4)H2PO4, and Al2O3, which are all attainable with low energy processing.[170] Nevertheless, it reacts in direct contact with Li metal[171] and also the other disadvantages of oxide ceramics (e.g., brittle and hard to process) remain. Recycling of ceramic oxides would be of great benefit to regain the used elements, however, as the concept of SSBs is not established, no specific processes have been developed yet.[163]
Sulfide glass electrolytes on the other hand can be processed more easily because they are deformable by cold pressing. However, moisture-free process conditions are necessary to avoid the formation of toxic sulfidic gases.[172] Furthermore, their ionic conductivities are higher than for oxides (Figure 11), as sulfide builds up a weaker coordinating framework in which Li ions shows a higher mobility.[173] Another advantage is their lower density of ≈2 g cm−3 compared to oxide ceramics. From the preparation perspective, the Li2S–P2S5 glass electrolytes are synthesized directly from the elements. While phosphor (P) and S are both abundant, white P is energy intensive to produce, highly reactive, and poisonous for humans and the environment.[174] The argyrodites with the structure Li5PS6X (X = Cl, Br, I) can be synthesized by ball milling as well from Li2S, P2S5, and LiX with no further annealing.[175] They further have a low crystalline density with X = Cl (1.86 g cm−3) and with X = Br (2.09 g cm−3).[176]
SEs can offer opportunities for safer cells with higher energy density since they do not contain volatile and flammable components and may enable the operation of LMB cells. Due to high Li contents and high production and processing efforts including elevated temperatures needed for processing and operation, current SEs will have difficulties to compete with liquid SOTA LIB electrolytes in terms of sustainability.
3.2 Sulfur || Lithium Metal Batteries
The combination of a sulfur–carbon (S–C) composite cathode with Li metal anode in a S || Li-battery (LSB) is from a material-related point a promising “beyond-LIB” technology. S is considered as sustainable cathode active material with very low price.[154, 177] The abundance of S is fairly high in the continental crust as it represents the 13th most abundant element.[178] Additionally, S is a waste product of crude oil and gas refining.[179] S can be further found pure or as sulfides and sulfates in nature.[174, 180] The production of S is a mature business and the resources are quite abundant which makes S, from an economic as well as ecologic point of view, a viable material for energy storage.[181] Within the scope of this work, the focus is put on environmental friendly approaches for LSBs categorized under sustainable aspects, while reaction mechanisms, challenges, and other aspects can be found elsewhere.[153]
LSBs will be of interest for large-scale or high specific energy (=low weight)-driven applications (e.g., aviation) since S and Li metal exhibit huge specific capacities (S: 1675 mAh g−1; Li: 3862 mAh g−1).[177] Despite these high theoretical capacities, practical values are typically far away from these expectations.[4, 7] Furthermore, production costs have to be considered as well since the biggest competitor, LIB, is a mature battery technology and production takes place already on large scale.[5] To increase the sustainability of LSBs, current research focuses on simply designed S–C composite electrodes prepared by, e.g., aqueous processing[93] and based on sustainable resources.[182] The electrolyte and Li metal are key components of an LSB as well and have to be considered for the carbon footprint and costs of this battery cell type. Biomass- or organic-derived polymeric electrolytes[156] play an important role in future LSBs since S and polysulfide solubility[183] might be reduced and toxic SOTA electrolyte components could be replaced. “Greener” anodes that can compete in terms of specific energy with Li metal are scarce and might be composed of Si-based materials.[184] Figure 12 shows two approaches toward greener LSBs, a starch-based solid polymer electrolyte and a binder system for the S–C cathode, which both are assumed to be low-cost, environmentally friendly, water-soluble, and allow for high S loadings.
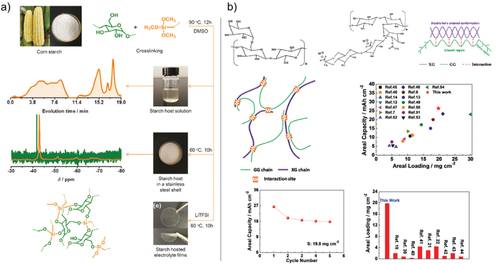
The starch-based electrolyte in Figure 12a represents a C–O–C functionalized network that is assumed to work similar to PEO.[156] The synthesis of a cross-linked starch form was used for a solid-state LSB with adequate performance.[156] An approach toward biogenic binders for LSBs shown in Figure 12b is based on polysaccharide containing compounds (xanthan gum and guar gum crosslinked via intermolecular binding effects).[185] These biopolymers are nontoxic, accessible through renewable resources, potentially low-cost and reveal new concepts for more sustainable S–C composite electrodes. It should be mentioned that the electrochemical stability window of organic- or bio-based materials are limited. As the cell voltage of S || Li cells is comparably moderate (<2.5 V for LSBs vs >3.5 V for LIBs), LSBs are very suitable for organic, renewable materials.
As a long cycle life is tremendously important for the sustainability of a cell, the strong capacity fading of LSBs is a major drawback. Many research approaches are aiming to extend the cycle life of LSBs in order to achieve practical and sustainable LSBs.[186] Peng et al. were recently able to transfer the concept of a biological self-healing process to an LSB that regulates the deposition and dissolution of the S species. By developing a polysulfide-mediated phase transfer, they achieved a S || Li cell with a high S loading (5.6 mg cm−2) that offers an optimized capacity of ≈3.7 mAh cm−2 (≈661 mAh g−1) and exhibits almost no decay over 2000 cycles.[187]
LSBs show a high potential for a sustainable battery technology, especially from a positive electrode material-based perspective. Nevertheless, current S || Li cells still need large amounts of Li in metal form and as Li+ ions stored in large electrolyte reservoirs. Going beyond Li, other potentially more sustainable metal-based S battery technologies draw increasingly attention of researchers and producers due to the abundance and high availability of S. It should be mentioned, that the typical pyrometallurgical and/or hydrometallurgical recycling of LSB cells may be accompanied by enormous H2S and/or SO2 production.
4 “Alternative-to-Li” Battery technologies
Beside of the Li ion and lithium metal battery cell technology, other alkali and earth alkaline chemistries were investigated for energy storage applications even long before LIB's commercialization. Early works were focused on Na, potassium (K), magnesium (Mg), calcium (Ca), Al, Fe, and Zn containing rechargeable batteries.[3, 188] The commercial success and the knowledge gained from the LIB technology revived these alternative cell chemistries acting as possible contingency plan in case of future of scarce resources scenarios. The here presented cell chemistries are classified into two main battery principles, metal-ion based batteries (MIBs) and dual-ion batteries (DIBs).
4.1 Metal-Ion Battery (MIBs) Principle
Proceeding from the rocking-chair mechanism of the LIB, metal-ion based battery systems are generally based on the motion of the metal cation from one electrode to the other.[3, 189] The electrolyte is containing metal cations in liquid solution (either aqueous or nonaqueous), in a solid state polymeric matrix, or in solid-state ion conductors like ceramics or glasses.[53, 190] Various host materials with different structures are known for insertion of metal ions. Conventional LIB host materials have often layered structures, e.g., graphite as negative electrode active material (anode) and NMC or LCO as positive electrode active material (cathode; see Figure 13a).[14]
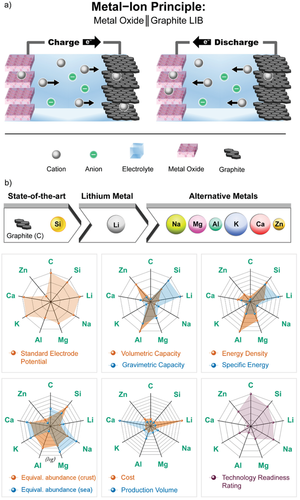
The benefits in regard of higher energy densities become obvious when an intercalation-based anode is replaced with an “alloying” material forming an intermetallic phase with Li[29] (such as Si, see Section 2.1) or by a metal electrode that relies on the electrodeposition/-dissolution process.[4] The LMB, however, still lacks a proven, commercially viable method against HSAL formation and remains therefore in the R&D stage.[191] Furthermore, a steady cost increase for Li-based compounds is expected in the future (see Li2CO3 price trend, Figure S1, Supporting Information)[180] which may allow alternative cell chemistries to become more desirable for mobile and especially for large-scale stationary applications.[192] Several metals are currently in discussion for rechargeable battery systems while abundance and expected developments of costs play a critical role.
