Overcoming the voltage losses caused by the acceptor-based interlayer in laminated indoor OPVs
Abstract
Harvesting indoor light to power electronic devices for the Internet of Things has become an application scenario for emerging photovoltaics, especially utilizing organic photovoltaics (OPVs). Combined liquid- and solid-state processing, such as printing and lamination used in industry for developing indoor OPVs, also provides a new opportunity to investigate the device structure, which is otherwise hardly possible based on the conventional approach due to solvent orthogonality. This study investigates the impact of fullerene-based acceptor interlayer on the performance of conjugated polymer–fullerene-based laminated OPVs for indoor applications. We observe open-circuit voltage (VOC) loss across the interface despite this arrangement being presumed to be ideal for optimal device performance. Incorporating insulating organic components such as polyethyleneimine (PEI) or polystyrene (PS) into fullerene interlayers decreases the work function of the cathode, leading to better energy level alignment with the active layer (AL) and reducing the VOC loss across the interface. Neutron reflectivity studies further uncover two different mechanisms behind the VOC increase upon the incorporation of these insulating organic components. The self-organized PEI layer could hinder the transfer of holes from the AL to the acceptor interlayer, while the gradient distribution of the PS-incorporated fullerene interlayer eliminates the thermalization losses. This work highlights the importance of structural dynamics near the extraction interfaces in OPVs and provides experimental demonstrations of interface investigation between solution-processed cathodic fullerene layer and bulk heterojunction AL.
1 INTRODUCTION
Solution-processed organic solar cells are a promising solar cell technology with the potential for scalable production using wet deposition techniques such as printing and coating leading to a low environmental impact.1 However, the combination of high power-conversion efficiencies and long lifetime require more understanding and development to compete with established technologies. A niche market for organic photovoltaics (OPVs), lately gaining much attention also in academic publications,2-4 is indoor applications to power, for example, connected sensors and electronic shelf labels. Under indoor climate, the main degradation challenges associated with elevated temperature and light-induced “burn-in” is not a limiting factor for a lifetime.
The interfaces in OPV devices, starting from the substrate–electrode interface to those within the active layer (AL), play a crucial role in determining the efficiency of the device.5, 6 Among these interfaces, two types, in particular, receive significant attention in typical heterojunction OPVs, namely, the semiconductor–semiconductor interface between the donor and acceptor7, 8 and the semiconductor–metal interface between the AL and electrodes.9, 10 The electronic properties of solution-processed OPVs are strongly dependent on morphological characteristics, which include semicrystalline size at the nanoscale, percolation at the mesoscale, and structural distribution at the macroscopic scale.11-13 The bulk heterojunction (BHJ) approach, which involves interpenetration of the donor and acceptor, is a commonly used technique in OPVs. However, it is challenging to predict the final morphology of the film from the solution.14 An ideal arrangement of donor and acceptor, characterized by highly ordered domains of donor and acceptor, with the donor predominantly at the anode electrode and the acceptor predominantly at the cathode electrode, has been suggested by simulations15, 16 (Figure 1A).
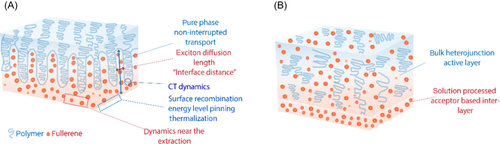
As depicted in Figure 1A, the ideal AL arrangement in OPVs requires careful consideration of multiple parameters to achieve high performance. One of the critical parameters is the charge transfer (CT) dynamics at the donor–acceptor interface, where the energy of the CT state (ECT) governs charge carrier separation and recombination, thereby impacting the maximum open-circuit potential.17, 18 Additionally, the effective charge generation is facilitated by a domain width that is two times the size of the exciton diffusion length.19 The ordered arrangement of donor and acceptor domains also ensures noninterrupted transport through the pure phase.15, 20 Furthermore, the energy alignment at the semiconductor–metal interface, which influences the open-circuit voltage (VOC) and device performance, is dictated by the electrical, chemical, optical, and film-forming properties of the transport layers.9, 21, 22 There have been numerous studies on the nonradiative recombination losses at the electrode–AL interface, including surface recombination, energy level pinning, and thermalization losses.21, 23, 24
While the ideal AL arrangement implies positive effects of pure donor (acceptor) compositions at the anode (cathode) side, recent studies demonstrated a counterintuitive approach to the ideal arrangement by adding a pure acceptor phase on the “wrong” anode electrode. Incorporation of the acceptor phase on the anode electrode enhances device VOC; however, the mechanism behind the increased VOC is still under debate. Ding and Forrest25 demonstrated that the VOC loss that comes from a static dipole at the active region–anode interface can be reduced by inserting a tunnel-thin acceptor layer on the anode. Kotadiya et al.26 reported that the acceptor incorporation on the anode leads to Ohmic contact formation that suppresses surface recombination losses, while Pranav et al.27 suggested that the suppression of the surface recombination is due to an enhanced built-in potential. Although these studies come in handy in understanding the interfacial energy losses and enhancing VOC output, they are limited by the thickness of the inserted tunnel-thin interlayer. As shown in these reports, when interlayer thickness exceeds the threshold of tunneling, charge transport will be hindered. Moreover, there are hardly any studies on the influence of the structural order of the acceptor-based interlayers on the energy loss of OPVs. A comprehensive understanding of the ideal arrangement near the electrode interface and strategies to mitigate energy losses are necessary for maximizing the potential of OPVs.
