Uprising Unconventional Nanobiomaterials: Peptoid Nanosheets as a Multi-Modular Platform for Advanced Biological Studies
Abstract
Peptoids are bio-inspired peptidomimetic polymers that can be designed to self-assemble into a variety of nanostructures. Among these different assemblies, peptoid nanosheets are the most studied. Peptoid nanosheets are 2D highly ordered nanostructures, able to free float in aqueous solutions while featuring versatile chemical displays that can be tuned to incorporate a plethora of functional units. In this review, the synthetic approach used to prepare sequence-defined oligomers and highlight their main characteristics is introduced. The ability of peptoids to fold into nanostructures is then reviewed with an extensive emphasis on peptoid nanosheets, and their physico–chemical characteristics, assembly mechanism, and stability. A particular focus is also placed on the variety of functionalization incorporated into the peptoid nanosheets to tune their properties toward specific applications, especially within the fields of biology and medicine. Finally, the comparison between peptoid nanosheets and other 2D nanomaterials is discussed to address the challenges in the current nanomaterials and underline the future development of peptoid nanosheets in the field of biology.
1 Introduction
Nanosheets (NSs) are a class of nanomaterials that represent a frontier in material sciences and are defined as 2D nanostructures, generally ranging from 1 to 100 nm in thickness, and with lateral dimensions ranging from nanometer to micrometer scale. They have an extremely thin morphology and high surface area and offer unique properties and useful applications across various fields (Figure 1). From energy storage and sensors to environmental and biomedical applications, nanosheets have revolutionized numerous scientific domains in the past few decades. Nanosheets can be composed of a wide range of materials, each of them featuring their own set of unique characteristics and potential applications.[1] In literature, we can find examples of 2D nanosheet-like materials derived from different origins such as inorganic nanomaterials, carbon nanomaterials, and bioorganic-based materials (Figure 1). Nanosheets of inorganic origin such as transition metal dichalcogenides (TMDCs), MXenes, black phosphorous, transition metal oxides, and Boron nitride are currently drawing attention for biological applications due to the ease of functionalization, and other physicochemical properties, such as mechanical, optical and electronic properties.[2] TMDC NSs are represented by the general formula MX2, and this class of materials includes MoS2, WS2, TiS2, MoSe2, and WSe2.[3] Chemically exfoliated TMDC NSs have proven to be exceptional fluorescence quenchers. As an example, high quenching efficiency of MoS2 NSs surpasses even that of graphene.[4, 5] Despite the great promises of this class of materials for drug and gene delivery, photothermal, and multimodal therapy, the current understanding of the toxicity of TMDC NSs is still in its early stages.[6] Metallenes such as stanene, bismuthene, and PdMo are another class of inorganic-based nanosheets comprising pure metal atoms with compact size and ultrathin nature that contribute to high specific surface area and active sites, enhancing various properties such as abundantly exposed metal atoms and robust localized surface plasmon resonance. These materials have shown potential in a wide range of biomedical applications, such as biosensing,[7] bioimaging,[8] and medicine due to their antibacterial,[9] anti-inflammatory,[10] and antitumor[11] properties. A good example of the antibacterial action of bimetallenes is a study conducted by He et al. where AuPd alloy nanosheets functionalized with a targeting peptide were investigated showing good biocompatibility and promising therapeutic potential for photothermal therapy to treat Staphylococcus aureus. Exfoliated TiO2 nanosheets are transition metal oxide nanosheets, which thanks to their low cytotoxicity and unique physicochemical properties, have become potential candidates for various biomedical applications.[12] For example, TiO2 nanosheets have been investigated for the immobilization of proteins such as myoglobin.[13] In addition to TiO2, researchers have also investigated titanium monoxide nanosheets (TiO NSs) for their potential biological applications, and these studies have revealed that these nanosheets possess antibacterial properties. When exposed to near-infrared laser irradiation, TiO NSs have demonstrated high photothermal conversion efficiency and sterilization capabilities which enable TiO NSs to exhibit strong bactericidal effects against both Gram-negative E. coli and Gram-positive methicillin-resistant S. aureus.