The eight most promising active elements (Si, Li, Na, Mg, Al, K, Ca, Zn) for the negative electrode of metal-ion batteries are compared to graphite (C) as SOTA material in nine different ecological and economic categories (Figure 13b). The radar charts are based on data summarized in Tables S2 and S3 (Supporting Information).
In the alkali metal group of the periodic system of elements (PSE), Li represents the most ideal metal anode material from an electrochemical point of view. Metallic Li has the lowest standard electrode potential (−3.04 V vs SHE), a high capacity per weight (theoretical gravimetric capacity of Qgrav,Li = 3861 mAh g−1), and a still favorable, but comparatively moderate theoretical volumetric capacity of Qvol,Li = 2042 mAh cm−3) due to a low density (0.53 g cm−3).[174, 193] A major aspect for the availability of a raw material is the natural abundance of its corresponding element on earth. In terms of battery cells, charge is stored or transferred over electrons in correlation with the Faraday law (Q = n·z·F, with Q being the total electric charge, n is the amount of substance in mole, z is the ion valency number, and F is the Faraday constant). The molal abundance based on the equivalent mass of a certain element in the earth crust (mol kg−1) and the molar equivalent mass abundance in sea water (mol L−1), respectively, listed in Tables S2 and S3 (Supporting Information), are for the battery context more meaningful than its gravimetric abundance because it allows a comparison of the elemental abundance based on the transferable number of electrons. For Li, a natural molal equivalent mass abundance of 0.00288 mol kg−1 is found in the earth crust. In contrast, K with 0.535 mol kg−1 and Na with 1.027 mol kg−1 are 180–350 times more abundant in the earth crust than Li. Besides that, rather vast Li reserves of more than 230 000 Mt are estimated to be found in seawater.[194] A comparison of the molar concentrations between Li and the other alkaline metal ions in seawater, however, show a comparable low concentration for Li (Li: 0.0259 mmol L−1; Na: 470 mmol L−1; K: 10.2 mmol L−1, see Table S3 in the Supporting Information). Even though Li, Na, and K are found in valuable amounts in oceanic water, sea water extraction is still a quite expensive process.[195] Therefore, Li is per se not scarce but still comparably less abundant than the other alkaline metals. Subsequently, the costs per mol/atom are much lower for Na and K than those of Li, especially when the high annual production amounts of soda ash and potash are considered (Li2CO3: 85 000 t a−1; 17 USD kg−1; Na2CO3: 55 000 000 t a−1, 0.15 USD kg−1; K2O: 42 000 000 t a−1, 0.74 USD kg−1).[180] A crucial aspect for producers are the locations and availability of alkali metal resource sites. Na is considered as the easiest accessible candidate since it can be obtained over Na chloride, which is stored in large amounts in globally distributed deposits. Among the alternative elements for battery chemistries beyond LIB and LMB in Figure 13b, Na, K, and Ca have the advantage of low standard electrode potentials (Na: −2.71 V vs SHE; Ca: −2.86 V vs SHE; K: −2.93 V vs SHE).[196] As alkaline earth metals can supply two electrons per atom for capacity storage, apart from Ca, also the more light weight Mg with a standard potential of −2.37 V versus SHE is considered as an interesting alternative material. When comparing possible specific energy and energy density values (calculated with the standard electrode potential versus SHE) of alkali and earth alkaline metals, Li shows the highest theoretical gravimetric capacity and the highest specific energy, which nominates it as an optimal choice for applications, when battery cell weight is most crucial. In terms of theoretical energy density (Wh L−1), only Mg and Ca have the potential to compete with Li and could exceed its energy density in theory (Table S2, Supporting Information). It has to be mentioned that the specific energy and energy density values shown in Figure 13 illustrate, however, only the theoretical values of the elements. In reality, the standard electrode potentials do typically not correspond to the actual, practical potentials of the metals in organic electrolyte solutions.[7] Further, the electrolyte composition can strongly affect the theoretical energy of a battery cell. While monovalent alkali salts with a monovalent anion can be generally considered as fully dissociating salt in appropriate solvents, bivalent alkaline earth metals are preferably soluble only in complexes, e.g., Mg2Cl22+ for SOTA Mg-based electrolytes.[197] Therefore, the weight and volume of the (solvated) salt complexes might curtail the practical gravimetric and volumetric capacities of alkaline earth battery cells. Despite promising theoretical values of alternative alkali and alkaline earth metals, a real practicability of these battery technologies is still lacking yet, and further groundbreaking fundamental research progress is necessary.
Al- and Zn-based batteries have returned into the focus of energy storage recently. Though less abundant than Li in the continental crust (molal equivalent mass abundance of Li: 2.88 mmol kg−1 vs Zn: 2.14 mmol kg−1), Zn is produced in a large ton-scale (13 000 000 t a−1) due to its application in the iron and steel industries, which leads to a comparably low Zn metal price (3.02 USD kg−1) compared to most alkali and alkaline earth metals (cost in Figure 13b are based on the related metal oxides).[174, 180, 198] Zn has a standard electrode potential of −0.76 V versus SHE resulting in a low specific energy compared to other anode metals. Nonetheless, the very high volumetric mass density (7.13 g cm−3)[174] enables a reasonable energy density (Figure 13).
Al became recently an attractive candidate for next generation battery technology. Al is the third most abundant element in the continental crust of the earth, only Si and oxygen (O) are more abundant.[178, 199] Since Al is used in many industrial applications (the production volume of alumina is 130 000 000 t a−1 and of bauxite 300 000 000 t a−1), its price is comparably low (alumina: 0.56 USD kg−1; bauxite: 0.03 USD kg−1; and Al metal: 2.54 USD kg−1) and thus can be considered as most interesting material of choice for large-scale energy applications, e.g., for stationary purposes.
The technology readiness rating (TRR) for the different elements (Figure 13b) is based on their related rechargeable MIB chemistries. Carbon, in form of graphite, marks as SOTA anode material the highest TRR (in five levels) of all systems within this comparison due to its commercial use in LIBs. Si is partly a constituent of modern LIB anodes, as well. Si, however, is up to now only implemented as Si/C composite blends with a small Si mass fraction and therefore rated with a TRR level of 4. Na and the Na-ion battery technologies are classified to level 4 due to a beginning commercialization of these systems, many possible cell chemistries and ongoing large efforts in research. Metal-based batteries, such as Zn-based rechargeable batteries, suffer in general under severe inhomogeneous deposition of the metal electrodes (“dendrites”) when realistic usage conditions are applied.[200] Despite the advantages of Zn metal as abundant anode material, this battery technology exhibits due to this reason only a TRR level of 3 so far. Li metal-based cells are rated in comparison with a TRR of 3.5 because first commercialized LMBs (S || Li) are available (e.g., from OXiS Energy[147]). K and the multivalent metals Mg, Al, and Ca are rated with a low TRR of <3. The main drawback of these systems are the absence of suitable electrolytes and high capacity, high-reversibility cathode materials for rechargeable battery cell systems.
4.2 Dual-Ion Battery Principle
Stationary battery systems are considered to become increasingly important in the next decades and therefore new cell technologies are necessary to fulfill the requirements of large-scale battery utilization.[201] In recent years, so-called DIBs have come into focus for that purpose. Contrary to the rocking chair mechanism based on the cation transfer between the electrodes, both, cations and anions of the electrolyte are taking part in the charge/discharge cycling process of DIBs.[3] During charge, cations from the electrolyte are inserted into or electrodeposited onto the anode, while anions are inserted into the positive electrode resulting in ion depletion in the electrolyte phase (Figure 14a). This is an important contrast to the rocking chair principle since the electrolyte has to be considered as active material in DIBs, whereas it is “only” ion transport medium in the former battery type. As part of the DIB family, the graphite || graphite battery (DGB) is one of the most investigated systems.[8] In the classical DGB, both electrodes consist of graphite in combination with a highly concentrated organic electrolyte or an ionic liquid-based electrolyte.[8, 202] Considering a larger amount of electrolyte and the limited capacity of graphite both for cations and anions, the specific energies of these systems are low compared to MIBs and especially LIBs.[8] Nonetheless, DIBs might show advantages in large-scale energy storage application because they do not depend on metals such as Co or Ni, which are expected to be increasingly demanded in future.