This study aims to increase the understanding of energy losses at the AL/cathode interface. We focus on BHJ ALs and interlayers based on fullerenes, where we investigate the impact of the fullerene-based acceptor interlayer on the performance of conjugated polymer–fullerene-based OPVs for indoor applications (Figure 1B). We focus on solution processing of bulk thick (nontunneling) acceptor-based interlayers, as the insensitivity of interlayers to film thickness is of critical importance for industrial upscaling.28 For many years, efforts have been made to achieve consistent work batch-to-batch variations, purification, and reproducibility in the context of fullerene-based OPV upscaling. While nonfullerene acceptors (NFAs) have pushed the power-conversion efficiency to a high value approaching 30% (at 2000 lx, warm light-emitting diode [LED]),29 such efforts for upscaling are still in their early stages with respect to NFAs, motivating us to use fullerenes as a case of study here, especially considering that our focus is for industrial applications. NFA-based interlayers are interesting for future case studies but are outside the scope of this manuscript. We employ an industrially applicable lamination technique,30 which enables an investigation of the interface between solution-processed cathode [6,6]-phenyl-C61-butyric acid methyl ester (PC61BM) and polymer:PC61BM-based OPVs, deposited from nonorthogonal solvents. Although the utilization of the interlayer PC61BM would intuitively give an optimal device performance, we found that the PC61BM interlayer leads to a reduced VOC. To address this challenge, we successfully incorporated insulating organic elements, specifically polyethyleneimine (PEI) and polystyrene (PS), into the PC61BM interlayers. This integration yielded two distinct benefits: a lowered work function (Wf) of the cathode and the potential repulsion of minority carriers for the PC61BM:PEI interlayer, as well as an enhanced energetic alignment between the interlayer and the AL for the PC61BM:PS interlayer, improving overall device performance. Neutron reflectivity (NR) studies further uncover that the fundamental mechanisms of decreased Wf are different in both cases: in the case of PEI incorporation, a tunnel-thin layer of self-arranged PEI on top of PC61BM could potentially eliminate hole transfer from the BHJ AL blend to the acceptor interlayer, leading to a reduced hole population on the highest occupied molecular orbital (HOMO) states, hence reducing the recombination losses; in the case of PS incorporation, the structural order of the film is improved, creating a gradient distribution of the PC61BM:PS mixture, hence decreasing the thermalization losses due to the acceptor interlayer. Our work provides one of the experimental demonstrations of interface investigation across the solution-processed cathodic fullerene layer and BHJ, highlighting the importance of understanding the morphological arrangement of the interlayers on the device VOC. Our finding is also aligned with recent development in perovskite solar cells, where fullerene is frequently used as an interlayer.31, 32
2 EXPERIMENTAL SECTION
2.1 Materials and solution preparation