[14] In the last few decades, carbon nanomaterials have raised enormous attention in the scientific community due to their physical, chemical, electrical, mechanical, thermal, and optical properties and due to their potential in applications that span from electronics to medicine.[15, 16] In this context, graphene-based nanosheets (GNS), which include both graphene and graphene oxides, have recently been widely investigated as drug delivery systems due to their ability to transport biomolecules and therapeutic drugs. Most advantages of GNS lie in their ultrahigh surface area composed only of carbon, facilitating highly efficient drug loading compared to alternative nanocarriers. However, the same advantage can also be a drawback, leading to enhanced interactions with blood components and the formation of precipitates with red blood cells or serum proteins after in vivo administration.[17]
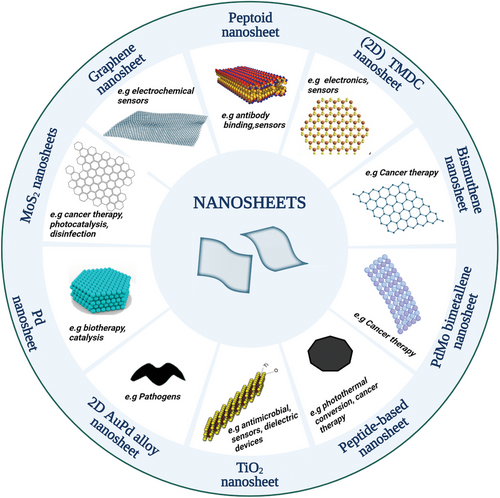
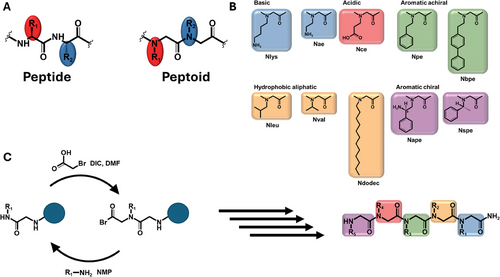
Nanosheets can also be formed from bioorganic components such as peptides and peptidomimetic polymers (Figure 1). Peptides play a fundamental role in the human body through various biological processes and form an intriguing biological material due to their unique properties.[18] Peptide backbone chirality and hydrogen bonding between amino and carbonyl groups of the peptide chains are the factors responsible for peptide folding into their well-known highly ordered secondary structures, for example, α-helices and β-sheets. After opportune design, peptides can self-assemble to form numerous nanostructures such as nanoparticles, nanotubes, and nanosheets. These assemblies occur through various types of interactions, such as electrostatic, hydrophobic, π−π interactions, and hydrogen bonding, and the design of the peptides takes advantage of the nature of amino acid residues including positive or negative charge along with their hydrophobic or hydrophilic properties.[19] Numerous reviews focus on the self-assembly of peptides into different functional nanomaterials, such as particles, fibers, tubes, sheets, and ribbons, and their versatile biomedical applications.[20, 21] One example is the study of amyloid-like peptide nanofibrils and their use as drug-delivery systems. This application can be achieved through different methods, either by using peptide-based drugs that inherently form fibrils, or by attaching drug molecules to specific peptide sequences capable of forming β-sheet structures.[22] Researchers have also developed classes of amphiphilic peptides that form high-aspect-ratio nanofibers in water through hydrophobic collapse and hydrogen bonding among peptides which form highly functional 3D networks upon addition of physiological electrolytes, which can be used as extracellular matrix-like scaffolds for regenerative medicine.[23, 24] Peptide nanosheets have also been investigated together with other nanomaterials. According to the literature, peptide nanosheets loaded with gold nanorods show excellent photothermal therapy efficacy in vitro and in vivo, minimal toxicity, and good biocompatibility, all of which point to their possible application in cancer therapy.[25] Despite their high potential and the current successful applications, peptide nanomaterial-based therapeutics have shown certain drawbacks such as limited stability and susceptibility to enzymatic degradation. To address these challenges, peptoids, a novel class of peptidomimetic polymers, have emerged as a promising alternative, offering an excellent platform for various applications, particularly in the range of biological and biomedical sciences. Peptoids are a class of synthetic compounds that can mimic the properties of peptides and proteins due to their structural similarity. Peptoids are composed of N-substituted glycine monomer units in which the side chains are linked to the nitrogen atoms (Figure 2A).[26] This structural difference from peptides makes peptoids biologically more robust as they become resistant to proteolysis.[27, 28] In addition, the absence of the NH group on peptoid backbone prevents the hydrogen bonding that instead occurs in peptides, impeding the adoption of secondary structures that we observe in proteins. Nonetheless, secondary structure motifs in peptoids can be introduced through peptoid design, selecting opportunely the peptoid monomers.