Electrodes based on organic materials instead of graphite have been successfully implemented in battery cells (MIB and DIB).[204] Battery technologies containing organic anode and cathode materials are called full-organic battery (FOB). Usually, p-type and n-type redox polymers with mostly amorphous structures are used as active materials, which can coordinate or insert cations and anions to/into their active host material (Figure 14b).[205]
4.2.1 Alkaline Metal-Based Batteries (Na, K)
The higher natural occurrence of Na and K in the earth crust and seawater compared to Li (see Tables S2 and S3 in the Supporting Information) and the globally more distributed deposits underline their potential as abundant battery materials, in case of a future shortcoming in Li production. Li and Na are produced via electrolysis in Downs cells, which consume large amounts of electrical energy. Since the separation of liquid K and potassium chloride (KCl) in an electrolytic bath is difficult, K metal is mainly produced by chemical reduction of KCl with metallic Na.[174] The accompanying consumption of Na makes K less attractive regarding sustainability aspects. Nevertheless, increasing demand might lead to further synthesis optimizations or even to the development of new synthesis concepts.
Na has been studied for a long time in energy storage devices and has found its way into some application areas such as stationary battery technology for power supply stabilization (mitigation of renewable energy fluctuations in power grids).[206] The high temperature S || Na battery (HTSSB), reported in the 1960s by Kummer and Weber from Ford Motor Company,[207] is composed of liquefied Na, a sodium beta-alumina (β-NaAl11O17) solid electrolyte, and a liquid S cathode.[207, 208] An operating temperature of ≈300 °C is necessary, which makes the HTSSB less attractive for mobile applications.[209] For large-scale stationary applications, heating of the battery has a minor impact on the energy balance because these batteries have a relatively high durability (long cycle life) and efficiency.[206, 209] Apart from the additional energy consumption during use, HTSSBs are a comparatively sustainable battery technology due to its abundant and low-cost materials (Na, Al2O3, and S) and its good recyclability. More than 99 wt% of an HTSSB battery (steel, copper, and aluminum) can be recycled. Only sodium must be handled as a hazardous material and recycling processes for sulfur and sodium are still in development.[210] The optimization of the battery principle led finally to the installation of HTSSBs in several countries, especially in Japan.[211] Various Japanese cities have installed HTSSBs to maintain the stabilization of the power grid, particularly, if energy is produced by fluctuating sources, such as wind or solar power. One of the major challenges of these batteries concerns the housing of the batteries, which has to withstand the harsh chemical conditions of liquid Na, S, and Na polysulfides and its vapors. Safety issues arise primarily from liquid/gaseous Na which can heavily react in contact with moisture (fires, explosions, etc.).[212] Developments toward intermediate (150–200 °C) or lower temperatures (25–150 °C) would lead to a significant improvement of the economic and ecological attraction of these systems.[207, 209, 213] One major problem to solve is that lowering the temperature of a S || Na battery cell increases the formation of high surface area Na deposits resulting in a severe impact on the safety and cycle life similar to LMBs.[214] Nevertheless, the pairing of Na and S as active materials remains still as one of the most promising battery concepts for more sustainable batteries in the future.
Na metal can also be combined with metal halides such as NiCl2, FeCl2, and ZnCl2. These battery concepts belong to the zero emission battery research activities (ZEBRA) batteries, which were developed and brought to prototype application in the 1980s.[215] Major success was achieved with the development of the NiCl2 || Na battery for electromobility as well with other MCl2 || Na combinations.[216] Even though the ZEBRA battery was successfully demonstrated as a robust and comparably safe energy storage system for EVs and was further optimized for more than 30 years, the utilization of this technology was only successful in a few application niches, e.g., submarines and as missile power supplies.[217] A significant drawback compared to room temperature battery chemistries has been the high operation temperature of ≈300 °C, which requires electrical energy from the battery for heating (=“thermal self-discharge”). Some approaches consider the use of ZEBRA batteries for large scale stationary storage applications similar to HTSSBs.[207, 218] From an ecological point of view, ZEBRA batteries containing cheap iron chloride as positive electrode material could play a supplementary role in future energy storage concepts.[207]
Besides Na metal batteries, Na-ion batteries (SIBs) with a working principle analogous to the LIB rocking chair mechanism are currently considered as potentially low-cost batteries.[209, 219] Here, hard carbons are used in many cases instead of graphite as anode material.[220] The reason is the very limited Na-ion storage ability of graphite which can form NaC64 as the highest sodiated graphitic intercalation compound (GIC) leading in best case to a specific capacity of <100 mAh g−1 (in comparison to LiC6 in LIBs of 372 mAh g−1).[221] For hard carbons, specific capacities of up to 300 mAh g−1 were reached,[222] however only part of this capacity can used in a safe manner. As positive electrode active materials, a broad variety of Na-containing inorganic compounds and partially analogues to LIB cathode active materials, such as olivines and layered oxides, were investigated for SIBs.[220, 223] Since the Na ion shows distinct differences to a Li ion, a simple adaption of the LIB development experience is not possible. For example, the bond between Na and oxygen (O) is less ionic and more covalent than for Li–O, which lowers the practically achievable potential of Na ion layered oxides.[224] Several efforts were made to achieve high potential positive electrode materials (>3.5 V vs Na|Na+) for Na ion intercalation or insertion.[220, 225] Layered oxide materials with a Ni2+/Ni4+ redox couple or anion redox activity could provide higher energy densities.[226] Another type of cathode materials are polyanionic compounds, e.g., Na3V2(PO4)3, which have a NASICON-type structure and exhibit long cycle life and outstanding ion diffusion properties.[227] Polyanionic materials based on Co2+/Co3+ (and other redox-active transition metals) and phosphate-derived anions show a broad range of discharge potentials >4 V versus Na|Na+.[228] While the discharge of NaCoPO4 takes place at 4.19 V versus Na|Na+, similar anionic compounds with a structure of Na4Co3(PO4)2P2O7 offer discharge potentials of 4.4 V versus Na|Na+.[229] Even though the practical specific energies of SIBs are unlikely to outpace the energies of LIBs, the abundance and lower costs of Na will further drive the development toward Na-based batteries that could play a major role in future, especially for stationary power supply.[230]
The combination of high abundancy and very low standard reduction potential of K|K+ (−2.93 V vs SHE) makes the potassium ion battery (PIB) and with some limits the potassium metal battery (PMB) very attractive for research as an alternative, nonaqueous rechargeable battery technology.[231] A cell set-up of a PMB was introduced in 2004 with Prussian blue as cathode material.[232] Prussian blue offers quite unique properties because it shows reversible and distinct K ion intercalation/de-intercalation, which is still rarely observed in literature.[232, 233] Besides intercalation-type cathodes, also O and S conversion-type cathode containing PMBs show promising results.[234] These approaches are interesting from an ecological point view likewise to the Na systems due to the high abundance of the active material elements without the use of Co or Ni. However, safety concerns of the K metal anode arise comparable to other metal anode based battery cell systems due to the formation of high surface area K deposits (HSAK; “dendrites”) that can lead to shortcuts accompanied by a thermal-runaway of the cell. Additionally, the melting point of K (63.6 °C) is significantly lower than that of Li (180.5 °C), or of Na (97.8 °C), resulting in a limited temperature range for operation.[174] The low melting point may be exploited for a “cell regeneration step” to melt high surface area K or “dead K” (electronically isolated K particles) as reported for Li,[235] which might circumvent the penetration of dendrites through the separator. In any case, the too low melting point of K metal makes the use of PMBs rather unlikely. Alloys based on other abundant materials with similar capacities to metallic K could be a promising alternative for advanced anode designs.[236]
Many efforts are made to investigate intercalation- or insertion-type anodes for PIBs. The redox potential of K-GICs, which is very close to the K|K+ standard redox potential, the formation of a stable KC8 phase, and the lowest ion solvation (and thus desolvation) enthalpy among Li+, Na+, K+, Mg2+, and Ca2+ ions in carbonate-based electrolytes, are favorable conditions for PIB anode development.[237] While first results on the electrochemical intercalation of K into graphite showed fast capacity fading and moderate rate capabilities,[237, 238] great progress could be achieved recently due to intense cell chemistry and electrolyte investigations.[239] In particular, highly concentrated electrolytes (e.g., 3.5 m potassium bis(fluorosulfonyl)imide in ethyl methyl carbonate) can enhance the capacity retention of a dual graphite battery cell significantly, up to 76% after 200 cycles.[240] Other carbon materials, like hard carbons, were studied as well, but their cycling performance remains moderate.[237]
In summary, Na-based batteries have experienced a long development history and will be likely a suitable candidate for future, hopefully more sustainable batteries. K-based systems represent an alternative, sustainable technology, however, they are still at an early research stage. A focus on host structures for Na or K ion batteries composed of abundant elements could contribute eminently to a higher performant and a more sustainable future of these battery cell types.