Commercial PBTZT-stat-BDTT was purchased from Raynergy Tek and PC61BM and phenyl-C71-butyric acid methyl ester (PC71BM) were obtained from NanoC. Poly [[6,7-difluoro[(2-hexyldecyl)oxy]-5,8-quinoxalinediyl]-2,5-thiophenediyl]] (PTQ10) with Mw of 49 kDa and Mn 17 kDa and poly[(2,6-(4,8-bis(5-(2-ethylhexyl-3-chloro)thiophen-2-yl)-benzo[1,2-b:4,5-b′]dithiophene))-alt-(5,5-(1′,3′-di-2-thienyl-5′,7′-bis(2-ethylhexyl)benzo[1′,2′-c:4′,5′-c′]dithiophene-4,8-dione)] (PM7) with Mw of 98 kDa and Mn 41 kDa were purchased from Brilliant Matters. O-xylene and methylnaphthalene (MN), diphenyl ether (DPE), and chloroform (CF) were obtained from Sigma-Aldrich and used as received. Commercial ETL inks formulations (SnO2 and ZnO) were purchased from Avantama and used after 10 min horn sonication. Commercial copper(I) thiocyanate solution (CuSCN) was purchased from Sigma-Aldrich. The PC61BM interlayer solution was prepared by dissolving 10 g/L PC61BM powder either in CF or o-xylene. A 10 g/L of PEI (branched with Mw 25 kDa and Mn 10 kDa; Sigma-Aldrich) formulation was prepared in CF, whereas 10 g/L PS (with Mw of 35 kDa; Sigma-Aldrich) formulation was dissolved in o-xylene and MN with a ratio of 85:25 (w/w). Optimized PC61BM:PEI formulation was obtained from 9 vol% of PEI in PC61BM solution. Optimized PC61BM:PS formulation was achieved from 11 vol% of PS in the PC61BM solution. Poly(3,4-ethylenedioxythiophene): polystyrene sulfonate (PEDOT:PSS) (Clevios PH1000) was obtained from Heraeus GmbH. The pristine PH1000 was mixed with ethylene glycol (from Sigma-Aldrich) and Capstone FS-30 surfactant (Dupont) in a volume ratio of 93.5:6:0.5 (PH1000:EG:FS-30) and further diluted in deionized water in a 2:1 ratio (v:v, ink:water). A measure of 125 μm heat-stabilized polyethylene terephthalate (PET) Melinex ST505 (Tekra) was utilized as a substrate.
2.2 Device fabrication and electrical characterization
Fabrication of laminated OPV devices started from the slot-die coating (Solar X3; FOM Technologies) PEDOT:PSS on top of PET foils, followed by baking at 130°C for 15 min. The cathode side of the device was completed by spin-coating 32 nm SnO2 and annealing at 115°C for 2 min in air. For reference AL/AL laminated devices, ALs were spin-coated on both cathode and anode stacks and were annealed at 80°C for 2 min in the glovebox. PBTZT-stat-BDTT and PC61BM were blended in a 1:1.5 (w:w) ratio in o-xylene:methylnaphthalene (85:15, v:v) at 45 g/L total concentration. PTQ10:PC61BM ink was prepared 1:1.5 (w:w) ratio in o-xylene:DPE (85:15, v:v) at 40 g/L total concentration.33 PM7:PC61BM ink was prepared 1:1.5 (w:w) ratio in o-xylene:DPE (85:15, v:v) at 45 g/L total concentration. For AL/acceptor-based interlayer laminated devices, PC61BM, PC61BM:PEI, or PC61BM:PS solutions were spin-coated on the cathode stack on top of SnO2 at 2000 r/min for 30 s and annealed at 80°C for 2 min in the glovebox. ALs were coated on the anode stack. The resulting stacks were prepatterned with a scalpel to isolate three active areas per substrate. The active area is subject to subjective errors of ±0.05 cm2. The cathode and anode stacks were laminated in the air using a roll laminator (GSS DH-650S; Graphical Solutions Scandinavia AB) at a roll temperature of 115°C and a force of ∼50 N (measured with a force sensor FlexiForce A201; Tekscan). In the end, the laminated devices were placed between two glass slides to provide mechanical support for easier handling. Additionally, silver paint (Agar AGG302) was applied to the exposed PEDOT:PSS contacts. It is important to note that the devices were not encapsulated.