[29] Indeed, peptoid folding is largely governed by side-chain interactions, giving rise to a multitude of supramolecular architectures, such as helices, ribbons, nanotubes, superhelices, and nanobelts.[30-37] To investigate the supramolecular assembly of peptoids and the diverse nanoarchitectures they form, researchers have employed a wide array of high-resolution structural characterization techniques, computational studies, mechanistic, and kinetic analyses.[38-40] The properties of these peptoid nanostructures have been investigated toward many applications, and a particular interest has been directed toward their use in biomedical applications. For example, crystalline peptoid nanoflower-like particles have been synthesized from fluorinated sequence-defined peptoids and investigated as a drug delivery system to carry mRNA and effectively deliver it to the cytosol.[41, 42] A recent study by Webster et al. has also demonstrated the potential of peptoids in developing mRNA therapeutics. They have formulated a large peptoid library able to form peptoid-based lipid nanoparticles, which can serve as a tunable delivery platform for developing mRNA therapeutics.[43] Recently, peptoids have also been used in tissue engineering due to their capacity to form synthetic hydrogels mimicking biological microenvironments. Morton et al. have investigated the use of peptoid-based crosslinkers demonstrating that the obtained hydrogels possessed tunable properties depending on peptoid composition, length, and concentration.[44] Recent research has also focused on using peptoid-based hydrogels for drug delivery. Coulter et al. have investigated the synthesis and the application of an enzyme-responsive material, based on in situ forming injectable peptoid-peptide hydrogel that allows low-molecular-weight drugs to be covalently conjugated.[45] Sebastian et al. have explored a one-pot multicomponent synthesis of a series of tripeptoids from which low molecular weight organic gelators can be synthesized. The obtained organogel and organo/hydrogels were used for drug encapsulation and stimuli-responsive release.[46] Peptoid-based hydrogels have demonstrated significant potential for enhancing immunomodulatory effects. The group of Rosales has shown how hydrogels cross-linked with peptoid-based helical structures can be used to generate materials of different stiffnesses and fixed network connectivity to modulate immunoregulatory response in human mesenchymal stromal cell in the presence of Interferon-Gamma.[44, 47] Peptoids themselves demonstrated significant biological applications beyond their self-assembled architectures. Wang et al. investigated how structurally well-defined peptoids can act as coatings to provide a tunable platform for protecting DNA origamis in a broad range of bioactive environments. The authors then demonstrated the applicability of this approach for drug delivery, bioimaging, and cell targeting.[42] The group of Guo showed that peptoids possessed excellent antifreeze properties and were nontoxic to cells, becoming excellent candidates to replace current commonly used antifreeze agents.[48, 49] Others reported biological activity of peptoids including their antimicrobial activity[50-53] and their use as therapeutics for neurodegenerative diseases, for example, in Alzheimer's disease.[54] Recently, studies have also investigated how peptoids bind to a protein and have provided high resolution structural information offering invaluable insights into the design of protein-targeting peptoids that can be used in several therapeutic applications.[55]
Among the various structurally well-defined peptoids and their diverse self-assembled architectures, a particular interest has revolved around 2D peptoid nanosheets due to their stable ultra-thin bidimensional morphology with large surface area enabling surface functionalization. 2D peptoid structures have been used as scaffolds for developing nacre-mimetic functional materials[56, 57] and for controlling the morphology of ZnO nanomaterials.[58] Chen and co-workers developed a new class of biomimetic catalysts using peptoid membranes as enzyme mimetics for catalytic organophosphate degradation.[59] Scientists have also explored the use of peptoids in developing antibiofouling membranes by adding an antimicrobial peptoid enabling the formation of a highly crosslinked and permeable separation layer.[60]
This review presents a comprehensive analysis of 2D peptoid nanosheets, reviewing their structural features, formation processes, and biological significance. Further, it explores the applications of peptoid nanosheets in various biological fields, highlighting their promising role in advancing biomedical sciences.