4.2.2 Alkaline Earth Metal-Based Batteries (Mg, Ca)
Alkaline earth metals such as Mg and Ca as bivalent elements offer in general the advantage of a two-electron-transfer per atom depending on the complexation of the cations in solutions. In case of the formation of metal-chloride complexes (e.g., Mg2Cl22+) for example, lower charges per atom are obtained. The high theoretical capacities, low standard redox potentials, and the high equivalent mass abundance of Mg (1.92 mol kg−1) and Ca (2.07 mol kg−1) in the continental crust and in seawater (Mg: 106.13 mmol L−1; Ca: 20.56 mmol L−1), offer the possibility of high-energy and sustainable battery chemistries which came into focus again for energy storage applications.[241] Moreover, their anodes are presumed to form less high surface area deposits than alkali metals upon charge.[242] Mg has a high melting point of 650 °C while Ca exhibits even a higher value of 839 °C.[174] Such high melting points are expected to be beneficial for future safety assessments. Despite all advantages, the drawbacks of Mg and Ca are based on their bivalent character as well. Generally, the metal surfaces have to be free of any layer/interphase (such as an SEI), as bivalent ions due to their larger Coulomb interaction with the interphase and its constituents could hardly transferred into the electrolyte and back, which does therefore generate very high overpotentials for electrodeposition/electrodissolution or even hinders the ion transfer process completely.[243] As a result, the electrolyte components have to be chemically and electrochemically stable toward the metal anodes, which severely limits the choice of electrolyte components. This stands in contrast to Li, where the decomposition layer on the metal (=SEI) is usually a well ion conducting interphase.[244] The first electrolytes for reversible electrodeposition and -dissolution of Mg in metal-type batteries were Grignard reagents.[242, 245] Many efforts, for example, by Aurbach and co-workers, have been made since to optimize the Grignard electrolytes and increase the Coulombic efficiency as well as stability toward the anode.[246] A breakthrough in Mg-electrolytes was achieved by utilizing organic magnesium aluminum chloride salts in ethereal solutions. Further development led to magnesium aluminum chloride complex-based electrolytes (MACC) which are composed of a mixture of MgCl2 and AlCl3 in ethereal solutions.[247] Recent studies show alternatives to the highly corrosive chloride-based electrolytes for example using fluorine-containing salts like MgTFSI2, which represents one of very few Mg salts that is soluble and can be used to reversibly deposit metallic Mg.[248] The situation for Ca-based systems is similar,[242] however, certain electrolyte formulations were predicted to be beneficial in terms of Ca solubility.[249] As a consequence, the electrodeposition of metallic Ca from Ca salts appears to allow a broader range of electrolyte formulations and was recently shown in carbonate-,[250] 1,2-dimethoxyethane, and tetrahydrofuran-based electrolytes.[251] Besides the electrolyte, insertion-type cathode materials for Mg- and Ca-cations are rare and mostly unexplored. While Chevrel phases like Mo6S8 were demonstrated to be feasible cathode-active materials with high reversibility but low working potentials for Mg,[252] recent studies about Ca cathode materials were focused on Prussian blue analogues, e.g., CaxMnFe(CN6)[253] and have been extended to several Ca-bearing minerals as potential cathodes.[254] Various attempts have been made to find more reversible insertion-based cathode materials, but the success is still limited.[242, 255] A promising mostly unexplored material class are MXenes with the general structure of Mn+1XnTx (M is a transition metal, e.g., Ti, V, Cr, Nb, or Mo, X is C, and/or N, n = 1, 2, or 3, Tx is represented by functional groups, e.g., OH, O, and/or F). MXenes can be used as a 2D host material for multivalent ion-based batteries, especially for Mg. This material class might offer low ion diffusion barriers with quite high surface areas (2D and 3D structures are known), often combined with virtually metallic conductivities.[256] Nevertheless, insertion-type materials might not overcome their discussed intrinsic limitations. Sulfur, as conversion cathode, seems to be more promising cathode candidate,[257] however with distinctly different charge/discharge mechanisms than in S||Li systems.[145, 258] Insertion/intercalation anodes for Ca and Mg are even more limited.
The complexity of the electrolyte/metal interplay and the lack of suitable cathodes lowers the probability of a rapid market entrance of these alkaline earth-based battery technologies. More work on organic and conversion-type materials cathode materials and progress regarding the reversibility and efficiency of Mg and Ca metal-based battery systems are necessary before the advantages of relatively high element abundancy and theoretical energy density can be harnessed for real application.