The current–density–voltage (J–V) curves were obtained by using a Keithley 2400 SourceMeter while the devices were under cool LED irradiation at a temperature ranging from 20°C to 25°C. The LED source's emission spectra and irradiance (Supporting Information: Figure S1) were measured using a high-precision fiber optics spectrometer (QE-Pro; Ocean Optics) and a Hamamatsu silicon photodiode S1133-01. Integrating the corresponding emission spectrum obtained34 from the specific device location, the illuminance, power density, and current density were calculated as 552 lx, 148 µW/cm2, and 67 µA/cm2, respectively.
2.3 Photoelectron spectroscopy (ultraviolet photoelectron spectroscopy [UPS])
The substrates used for UPS and NR were cleaned via sonication in detergent, followed by sequential washing in deionized water, acetone, and 2-propanol. The films (18–30 nm) of fullerene-based interlayers were spin-coated and annealed in the glovebox according to the previously mentioned fabrication methods. The samples were transferred to the load lock chamber of the ultrahigh vacuum system. The UPS experiments were performed in a home-designed spectrometer. The excitation source was monochromatic He I radiation with a photon energy of 21.22 eV. The WF was derived from the secondary electron cutoff and the vertical ionization potential from the frontier edge of the occupied density of states with an error margin of 0.05 eV. All the measurements were executed with a base pressure lower than 1 × 10−9 mbar.
2.4 External quantum efficiency (EQE)
The Solar Cell Spectral Response Measurement System QE-R3011 from Enli Technology Co., Ltd. was employed for EQE. To obtain the EQEEL (external quantum efficiency—electroluminescence) values, an in-house built system was utilized. This system consisted of a Hamamatsu silicon photodiode 1010B, a Keithley 2400 SourceMeter for voltage supply and recording injected currents, and a Keithley 485 picoammeter to measure the intensity of emitted light.
2.5 Fourier transform photocurrent spectroscopy-EQE (FTPS-EQE)
For FTPS-EQE measurements, a Bruker Optics Vertex 70 spectrometer was employed. The spectrometer was equipped with a quartz tungsten halogen lamp, a quartz beam splitter, and an external detector option. To amplify the photocurrent produced when the photovoltaic devices were illuminated with modulated light from a Fourier transform infrared spectroscopy (FTIR), a low-noise current amplifier (SR570) was used. The output voltage from the current amplifier was then fed back into the external detector port of the FTIR, enabling the FTIR's software to collect the photocurrent spectrum.
2.6 NR
The NR experiments were carried out using the reflectometer MORPHEUS located at SINQ (Paul Scherer Institut). During NR experiments, a neutron beam was directed at the sample surface under a small angle (θ), reflected at each composition interface, and detected. The samples were mounted vertically, and the reflected beam was detected using a He-3 detector. The intensity of the reflected beam was measured as a function of the scattering vector normal to the surface Qz = 4π(sin θ)/λ, with λ being the neutron wavelength. The wavelength used in the experiments was 4.8 Å. Samples were measured from 0° to 3° in 2θ.
2.7 Atomic force microscopy (AFM)
AFM measurements were performed with a dimension 3100 system using antimony-doped silicon cantilevers in tapping mode NR.
3 RESULTS AND DISCUSSION
Our study was conducted on polymer:fullerene BHJ AL PBTZT-stat-BDTT:PC61BM- (Supporting Information: Figure S2) based OPV devices, characterized under typical indoor illumination. The devices were fabricated via the lamination method on flexible PET substrates. Cathode and anode stacks were processed independently, and placed in between two counter-rotating rolls to join them using lamination under certain pressure and temperature, shown in Figure 2A (see Supporting Information for further details). The anode side of the stack includes a slot-die-coated highly conductive PEDOT:PSS layer, which works as the electrode and hole-transport layer, and a spin-coated BHJ AL. The cathode stack consists of a slot-die-coated PEDOT:PSS layer as the electrode, a spin-coated SnO2 followed by a spin-coated BHJ AL (Figure 2C). These laminated semitransparent devices show a fill factor (FF) of 0.75, short-circuit current density JSC of 33 µA/cm2, and VOC of 0.62 V under 552 lx illumination conditions (equivalent to an irradiance of 148 µW/cm2, see details in Supporting Information: Figure S1).