2 Solid Phase Peptoid Synthesis Approaches
Peptoids can be synthesized using both solution and solid phase protocols.[61-63] The most common approach that offers automated synthesis of longer sequences with high purity is the solid-phase sub-monomer procedure. In this approach, the first step is backbone elongation where haloacetic acid (e.g., bromoacetic acid) activated with N,N'-Diisopropylcarbodiimide (DIC) reacts with an amine linked to the solid support, usually a rink amide resin, followed by a nitrogen atom and side chain incorporation with a primary amine that defines the side chain structure (Figure 2B).[26, 64] Peptoids are then cleaved from the resin using a cleavage cocktail mainly constituted of trifluoroacetic acid (TFA), allowing at the same time, deprotection of the protecting groups on the side chains (e.g. Boc). The solid-phase sub-monomeric method allows the synthesis of polypeptoids ranging from 6 to 50 monomers, with satisfactory yields and precise control of peptoid chain length, side-chain chemistry, and sequence. A variety of amines are commercially available, allowing peptoids to be designed and synthetized by incorporating side chains with different chemical properties, especially in terms of hydrophobicity and net charge of the side chain (Figure 2C).[63, 65, 51] Peptoid synthesis, although well established, is still being developed focusing on new approaches to improve efficiency in terms of time and yield and to explore future possibilities on synthesis scaling up, automatization, new nanoarchitectures, and sustainability.[65, 66] Solid phase peptoid synthesis involves significant consumption of hazardous solvents such as N,N-dimethylformamide and N-methyl-2-pyrrolidone as they are used in large quantities for resin swelling, washing, and reagent preparation, which limits the sustainability of peptoid synthesis. In response to these issues, recent research has focused on developing greener approaches to peptoid synthesis. Clapperton et al. have reported solid-phase synthesis of peptoids with complete elimination of hazardous solvents by replacing them with greener solvents such as gamma-valerolactone, dimethyl sulfoxide, ethyl acetate, and binary mixtures to minimize the environmental impact. They have shown that the purity and yield of the final peptoids are not significantly affected when compared to those synthesized in traditional solvents.[67]
Solid-phase synthesis of polypeptoids as described also allows the chemical incorporation of other biological groups such as polypeptides and fatty acids.[65, 68] Other motifs such as chromophores and fluorophores can also be chemically incorporated for both peptoid functionalization and physicochemical characterization. Depending on the properties and stability of the functional groups and on the applications of the peptoid of interest, these functionalities can be incorporated into the peptoid before or after the cleavage from the resin or either after the formation of the peptoid nanostructures.[69-71] These functional groups give the peptoids peculiar physicochemical and biological properties that can be further explored to design functional materials. In addition to solid-phase synthesis, peptoids are also being synthesized using other synthetic procedures focusing on efficiency, yield, and sustainability. To mitigate the sustainability issues, Soumanou et al. reported synthesis of peptoids in aqueous media. They have investigated the optimum conditions for chain elongation in a unique N to C direction and have presented a convenient one-pot protocol for the incorporation of each peptoid residue.[72]
3 Peptoid Self-Assembly in 3D Architecture
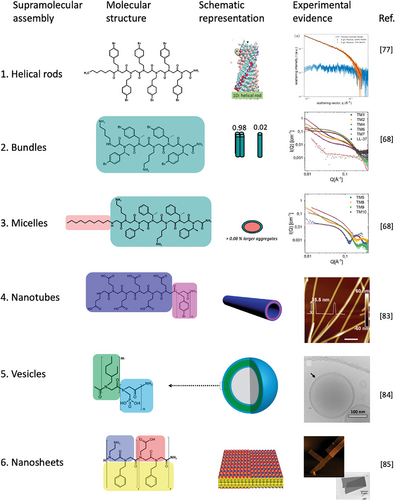
By precise control of the sequence and spatial disposition of hydrophobic and polar hydrophilic, noncharged, zwitterionic, and positively or negatively charged side-chain monomers, one can design amphiphilic polypeptoids (Figure 3 and Table 1).[29] These are thermodynamically prompt to acquire a defined intramolecular protein-like fold or to self-aggregate in an organized and controllable fashion, giving rise to a variety of supramolecular ensembles.[73] Self-association takes place in many peptides and protein systems and is frequently crucial, so these organized assemblies display their biological activity.[74, 75] Figure 3 highlights some examples of peptoid 3D nanoarchitectures that are reported in the literature and techniques by which they have been characterized. The summarized self-assembly of peptoids into various secondary structures highlights their potential as versatile and robust materials for applications in nanotechnology. In the following sections, several secondary structures are discussed followed by an extensive description and discussion of nanosheet structures with their promising applications in biology.