4.2.3 Further Multivalent Metal-Based Batteries (Al, Zn)
Besides alkaline and earth alkaline metals, Al and Zn are considered for new battery chemistries.[241, 259] The main advantage of Al is its abundance (9.15 mol kg−1 equivalent mass abundance in the continental crust, see Table S3 in the Supporting Information), which outranges all other metals discussed in this work. Moreover, trivalent Al ions exhibits high charge capacity with the smallest ionic radius compared to the other metals discussed herein, which are promising properties for high energy density batteries.[260] Another beneficial point could be an increased safety of a battery based on an Al anode due to the high melting point (660 °C) and low reactivity due to a native Al oxide layer. Al metal batteries (AMB), mostly primary, exhibit a long history of research.[217, 261] In the 1850s, the first Al electrode was presented for aqueous battery-type applications, first as cathode, later on as anode.[262] Like for all other metal-based systems, high efforts have been made to utilize aprotic organic electrolytes for a reversible Al ion transfer between the electrodes. Similar to Mg, Al ion-based electrolytes are quite complex. The coordination of small, trivalent Al ions remains as one of the main challenges of Al-batteries because only a few Al salts are known, which can completely dissociate in aprotic solvents.[263] Therefore, current studies focus on ionic liquid-based systems, mostly combining AlCl3 with imidazolium-based chlorides, which comprise complex ionic species capable to electrodeposit and -dissolve Al or Al3+.[260, 261, 263] Similar to alkaline earth metals, suitable cathode materials are scarce.[264] The strong Coulombic attraction due to the high charge density of trivalent, “hard” Al cations led to a limited reversible intercalation into cathode structures accompanied with low diffusion rates because of strong electrostatic interactions.[265] Many published intercalation-type cathode materials are based on vanadium-, manganese-, titanium-, or molybdenum oxides, metal-sulfides as well as Prussian blue compounds.[260, 263, 265] Conversion-type cathodes with S or O have been proposed for Al-metal batteries but still suffer from a low cycling stability in case of S || Al cells[266] and a low reversibility in O2 || Al cells.[267]
Besides Al-based batteries, Zn-based (as like some Fe-based) technologies are one of the most promising aqueous battery systems since the metal shows good electrochemical stability toward water-based electrolytes compared to other metal-based batteries mentioned in this review.[268] The evolution of hydrogen is kinetically slowed down due to high overpotentials for the reduction reaction of water on Zn metal.[269] Aqueous Zn ion batteries, including flexible approaches for wearable electronics,[270] are evolving quickly and great research efforts are invested to find suitable, sustainable cathode materials which allow for reversible Zn ion intercalation/deintercalation.[271] The combination with oxygen as cathode-active material is rendered as most favorable since a high theoretical energy density and specific energy are expected (4091 Wh L−1; 1087 Wh kg−1).[272] Nevertheless, disadvantageous for rechargeable Zn-batteries is the formation of high surface area Zn deposits (HSAZ, “dendrites”) in alkaline solutions, which leads to an unpredictable and irreproducible end of life of O2 || Zn cells.[269, 273] The utilization of pH-neutral aqueous electrolytes is proposed to reduce the formation of HSAZ, which could further improve the cell life time due to the less corrosive components.[274] Primary O2 || Zn batteries, patented in 1860 and commercially available already in 1930, are a well-developed electrochemical energy storage technology.[275] The long-term experience of primary O2 || Zn systems stimulate high interest in achieving rechargeable batteries of the same kind.[276] Zn-based systems are considered as one possible candidate of the next generation of mass-produced and environmentally friendly, water-based battery cells to replace mature but quite environmentally burdening battery technologies such as metal-hydride and lead-acid batteries. Especially the O2 || Zn battery gained increasing attention in the last years because it might overcome the limitations of other air cathode-based secondary battery principles like O2 || Li and O2 || Al.[276, 277]
Rechargeable O2 || Zn cells could offer beneficial properties for a green battery system such as low cost due to large-scale production of Zn (even though Zn is in molal quantities less abundant than Li) as well as high safety and environmental friendliness due to aqueous electrolytes, comprising nontoxic materials, and the prevention of formation of hazardous compounds.[276] Besides the concerns toward HSAZ formation on the metal Zn anode, challenges on the cathode side, which is commonly comprised of a (thin) catalytic active layer on a gas diffusion electrode (GDE), has not been solved yet despite the technological knowledge from the maturity of the primary cells. GDEs consist of a carbon matrix enclosed by a water nonpermeable polymer.[276, 278] The catalyst of the GDE remains a key issue of metal/air systems and is extensively investigated because it has to facilitate both, the O-reduction and -evolution reaction.[279] Today's research activities in electrocatalysis focus on the search of a highly efficient, low cost, and nonpolluting candidate.[276, 280] In contrast to commonly used noble metal or metal oxide-based catalyst layers, new sustainable approaches are based on carbon-derived materials, e.g., N and S codoped hierarchically porous carbons, and metal–organic frameworks.[276, 281] Besides the high expectations of O2-based batteries, a major obstacle for the long-term practicality of O2 || metal cells using environmental air is the possible ingress of undesired air constituents like CO2 and moisture. While the former may form solid carbonates clogging the pores of the GDE (depending on the pH of the aqueous electrolyte), the latter may lead to large water uptake in geographical regions with high humidity or to drying out of the cell in very dry climates.[282]
From an ecological point-of-view, Zn-based systems might be a very promising, green battery technology due to the combination of high material availability and good compatibility with aqueous electrolytes. The energy densities of these systems are, however, limited by a low discharge potential because of the narrow voltage window of aqueous electrolytes. When volume and weight of the battery do not matter, e.g., for large-scale energy storage, the Zn-based system could be a highly promising sustainable battery concept. In contrast to this, Al-based battery systems can be a promising approach for high-energy battery applications. The development of Al batteries is still at a very early stage though and this battery technology has much room for improvement in terms of sustainability since all suitable electrolytes so far contain complex and highly corrosive chemicals.[283]
4.2.4 Graphite-Based DIBs (Graphite || Metal, Graphite || Graphite)
Carbon-based electrodes are not only used as negative electrodes to host metal cations, but can be used as anion-hosting positive electrode material in a dual-ion battery (see DIB principle; Section 4).[8, 202, 284] The most developed DIB concept is the graphite || graphite battery (DGB), depicted in Figure 14. While graphite is one of the best investigated host materials for anions, alternative materials such as organic and metal-organic materials are considered for the positive DIB electrode as well.[285] Bare metals, intercalation- or conversion-type materials can be used for the negative electrode. Metal cations are stored either due to electrodeposition or due to an intercalation process. DIBs and DGBs have been successfully established with Li-based electrolytes using either ionic liquid-based or highly concentrated organic solvent-based electrolytes (HCEs).[286] Fundamental insights into the theoretical working principle,[287] degradation mechanisms,[278] and effects of the electrolyte salts onto the cell performance[288] complete more and more the knowledge of DIB systems and promote their usability. Different metals, e.g., Ca and Al, are possible as negative active material, facilitating new opportunities in sustainability for energy storage.[289] DIBs are primarily considered for stationary applications which have focus on raw material cost, processing cost and material availability rather than energy density.[7] The often fast ion intercalation kinetics of DIBs favor an application as grid storage for intermittent energy sources such as renewable energies.[290] Since graphite constitutes the largest fraction in DGBs and a main component in graphite || metal DIBs, the exploration of new, environmental benign production ways for graphite is very important for large-scale applications based on DIBs (see Section 2.1).
In the context of sustainability, the introduction of Mg-, Al-, and Ca-based DIBs is highly desirable since these are more abundant and offer theoretically higher energy densities than Li-, Na-, and K-based systems.[289, 291] Zhang et al. developed an Al-graphite DIB (AGDIB) cell combining an Al current collector as negative electrode, an intercalation-type graphite-based positive electrode with an Li salt containing electrolyte (Figure 15a).[289]
In contrast to an electrodeposition mechanism, the negative electrode reaction is based on a (not fully reversible) alloying reaction of Li ions from the electrolyte with the Al current collector. On the positive electrode, the PF6− anions intercalate concurrently into graphite during charging. This cell type offers a capacity of 104 mAh g−1 (referenced to the mass of the graphite positive electrode) at a charge rate of 2C with 88% capacity retention after 200 cycles constant current cycling.[289] Further developments on AGDIBs have been reported by Wang et al. who introduced a new concept that comprise the intercalation of two anion species (PF6− and AlF4−) into a GIC host material, while Al foil was used as anode. This “two-anion” approach resulted in a high cycling stability of 600 cycles under asymmetric charge/discharge current densities (charge: 1000 mA g−1; discharge 100 mA g−1) at a capacity of ≈100 mAh g−1 with 99% Coulombic efficiency.[293] By tuning the carbon-based cathode, Chen et al. demonstrated an extremely stable, high power approach for AGDIBs based on a sophisticated design of a highly oriented film of graphene layers, which is called 3H3C (“trihigh tricontinuous”) graphene film cathode. The material was designed to allow an effective channeling of ions while being electronically conducting in all three dimensions (Figure 15b). The highly reversible AlCl4 ion storage within the graphene structure enables charge/discharge cycling for over 250 000 cycles at 1 A g−1 (Figure 15c) with almost no loss of capacity.[292] Indicated by plateau regions in charge/discharge curves, Faradaic reactions are assumed in the energy storage process of the cell, although capacitive effects could often not be ruled out in novel DIB systems. Despite the promising results for Al-based DIBs, reports on other multivalent ion systems are rare. First results on Ca-based as well as Zn-based DIBs with graphite cathode have been published recently.[294]
DGBs and other DIBs are considered as promising candidates for large-scale energy storage since these technologies have the potential to be low cost and sustainable depending on conditions of electrolyte and graphite production, as well as electrode processing. The symmetric battery cell set-up is also attractive from an economic point of view since a possible simplified fabrication process could lower further the production and recycling costs. With the availability of environmentally-friendly produced graphite and electrolyte, DIBs are a promising option for sustainable, large-scale, stationary battery applications, especially if systems with multivalent ions can be utilized.[8, 202]
4.2.5 Organic-Electrode Based Battery Cells
Even though organics are prone to be green, a realistic picture is more complex. Most organic compounds, which are reported as electrode-active materials for rechargeable batteries nowadays, originate from fossil resources. New approaches toward more “green batteries” focus on organic battery components from renewable sources.[295]
A classification of organic molecules for energy storage and an overview about organic battery types and their working mechanisms is presented in Figure 16.[296] Organic electrode-active materials can be characterized as p-type, n-type, and bipolar, compounds (Figure 16a). p-type materials are used as cathode-active compounds and can be oxidized during the charging process to form stable cationic or stabilized radical species.[297] n-type molecules can be used either as cathode- or as anode-active materials since they can be reduced electrochemically, while mostly a neutral state is achieved. Negatively charged species can be achieved by reduction of neutral n-type materials. In MIB and DIB designs, p-type molecules are used as cathode-active material while n-type compounds work as anode materials (Figure 16b).[205, 298] If both anode and cathode are represented by redox-active organic molecules, the assembled cell is classified as full-organic MIB or DIB (Figure 14b).[203, 299] Bipolar molecules are bifunctional, donor–acceptor materials and can release or store electrons during charge. Typical bipolar redox-active molecules such as conducting polymers can be used for electrochemical capacitors (ECs), which are based on a capacitive charge storage mechanism with faradaic contributions, often referred to as pseudocapacitors.[296, 300] Examples for p-type, n-type, and bipolar, molecules are presented in Figure 16a. A generic advantage of organic-based energy materials relies on the possibility to combine different organic-active materials for rechargeable batteries independently from the cell chemistry and the electrolyte constituents.