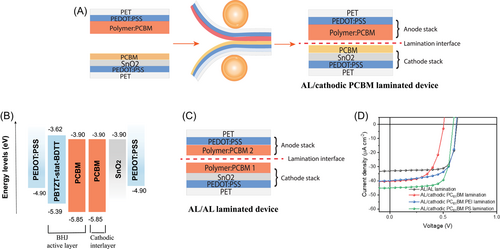
To approach what could be considered a naive “ideal” morphology model (Figure 1A), we now want to add a pristine electron-accepting PC61BM layer between the PBTZT-stat-BDTT:PC61BM AL and the SnO2 interlayer to investigate whether the pure phase of the acceptor layer deposited on the favorable electrode would lead to optimal performance. The above-mentioned lamination technique provides the possibility to study the interface of the solution-processed BHJ AL and cathodic fullerene layer, without the risk of dissolving the underlying layer. Since the BHJ AL has a favorable pristine acceptor phase as an interlayer close to it, one would assume that in ideal scenario energy levels are well aligned for achieving a high device performance (Figure 2B).
Figure 2D shows the J–V characteristics of BHJ AL/AL laminated devices compared to BHJ AL/cathodic PC61BM laminated devices under 552 lx light illumination. Surprisingly, the pure fullerene acceptor phase between the AL and SnO2 to the cathode stack shifts the (J–V) characteristics strongly to lower VOC and FF, whereas JSC has increased moderately. The VOC difference between devices with BHJ AL/AL lamination and BHJ AL/cathodic PC61BM lamination is 120 ± 20 mV. Since the VOC loss occurs when the AL is changed to the acceptor interlayer, we evaluate the origin of the VOC loss at the cathode stack by examining alternative interlayers. By altering the first interlayer in the cathode stack from SnO2 to ZnO as well as changing the cathodic acceptor-based interlayer PC61BM layer to PC71BM layer (Supporting Information: Figure S3A,B), we observe similar VOC losses. Therefore, we conclude that the VOC loss likely arises from the interface between the AL and PC61BM interlayer.
3.1 Elimination of VOC losses by the utilization of PC61BM:PEI and PC61BM:PS cathodic acceptor-based interlayers
Studies on perovskite solar cells with fullerenes as an interlayer have shown that fullerenes can result in nonradiative recombination losses. It was shown that the critical loss process in high-performing p–i–n-type solar cells is a surface-mediated process that occurs in the first monolayer of fullerenes.35 There have been studies where PCBM interlayers were modified with insulating molecules (e.g., PEI or PS) to achieve high-quality pinhole-free films, resulting in passivation of the trap states at the perovskite surface and a reduction of nonradiative recombination losses.31, 32 These studies provide a strategy to mitigate the VOC losses across the perovskite–interlayer interface.
Inspired by the developments in perovskite solar cells, we have implemented these strategies to investigate the effects of these insulating molecules mixed with PC61BM on the performance of the laminated OPVs. Devices with anode stacks with BHJ AL laminated to cathode stacks with PC61BM:PEI (9 vol% PEI) show an increased VOC of 0.62 V and FF of 0.68, and interlayer PC61BM:PS (11 vol% PS) display an improved VOC of 0.60 V and FF of 0.74, compared to 0.49 V VOC and 0.60 FF for pristine PC61BM-based interlayer (Figure 2D). Modified cathodic PC61BM interlayer-based devices exhibit similar VOC as BHJ AL/AL laminated devices (Table 1). These results strongly suggest that thermalization loss at the pure phase of PC61BM on the cathode electrode could result in the loss of VOC. By diluting the cathodic PC61BM with either PEI or PS, we speculate that the thermalization losses are eliminated and conditions for a better energetic alignment are created. To investigate the sensitivity of PS and PEI to PC61BM vol%, three stoichiometries were evaluated, all with a similar increase of VOC compared to pure PC61BM cathode (Supporting Information: Figures S4 and S5).
| Lamination interface | Pin (µW/cm2) | JSC (µA/cm2) | VOC (V) | FF | Pout (µW/cm2) | PCE (%) |
|---|---|---|---|---|---|---|
| AL/AL | 148 | 33 ± 5 | 0.62 | 0.76 | 15.50 | 10.47 |
| AL/pristine PC61BM | 148 | 40 ± 5 | 0.49 | 0.60 | 11.70 | 7.90 |
| AL/PC61BM:PEI | 148 | 40 ± 5 | 0.62 | 0.68 | 16.80 | 11.35 |
| AL/PC61BM:PS | 148 | 44 ± 5 | 0.60 | 0.74 | 19.50 | 13.17 |
- Abbreviations: AL, active layer; BHJ, bulk heterojunction; PC61BM, [6,6]-phenyl-C61-butyric acid methyl ester; PC61BM:PEI, [6,6]-phenyl-C61-butyric acid methyl ester polyethyleneimine; PC61BM:PS, [6,6]-phenyl-C61-butyric acid methyl ester polystyrene; PEDOT, poly(3,4-ethylenedioxythiophene); PSS, polystyrene sulfonate.
