Implantable and biodegradable closed-loop devices for autonomous electrotherapy
Abstract
Implantable electronic devices as a valuable biomedical tool are used to treat chronic diseases. However, traditional pacemakers exist a serious risk of complications. A biodegradable, closed-loop sensor-actuator system is developed for cardiac rhythm monitoring. This system could achieve autonomous electrotherapy.
Implantable electronic devices as a valuable biomedical tool are used to treat chronic diseases such as cardiac arrhythmias by monitoring in vivo physiological responses using wireless communication.1, 2 To be implantable in vivo, devices must meet the requirements of implantable properties, including excellent biocompatibility, biodegradability, miniaturization, and lower energy consumption. These advantages are driving the broad applications of implantable devices in the fields of smart gastric and cardiac pacemakers, cochlear implants, implantable cardioverter defibrillators, and brain–computer interactions.3 Therefore, realizing superior implantable device properties is both an opportunity and a challenge for medical and healthcare delivery, particularly autonomous electrotherapy of cardiac disease.1, 4, 5 As the cornerstone of cardiac disease therapy, existing platforms have significant limitations as they rely on conventional electronic hardware, monitoring systems, and interfaces to the body. First, bacteria can form a biofilm on the device, posing a risk of infection. Second, the external modules of the power supply and control system are not fully implanted, which would impede patient movement and clinical care. Third, surgical removal of temporary pacing devices poses additional risks. These features lengthen hospital stays and increase risk of complications because they require external devices plugged into the outlet. Compared to conventional pacemakers, the transient cardiac pacing system not only functions as a supplement to conventional pacemakers, but also forms a permanent pacing therapy for the treatment of postoperative bradycardia. To overcome the defect of traditional temporary pacemakers, a wireless, battery-free, fully implantable pacemaker for postoperative cardiac rate and rhythm control has been reported.6 Importantly, the pacemaker was timed to completely degrade and disappear through natural biological processes. This research lays the foundation for the next generation of postoperative temporary pacing techniques. Based on cardiac pacemakers, a conceptually similar closed-loop control scheme is used to achieve autonomous adaptive regulation of one or more fundamental physiological parameters to achieve set points without human intervention.
Recently, Rogers's group developed a biodegradable, closed-loop sensor-actuator system for cardiac rhythm monitoring.7 This system could achieve effective cardiac function therapy in patients at postoperative risk of bradycardia. The transient closed-loop wireless network system with three modules and seven key components (ⅰ–ⅶ) (Figure 1A). Module 1 was a fully implantable, bioresorbable module consisting of a temporary, bioresorbable, stretchable epicardial pacemaker (ⅰ), a bioresorbable steroid-eluting interface (ⅱ), and a subcutaneous, bioresorbable power harvesting unit (ⅲ). The bioresorbable module was used for the power of epicardial pacing. In addition, Module 2 consisted of a set of skin-interfaced modules, which was composed of a set of soft, skin-interfaced sensors that captured electrocardiograms (ECGs), heart rate (HR), respiratory information, physical activity, and cerebral hemodynamics (ⅳ), a wireless radiofrequency module (ⅴ), and a soft skin-interfaced haptic actuator (ⅵ). This module could transmit various physiological data to the control module for real-time data visualization and algorithm control. Moreover, Module 3 was an external control module, which was a handheld device for real-time visualization, storage, and analysis of data (ⅶ). These modules were interconnected into a closed-loop system for wireless network (Figure 1B).
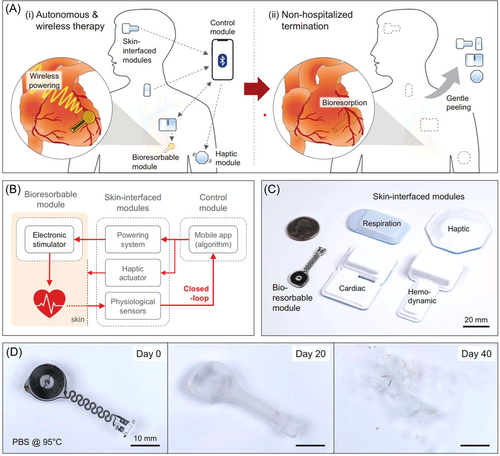
The soft and flexible designs allowed the module to be placed in different target positions on the body (Figure 1C). For the temporary cardiac pacing system, the bioresorbable module was able to dissolve in vivo and the skin-interfaced module was easily peeled from the skin (Figure 1D). It eliminated the need for surgery and reduced the risk of surgery. It is demonstrated that dexamethasone acetate (DMA) was released from steroid-eluting patches of polylactic acid-glycolic acid copolymer (PLGA). It minimized local inflammation and fibrosis during cardiac pacing. Moreover, the functional lifespan of a bioresorbable conductor enabled about 1 month under simulated physiological conditions.
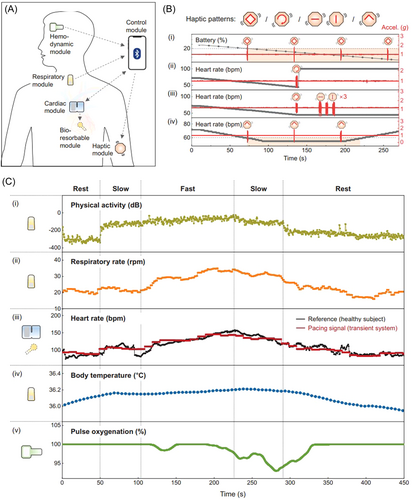
Transient closed-loop systems have been demonstrated to have the function of algorithmic identification for autonomous therapy based on the ECG characteristics of abnormal cardiac activity (Figure 2A). The bradycardia threshold was set at 54 beats per minute (bpm), the system automatically initiated pacing (100 bpm). After a predetermined pacing duration of 10 s, the system automatically stopped pacing and evaluated the underlying intrinsic ECG signal to determine if additional pacing therapy was needed. In addition, the module network also had the function of informing the patient of the remaining battery life (i), the proper operation of the cardiac module (ii), instances of malfunction of the other modules (iii), and symptoms of bradycardia (iv). The module provided haptic input through different patterns of vibration (Figure 2B). Meanwhile, it was activated to facilitate the positioning of the cardiac module during installation. The physiological parameters, physical activity (i), respiratory rate (ⅱ), heart rate (ⅲ), body temperature (ⅳ), and pulse oxygenation (ⅴ) were measured to provide important information for postoperative patients (Figure 2C).
The transient, implantable, and closed-loop represents a distributed, wireless bioelectronics technology. It could provide autonomous electrotherapy within a time frame that meets postoperative needs and captures detailed information from various locations of the body. The technology improves not only ambulatory monitoring but also remote monitoring for patients living in underserved areas. Complementing traditional biomedical devices, implantable closed-loop technology provides a framework for treating various diseases. Implantable electronic devices offer new solutions for miniaturized, degradable, and implantable energy devices. It is significant potential for the development of degradable implantable electronic medical devices in the future.
ACKNOWLEDGEMENT
The authors acknowledge financial support from the Ministry of Science and Technology of China (2018YFA0703200), National Natural Science Foundation of China (51973154), National Innovation Group “Organic Integrated Circuit Core Material Foundation” (52121002), and Natural Science Foundation of Tianjin (20JCZDJC00680). In addition, financial support was provided by the Haihe Laboratory of Sustainable Chemical Transformations.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.