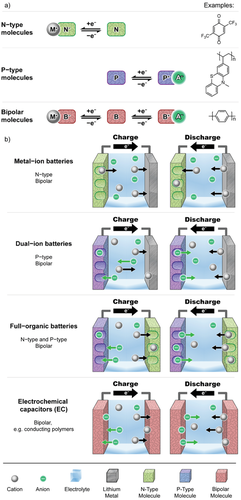
The size of organic redox-active materials for energy storage ranges from small molecules to larger macromolecules, up to polymers.[205, 295, 297, 298, 301] Small molecules are prone to provide high specific capacities, whereas polymers (conjugated and nonconjugated) show usually lower solubility and extended cycle life. A versatile example for organic energy storage materials are quinones and their derivatives.[302] These widely studied compounds contain carbonyl groups, which can be reduced while inserting a cation.[303] Already in 2009, a promising candidate was presented by Song et al., the poly(anthraquinonyl sulfide) (PAQS), that can provide a specific capacity of 198 mAh g−1 due to a comparably low molecular mass and a two-electron transfer mechanism.[304] The ecological advantage of organic carbonyl-based materials, especially quinone-derivatives, is presented by the precursors which can often be found in nature.[305] Biogenic molecules, e.g., myoinositol[306] or lignin,[307] are promising precursor candidates for low cost and ecofriendly energy storage materials.[305, 308]
A broad range of the reported organic battery materials are synthesized from petrochemical precursors. The necessity of more sustainable resources is therefore a main issue. Figure 17a reveals possible biogenic resources for organic materials, which could be used as redox-active materials in batteries. Nature-derived redox-active molecules are mostly composed of light and ecofriendly elements, such as H, C, N, O, P, and S. In nature employed biomolecules are characterized by highly efficient and reversible redox reactions and represent an inspiring source for new electrochemical concepts.[308, 310] For example, ubiquinone (coenzyme Q10) and plastoquinone (cofactor in photosystem II) are able of mediating electrons and protons,[311] and could be utilized as redox-active organic electrode materials without further modifications. Furthermore, bacteriophages or biological materials, like fibroin, leaves or certain biowaste, e.g., coffee bean and tea residues, can be used as templates for porous carbonaceous compounds or as substrates for other active materials as depicted in Figure 17a (blue frame).[308]
One of the most promising biogenic material is lignin, as it is the second most abundant biopolymer, can be produced by the extraction of lignin-based materials from the bark of trees, and contains redox-active polyphenol-type groups, which can be modified for energy storage purposes.[312] In Figure 17b, a synthetic approach by Milczarek et al. is presented for the production of redox-active and electronically conductive lignosulfonate/polypyrrole networks that can be applied as electrode active materials for capacitors.[309, 313] These carbon additive-free cathode materials synthesized from renewable bio-based precursors demonstrate the opportunities of the “biorefinery” approach.[314] Inspired by this work, several other groups follow similar ideas and developed further improvements in the field of biogenic active materials.[300, 315]
Furthermore, the anode of organic-cathode based batteries can cover a broad range of metals. Besides organic material || Li batteries,[316] Na-,[317] K-,[318] Mg-,[319] Ca-,[320] and Zn-based[321] cell assemblies have been reported.[299, 322] This extraordinary versatility makes redox-active organic molecules attractive for the development of more sustainable energy storage in various application fields with a possibility for large scale production and “green” fingerprints.[323] Following the first promising results, the research in the field of biogenic redox-active materials just takes off and is expected to intensify in the future. More efforts are necessary to find versatile bio-based molecules, which can be used in batteries with reasonable energy densities and cost.[324] Further on, the applicability of printable processing techniques will be beneficial for later commercialization,[325] especially for bio-derived redox-active materials. A major ethical problem for mass production of organic molecules from renewables can arise from the competition in agriculture to produce industrial materials instead of food. A fair balance has to be found here between the needs of modern society, social aspects, and environmental conservation.[295, 326]
4.2.6 Comment on the Availability of Resources for Alternative Systems
The abundance of resources is a simple measure to classify the sustainability of a possible material. Nevertheless, the geographical location of deposits accompanied with unclear political situations can make a resource, even though abundant, to a critical good.[327] The European Union (EU), as well as some other industrial countries, regularly publish reports on critical raw materials (CRM).[328] It is notable that some of the materials, which are considered to be part of a more sustainable energy storage system, can be found on this list. One example, natural graphite, which is needed in the EU not only for the production of LIB anodes, is considered as critical material because 76% of mined, natural graphite is imported from countries outside of Europe (China: 63%, Brazil: 13%).[328] Further, Mg shows a quite high abundancy in the earth crust, but is also considered as critical raw material by the EU due to its application in many different product areas (e.g., lightweight metal alloys).[12] An overview about the most important CRMs, which could concern a battery cell production in the EU, is displayed in Table S4 (Supporting Information) giving an idea about the distribution and supply bottlenecks of certain resources. The USA also enlists similar CRMs[12] which are mostly classified “critical” due to the import dependency from China. Goods as Co, Mn, and Li can be found on USA's CRM list and are essential for the LIB cell production in the USA, e.g., for the joint venture of Panasonic and Tesla.[136] Additionally, V is stated as a rare element for the use in grid-scale battery applications. In contrast, Mn and Li are not on the EU list of CRMs, but might follow if battery production is expanded in Europe.[329]
In general, the estimation and assignment of critical materials seems to be too simplistic in the general discussion. For instance, it should be regarded that not only raw material abundance and deposit locations, but also material mining, transport, processing and recycling needs to be taken into account, both in the cost and carbon footprint calculation of mobile and especially stationary battery systems[330] (Figure 18).
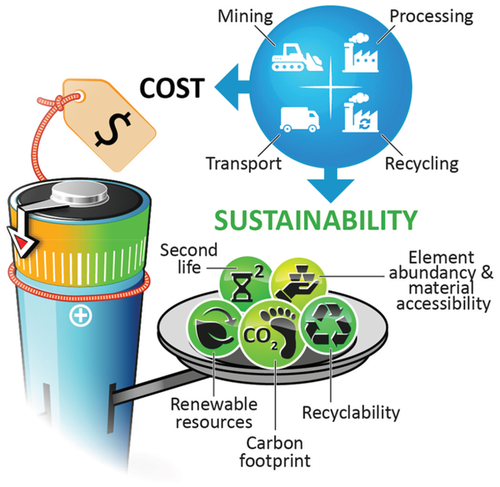
Furthermore, organic precursor-based materials, such as graphite, can be also obtained from synthesis, thus making natural graphite replacement possible. At the end, the situation is more complex and diverse than most of the available studies tell.