To examine the generality of the energy loss across the interface of polymer:PC61BM and interlayer PC61BM, we have investigated two more donor materials, PTQ10 and PM7 with PC61BM as the acceptor. The molecular structures of the materials used in the investigation are shown in Supporting Information: Figure S6A,B. We find that the previously observed voltage loss when PC61BM was used as an interlayer and performance enhancement with PC61BM interlayer is applicable to all the tested donor materials (Supporting Information: Figure S7A,B).
To gain further insights on VOC loss in these interlayers, we utilized FTPS-EQE and EQEEL and analyzed the contributions of radiative and nonradiative charge recombination (Supporting Information: Figure S8). Additionally, we determined the energy gap (Eg) by examining the derivative of the EQE edge,36, 37 which was found to be 1.81 eV despite the variations in the interlayer. The radiative loss, calculated using the formula , remains constant (1.00 eV) despite changes in the interlayer. This suggests that radiative recombination makes negligible contributions to the difference of the VOC loss. The nonradiative loss, calculated using the formula , yields a value of 0.41 V for the PC61BM interlayer, while PC61BM:PEI and PC61BM:PS interlayer-based devices show a value of 0.36 V, largely suppressing the VOC loss compared to pristine PC61BM interlayer-based devices. In summary, our findings indicate that VOC loss in fullerene-based interlayers is not primarily influenced by radiative recombination, but rather by nonradiative recombination losses. Introducing insulating molecules into the PC61BM interlayer effectively mitigates these nonradiative losses at the interfaces between the absorbing layer and cathodic acceptor interlayers.
3.2 Interfacial energy loss dependence on energy levels of the device
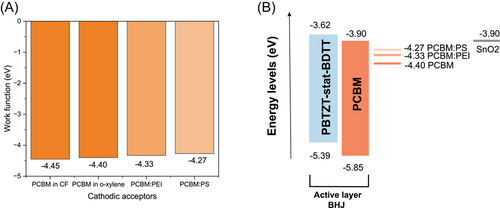
To investigate why the mixture of insulating molecules with PC61BM can improve the device VOC compared to the pristine PC61BM, UPS measurement was performed on fullerene-based interface layers (Supporting Information: Figure S9). It was found that the Wf of the pristine PC61BM varies from 4.40 to 4.45 eV depending on the processing solvent (Figure 3A). The energy diagram of the devices with and without modification of PC61BM interlayer is illustrated in Figure 3B. The addition of PEI into PC61BM lowers the Wf to 4.33 eV, while PC61BM:PS also shows a reduced Wf of 4.27 eV. One would assume that the energy level alignment was intuitively well matched from the pristine PC61BM interface layer to polymer:PC61BM-based AL. However, we observed a significant VOC loss as shown in Figure 2D. The reduced Wf due to the addition of either PEI or PS to PC61BM resulted in an improved VOC compared to pristine PC61BM-based devices. For PC61BM:PEI interlayer compared to pristine PC61BM, we see a VOC difference of 0.13 V and a Wf difference of 0.12 eV, while for PC61BM:PS interlayer compared to pristine PC61BM, the difference in VOC is 0.11 V and in Wf is 0.13 eV, resulting in a change in Wf and VOC of similar magnitudes. This indicates that reduced Wf leads to a better energetic alignment across the AL and acceptor-based interlayer, increasing the device VOC.38, 39 Possible mechanisms for the reduced Wf of the insulating molecule incorporated acceptor interlayers are discussed in the following section.