5 Second Life and Recycling
All known secondary battery technologies are prone to capacity degradation upon operation and storage. At a battery's end of life (cycle and/or shelf life), which is currently defined for EVs at a state of health below 80% of its initial capacity, it can either be transferred to a secondary, often stationary application (second life), or needs to be recycled in order to retrieve valuable raw materials and therefore closing the material life cycle. Lead–acid batteries, for example, have the biggest share in the battery market not only due to their high maturity and their low costs, but also due to efficient recycling procedures that return more than 85% of spent batteries materials into new batteries.[331]
5.1 Second Life
Over time and due to usage, batteries lose energy storage capability and power, which can reduce the use value in the respective application. For example, an aged traction battery of a car leads to a reduced driving range that does often not satisfy the need of its user anymore.[332] Nevertheless, these batteries still retain a significant energy storage capability and can serve as storage devices for different applications in a second life, mostly as stationary energy storage for the intermittent energy flow of renewable sources such as wind or solar energy.[333] Another second life application for batteries could be as large power bank for homes, EV charging stations, or grid storage in general.[334] For all these purposes, the lifetime costs have a higher priority than energy density[335] and the requirements concerning temperature range and current rates are lower than for EVs. The environmental benefit of second life concepts is that the very high carbon footprint of the battery production can be distributed (diluted) over more years of usage. A recent work about the life cycle assessment of the utilization of repurposed EV batteries to increase photovoltaic (PV) self-consumption in homes published by Bobba et al. underlined that “the environmental benefits obtained are greater when a repurposed EV battery is used in place of a fresh storage battery.”[336] A larger case study of Sathre et al. for California projects that the second life use of retired plug-in hybrid electric vehicle (PEV) batteries can deliver 15 TWh per year, which would lead to a reduction of GHG emissions by about 7 million tons CO2 equivalents per year in 2050.[337] Although the projected electric energy would be only 5% of the current total electricity use in California and uncertainties of the analysis cannot be excluded, this study illustrates the environmental value of the second life application even for smaller PEV batteries. When larger traction batteries are considered, the second life EV battery market could be a lucrative option for car manufacturers or licensed partners, who are able to track the history and prepare the battery for the next application step.[338]
Several criteria have to be met that the second use of batteries is beneficial concerning environmental, economic, and safety aspects. First, the size and type of battery has to fit the energy storage device intended for its second use. The batteries have to be removed from the former device and analyzed for their remaining energy storage capability and safety status before a further use is possible. Additionally, the whole battery or battery module has to be adapted to the new energy storage device including the cooling possibilities and battery management system. Therefore, the second life storage application should be designed for a certain battery or module from a specific parent application such as a certain electric vehicle model (e.g., Tesla Model 3, BMW i3, VW ID.3) since each battery is different in size, cell chemistry, and cooling system.[5] Today, car manufacturing companies are one of few producers of large, standardized battery packs, who can also track the usage history of the battery, which is essential to establish safe and reliable second life applications.[335]
Further, effects of an altered requirement profile in second life applications on the aging and performance aspects of the battery are scarcely investigated so far.[339] The amount of charge/discharge cycles can be much higher in stationary storage than in EV application, thus the “cycling stress” on the battery can accelerate battery aging in its second life more than in the first life. The current trend of growing battery capacity in EVs to extend the driving range could also lead to a lower total number of full charge/discharge cycles in an EV battery lifetime since the same mileage can be achieved. Therefore, future EV batteries may be designed for a smaller total cycle number when energy density and other EV requirements can be increased. However, the amounts of cycles necessary for stationary storage could be much higher. This discrepancy in the requirements between mobile and stationary energy storage might limit the practicability of second battery life concepts.
Although the life-prolonging usage of a battery in a new application area improves its ecological footprint, it has yet to be demonstrated that it is more economically profitable compared to an immediately recycling of the used battery materials accompanied with the production of new batteries. Large amounts of precious Co is contained in the early generations of LIB chemistries, which will not being returned to the battery market via recycling, when these batteries are used for further energy storage in a second life.[340] Considering the price of the present EV chemistries, it may be economically advantageous rather to design a low-cost, not raw-material critical chemistry for stationary energy storage today. This may change in future, when new battery chemistries with less amounts of critical raw materials are available.
5.2 Recycling
As the worldwide demand and production capacities for LIBs (≈160 GWh in 2018) scale up dynamically at compound annual growth rates (CAGR) of 24%,[6] also the amount of required raw materials increases rapidly. In the light of material scarcity of individual raw materials such as Co, Li, and possibly Ni for the cathode and natural graphite for the anode, it is imperative to recycle the growing number of spent batteries,[341] from an ecological, economical, and possibly geopolitical point of view.[11] The generic gravimetric composition of an EV battery system is shown in Figure 19, which illustrates that active materials (excluding the current collectors) account for roughly one quarter to the system mass.
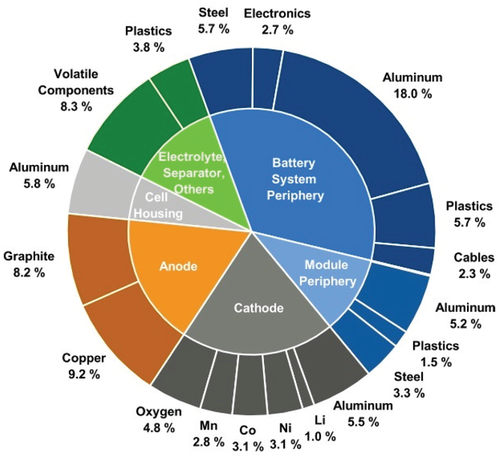
The legislation for battery recycling varies for different regions of the world. According to the battery directive 2006/66/EC of the European Union, member states need to achieve a collection rate of at least 45% and a minimum recycling efficiency of 50 wt% or more.[343] Recycling targets outside the EU are often lower or even voluntary. As the amount of battery waste is expected to increase at a fast pace, lawmakers worldwide will need to revise legislation for battery recycling.
The main recycling challenges are to adapt to the increasing number of battery cells, the larger cell dimensions from spent automotive batteries, and potential changes in the cell chemistry. Currently, the pyro-hydrometallurgical route constitutes the most important recycling processes of LIBs.[344] Major advantages of this route include the possibility to process spent LIBs together with other battery chemistries, including Ni metal hydride or primary alkaline batteries. Furthermore, batteries do not need to be fully discharged prior to recycling, as they are directly burnt in the initial pyrometallurgical step. The worn-out batteries are mixed with slag formers, coke, and other reducing agents and slowly heated up in a vertical furnace to temperatures up to 1450 °C. In this process, two fractions are formed, namely a slag, which contains Al, Si, Ca, and Fe, and an alloy fraction, which includes the valuable transition metals Cu, Co, and Ni. The alloy fraction is then refined in a hydrometallurgical process, which involves the dissolution of the alloy in sulfuric acid and the successive extraction of Cu, Co, and Ni in numerous steps.
Li only accounts for a small mass percentage of current LIBs. Nevertheless, hydrometallurgical processes are developed, which aim at the recovery of Li from the slag and flue dust of the pyrometallurgical step. However, this elaborated Li recovery process is not profitable until now. A major disadvantage of the pyro-/hydrometallurgical recycling process lies in the fact that major cell components, including the graphitic anode and the liquid electrolyte, are irreversibly lost in the initial pyrometallurgical step.[345]
Besides the pyro-hydrometallurgical process, also alternative recycling processes for LIBs have been established. For instance, the mechanical-hydrometallurgical process, which was initially developed for recycling of primary Li batteries, may be applied for rechargeable LIBs, too. However, as it mainly aims at the recovery of Li (whose mass fraction is low in LIBs) and involves an expensive, cryogenic step involving liquid nitrogen, it is not considered an economically viable option for recycling of LIBs at a large scale.
In Germany, another mechanical-hydrometallurgical process is currently under development in the course of a joint research effort between academia and industry within the LithoRec project, which focuses on recycling of automotive batteries (EVs and HEVs).[345] The goal lies in the development of a novel, energy-efficient recycling route in order to achieve high recovery rates of xEV-batteries. A major emphasis of the LithoRec process lies in the mechanical separation,[342] classification, and sorting of the shredded cell components, before they are introduced to a hydrometallurgical step.[36] A schematic overview of the entire LithoRec process is shown in Figure 20.
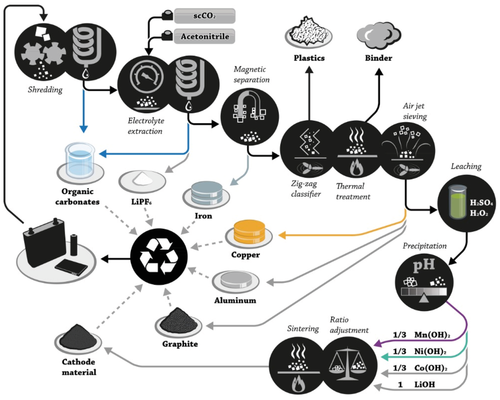
In addition to the costly transition metals Cu, Ni, and Co, also the combustible cell components (i.e., graphite anode,[346] carbonate-based liquid electrolyte[347]) as well as battery-grade Li shall be recovered. The regained Ni and Co could be used to resynthesize Li[Ni1/3Co1/3Mn1/3]O2 (NCM) cathode material,[348] which showed no negative effect on the electrochemical performance despite the presence of certain impurities from the Al current collector. The LithoRec process was evaluated in terms of its ecological and economic feasibility. While it has a high ecological impact, it may only be economically viable at very high throughputs.[349]
The ideal solution from an ecological point of view is the direct recycling process of active battery materials. The main advantage of direct recycling is that the energy-intensive decomposition of the used materials into the educts and the resynthesis into new active materials can be entirely omitted.[350] There are, however, several obstacles to overcome, including the separation of active materials from the rest of the components in a simple and scalable way and ensuring a high quality of the materials, which is difficult due to the inevitable deterioration of the materials during usage. Although several promising results have been achieved,[346, 351] and even if the regained materials reach their original quality, the fast development progress of new battery active materials over the time of battery usage (>8 years) will make the regained active material most likely inferior to materials at the market at that time. When standardized components are available, direct recycling of other battery parts, e.g., the housing or current collectors, could strongly enhance the economic and environmental value of the battery in the future.
6 Battery Development Strategies for a More Sustainable Future
With the transition toward electrical energy usage in more and more situations of our life, the necessity to store energy drives the continuous growing demand for efficient batteries. The resulting rise of battery mass production raises the important question about the energy balance and sustainability of current and future battery technologies. The production and development of LIBs, considered as today's SOTA battery technology, however, is characterized mainly by application-oriented key performance factors (e.g., energy density, power density, cost, etc.). High energy consumption of material and battery production, depleting resources of critical raw materials and low recycling rates contradict sustainability aspects of current LIBs (Figure 21a) and could lead to a severe environmental impact and uncertain production conditions in a possible LIB-rich future.
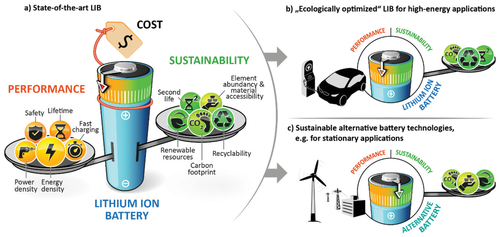
Our review of greener alternative materials and concepts for the LIB technology shows that nearly all parts of a conventional LIB could be replaced with materials fulfilling most of the depicted sustainability aspects. At present, a “completely green” LIB cell, however, would be unlikely to offer also a performance comparable to current LIB cells. The reason is that the evolutionarily developed present LIB materials are selected to match the high requirements on the electrochemical processes, whereas the green alternatives exhibit one or more material-inherent drawbacks regarding energy density, cycle life, etc., and are often not cost-competitive—at least, yet.
From our point of view, two different development strategies could achieve a more sustainable future for electrochemical storage technologies depending on the application field. First, portable and mobile applications (e.g., consumer electronics, e-mobility, aviation) rely on a safe, fast-charging energy storage with focus on high energy density and sufficient power, hitherto realizable only by LIBs (Figure 21b). Besides an incremental improvement of the LIB carbon footprint due to ecological advances of the inactive materials (e.g., binder, housing) and processing (e.g., water-based production), the main contributors to a more sustainable high-energy LIB are an increased lifetime in combination with second life applications and especially an effective recovery of the battery materials via stringent recycling. Nowadays, high energy densities, which are crucial for mobile applications, can be solely delivered by a few active material combinations underlining the importance of “closing the battery material life cycle” (Figure 2). A few “alternative-to-Li” (=“post-Li”) technologies (e.g., Al- and Mg-based approaches) may—under specific conditions—offer high enough energy contents and might be greener, complementary energy storage systems to the LIB. All these concepts are, nevertheless, still in early R&D stages and immature for actual application and probably also not for a fair comparison with practical LIBs.[7] When volumetric energy density is less critical, Li ion materials based on more abundant resources (e.g., LFP) become more interesting either for specialized high-power applications (e.g., public transport vehicles) or for small commercial home energy storage systems. In case of large stationary storage applications, when volume and weight of the battery system do not matter, the inevitable large amounts of active materials determine cost, material availability, energy efficiency and operation lifetime as the most important criteria (Figure 21c). This application area might be a bright opportunity for alternative battery technologies based on abundant (e.g., Na, S) and/or renewable materials (e.g., organic compounds), which offer lower energy densities than LIBs and LMBs but are considered to be more independent from critical resources, potentially lower cost and more environmentally friendly. Following the current trend toward more renewable and decentralized energy production, new efficient, cheap and sustainable energy storage technologies are urgently needed.
Both ways, establishing a closed battery life cycle through the recovery of critical materials and the development of new cell technologies including more accessible and abundant materials cannot only lead to a higher sustainability but can also save costs and reduce the import dependency of resources, thus overcoming three main challenges of a battery-rich future.
Acknowledgements
The authors wish to thank the Ministry of Economic Affairs, Innovation, Digitalization, Energy of the State North Rhine-Westphalia in Germany (MWIDE NRW), the German Federal Ministry of Education and Research (BMBF), and the German Federal Ministry for Economic Affairs and Energy (BMWi) for funding this work in the projects “GrEEn” (313-W044A), “MgMeAnS” (03XP0140), and “Go3” (03ETE002D), respectively. The authors thank further Andre Bar for his great graphical contributions to this manuscript.
Conflict of Interest
The authors declare no conflict of interest.
Biographies

Simon Dühnen is a postdoctoral researcher at the MEET Battery Research Center at Münster University. His research interests cover green and renewable battery materials for the development of sustainable energy storage. His research focus lies on the investigation of novel battery active materials (e.g., metal–organic frameworks) and the exploration of new cell concepts (e.g., dual-ion batteries). He is coordinating the German joint research project “GrEEn—Green Electrochemical Energy Storage,” a collaboration of several universities and research institutes developing a new battery concept based on sustainable materials and recycling approaches.

Johannes Betz conducts research at the MEET Battery Research Center on cathode synthesis and electrochemical investigations for lithium batteries. After doing an internship focusing on safety investigations for lithium ion batteries at Robert Bosch GmbH in Renningen, Germany, he visited Prof. Steve Martin's group at the Iowa State University in Ames, USA, to investigate ion-conducting glasses. He received his master's degree in 2016 at the MEET Battery Research Center under Prof. Martin Winter, where he investigated different layered transition metal oxide cathode materials. Since then, he has conducted studies on various methods to improve the energy content of lithium cells at material level.

Martin Winter has been researching in the field of electrochemical energy storage and conversion for almost 30 years. His focus is on the development of new materials, components, and cell design for lithium-ion and lithium-metal batteries and alternative battery systems. He is founder and scientific director of the MEET Battery Research Center at Münster University and of the Helmholtz-Institute Münster (HI MS), a division of Forschungszentrum Jülich.