3.3 Interfacial energy loss dependence on the vertical distribution of the acceptor-based interlayers
Since the addition of PEI or PS in PC61BM film helps to reduce the VOC loss, we are motivated to understand the mechanism behind the device VOC increase and Wf reduction. Therefore, we move forward to investigate the interaction between PC61BM and PEI or PC61BM and PS, especially considering that microstructure-related aspects like vertical phase separation could be very important. In the field of OPVs, NR has been employed as a technique for exploring the vertical stratification of BHJ ALs.40, 41 In this study, we have employed NR as a means of investigating the influence of interlayer's vertical arrangement on the performance of OPV devices. NR was performed to determine component distribution normal to the surface, enabling us to see the influence of PEI or PS on PC61BM. The PC61BM:PEI in CF or PC61BM:PS in o-xylene were deposited on SnO2/SiOx/Si substrate to ensure the corresponding cathode interface properties of AL/cathodic PC61BM laminated devices. The experimentally measured reflectivity curves were fitted using GenX (details in Supporting Information: Figure S10) to obtain the neutron scattering length density (SLD) distributions. The corresponding SLD depth profiles derived from fitting are shown in Figure 4A–C. Fitted SLDs of SnO2, PC61BM from o-xylene, PEI, and PS were found to be 3 × 10−6 Å−2, 3.8 × 10−6 Å−2, 2 × 10−6 Å−2, and 1.2 × 10−6 Å−2, respectively. Note that the SLD of the interface between the Si substrate and SiOx is shown by the abrupt increase of SLD located at 0 Å in the depth profile. On top of the SiOx, the thickness of the SnO2 layer ranged from 320 to 450 Å. Since the gradual change in SLD from one layer to the next represents the roughness of the film, the comparison of Figure 4A–C indicates that the roughness of the PC61BM layer at the air interface is decreased upon the addition of PEI or PS, consistent with the results obtained using AFM in Supporting Information: Figure S11.
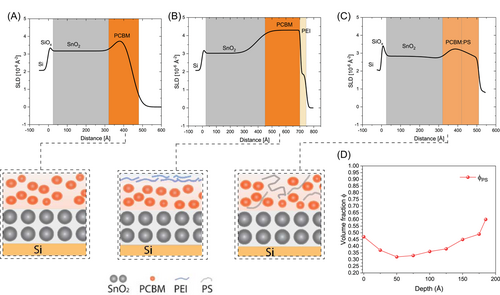
Upon the addition of PEI to PC61BM, the surface of the film (air interface) is significantly enriched with a layer of ~5 nm PEI with an SLD of 2 × 10−6 Å−2, depicting strong surface segregation of PEI from PC61BM (Figure 4B). This is different from pristine PC61BM (Figure 4A), which is composed of one layer with a thickness of 17 nm. We find that PC61BM on top of SnO2 is characterized by an SLD of 4.1 × 10−6 Å−2, implying that the blend has a composition of around 23 nm pure PC61BM. The SLD value of pure phase PC61BM in the PC61BM:PEI blend shows a different value from the SLD of the pristine PC61BM (Figure 4A) due to the processing from different solvents. The results indicate total segregation of PEI from PC61BM. We believe that the phase segregation of PEI on the top surface originates from the surface energy and chemical structure difference. With the NR results on the vertical phase separation, we can now rationalize why the PEI addition enhances the VOC. The reduced VOC observed in pure acceptor interlayer devices we tentatively ascribe to the ambipolar nature of PC61BM,42, 43 which facilitates the transfer of both electrons and holes. This characteristic increases the likelihood of hole (minority carrier) back-transfer from the BHJ polymer HOMO to the HOMO of the acceptor interlayer, leading to recombination losses in the pure cathodic PC61BM phase. Conversely, the presence of self-arranged PEI between the AL and acceptor interlayer potentially eliminates hole transfer from the AL blend to the pure acceptor interlayer, resulting in an increased VOC and decreased Wf. The increased PC61BM content has a strong influence on the device VOC and FF, which could be translated to longer extraction times and more likelihood of recombination events (Supporting Information: Figure S12).
In the case of PS-added PC61BM, the vertical phase separation is different from PEI-added PC61BM, although we also observed Wf reduction from the UPS measurements. In this case, a gradient film is observed on top of the SnO2 layer. To quantify the vertical distribution of the components, one can estimate the volume fraction of PC61BM and PS. A mass conversion equation was used to calculate and : where is the volume fraction of PS. The PC61BM:PS gradient film exhibits three regions with PC61BM ratios of 53 vol%, 68 vol%, and 40 vol% at the SnO2 interface, in the middle part, and at the air interface (Figure 4D). Overall, PC61BM:PS mixture at the bottom, PC61BM enrichment in the middle, and PS enrichment at the top surface area interpreted by the NR fitting. Therefore, the PS addition has a different mechanism for improving the device VOC compared to the self-assembled layer of PC61BM:PEI as stated above. The mixture between PS and PC61BM enhances the structural ordering PC61BM, resulting in a narrower width of the LUMO, and thereby decreasing the thermalization losses. This reduction in the thermalization losses leads to a decrease in the negative pinning energy and a subsequent decrease in the Wf at the contact.
Based on the observed changes in vertical arrangement, Wf, and pinning energy, it is speculated that the electron affinity (EA) of PC61BM interlayers also undergoes modifications when using insulating molecules. This phenomenon can be attributed to two potential reasons. First, the introduction of a PS “wetting layer” at the interface between PC61BM and the cathode may result in a decoupling effect.10 This implies that the screening of electrons in the PC61BM LUMO by the cathode is reduced, making the interface EA more closely resemble the bulk EA. Consequently, thermalization losses decrease.
The second possibility is that the introduction of PS induces changes in the film morphology, thereby affecting the stacking of PC61BM. This alteration may lead to a sharper edge in the LUMO density of states at the interface. Consequently, the density of states does not extend as far into the energy gap, resulting in reduced thermalization losses. Both scenarios ultimately contribute to an improved energy level matching at the interface of the BHJ AL–cathodic acceptor interlayer, as discussed in our manuscript.
Our findings demonstrate the potential to significantly reduce VOC loss across the AL and cathodic interlayer interface. This paves the way for the development of materials with improved interface properties and decreased energy losses. These results open new avenues for the design and optimization of indoor OPVs for industrial applications. Further investigations and optimizations are warranted to fully explore the potential of these advancements in enhancing device performance and efficiency.
4 CONCLUSIONS
In conclusion, inspired by the ideal morphology of OPV devices, we have investigated the effects of inserting pristine PC61BM as an interlayer between the AL and the cathode electrode enabled by the industrially viable method, lamination. We find that the addition of the pure fullerene acceptor phase strongly decreases the VOC of OPV devices. Our investigation shows that the VOC loss arises from the interface between the AL and the PC61BM interlayer. The VOC loss is mitigated upon the addition of insulating molecules (e.g., PEI or PS) into PC61BM. In spite of similar efforts in improving the VOC, UPS and NR measurements elucidate that PEI and PS have fundamentally different mechanisms for decreasing the VOC losses. Upon adding PEI to OPV devices, a self-assembled tunnel layer forms spontaneously between the AL and PC61BM interlayers. This layer may reduce hole transfer from the BHJ AL to the acceptor blend, thereby potentially eliminating the hole population at the acceptor interlayer's HOMO and reducing recombination losses. The change in the Wf and the vertical arrangement of PC61BM with PS insulating matrix can lead to reduced thermalization losses, facilitating an improved energetic alignment across the interface. Our results highlight the importance of the rational design of interlayer materials for industrial development of OPVs. This work underscores the critical role of deliberate interlayer material design in driving the industrial advancement of indoor OPVs. The insights gained from this study provide valuable guidance for researchers and engineers working toward the practical implementation of efficient and commercially viable OPV devices.
ACKNOWLEDGMENTS
The authors acknowledge the beamline on the neutron reflectometer MORPHEUS located at SINQ, Paul Scherer Institut, Villigen, Switzerland; Dr. Xabier Rodriguez-Martinez for the acquisition of indoor light irradiance; Dr. Tiankai Zhang for the help in acquisition of the AFM data. G. B., J. B., and F. G. acknowledge the Swedish Foundation for Strategic Research (SSF) (No. ID20-0105). A. Z. and F. E. acknowledge the Swedish Research Council (LiU No. 2019 00653) for financial support.
CONFLICTS OF INTEREST STATEMENT
Jonas Bergqvist and Thomas Österberg are cofounders of, and employed by, Epishine AB, which develops and manufactures organic photovoltaic devices for light energy harvesting.
Open Research
DATA AVAILABILITY STATEMENT
Data that support the findings of this study are available in the Supplementary Material of this article and are also available from the corresponding author upon reasonable request.