 |
3.1 1D Helical Rods
The stacking of peptoids into twisted one-dimensional filaments may represent an initial step in forming larger, more complex aggregates, such as nanotubes, nanosheets, and nanovesicles, in solution. The ability of diblock co-polypeptoids to assemble into superhelices was first reported by Murnen et al. in 2010.[76] More recently, 1D helical rods were reported by Zhao et al.,[77] who observed these assemblies after spontaneous aggregation of Peptoid 1 (Table 1) in water and organic solvent mixtures (acetonitrile or methanol).[77] The existence of 1D helical rods at these conditions was indicated by Molecular Dynamic (MD) simulations and energy calculation theoretical studies that showed favorable energetic stabilization of this arrangement with ΔGa ≈ −26 kJ mol−1. The structural properties were then confirmed experimentally using small-angle X-ray scattering (SAXS). Scattering data were fitted by the form factor of randomly oriented, monodisperse flexible cylinders of 28 Å in diameter that corresponded to the length of a single Peptoid 1. 1D helical rods were also imaged by atomic force microscopy (AFM), and the measured diameter of 2.5 nm agreed with SAXS and MD simulations.[77] Other helical structures formed by different peptoids have also been reported in the past year. Yang et al. have designed a novel dual-substituted peptoid which formed an extended helical chain that promoted further supramolecular self-assembly into nanosheets and flower-like superstructures.[78] Recent theoretical studies have focused on investigating how modification in sidechains, influences helical conformations in peptoids. These studies aimed to enhance the understanding of supramolecular self-assembly of forming peptoid helices.[79]
3.2 Bundles
The initial characterization of peptoid bundles was conducted using SAXS, investigating the self-assembly of peptoids with diverse antimicrobial sequences, as well as their counterparts with single-chain fatty acids attached to the N-terminal of the peptoids.[68] The alternating pattern of hydrophobic and hydrophilic side chains, as seen in Peptoid 2 (Figure 3), combined with its tendency to aggregate, led to the formation of an amphiphilic helical structure, with hydrophobic side chains positioned on one side of the helix and hydrophilic side chains on the opposite side. Helices then bundled together into dimers, trimers, or tetramers to avoid the hydrophobic faces of the helices being exposed to the surrounding water molecules, forming the observed helical bundles (Figure 3). Peptoid length and the presence of para-brominated Nspe side chains were also shown to affect helical conformation acquisition, thus affecting self-assembly. Taking advantage of the ability of these peptoids to form bundles, Zhao et al. studied the complexation strength between poly(acrylic acid) microgels and a series of cationic peptoids and found that the supramolecular structures increased the complexation strength, suggesting that these supramolecular systems may be suitable for in vivo antimicrobial applications.[80]
3.3 Micelles and Elongated Micelles
Micelles and elongated micelles are other structural motifs seen in peptoids. These structures are made by an assembly of peptoids and lipopeptoid chains (e.g. Peptoid 3).[68] In this context, the hydrophobic fatty acid segments dictate the supramolecular assembly, influencing the size, shape, and relative proportions of each possible aggregate form.[68, 81, 82] Lau et al. extensively characterized micelles formed by lipopeptoids, characterized by a 16-carbon long hydrophobic tail at the N-terminal and different sequences containing hydrophobic Npe monomers, and hydrophilic positively charged Nae and negatively charged Nce monomers (e.g. Peptoid 4 and 5).[81] SAXS, Cryo-TEM and DLS measurements, confirmed spherical micelles formation by lipopeptoids, with a radius ranging from 2.1 to 3.5 nm, hydrophobic core radius of 1.7 nm, and shell radius of 1.1 nm. Critical aggregation concentrations (CAC) were measured by fluorescence, ranging between 0.4 and 1.0 mm. The measured micelle hydrophobic core radii reflected the length of the fatty acid at the N-terminal, indicating that this property can be modulated according to the characteristics of the synthesized lipopeptoids.[81] The same molecule can form aggregates with different sizes, shapes, and molecular packing depending on the conditions. Furthermore, cylindrical micelles have been described by med by β-cyclodextrin (CD) functionalized peptoids (Peptoid 6). Jiao et al.[82] These are formed by β-cyclodextrin (CD) functionalized peptoids (Peptoid 6). Obtained cylindrical micelles further assembled into membranes and intertwined ribbons on substrates in aqueous solution, depending on the choice of solution and substrate (mica and quartz) conditions. Micelle assembly mechanism, investigated by in situ atomic force microscopy (in situ AFM), occurred via two steps. First, smaller particles were transformed into worm-like micelles, which then extended and coalesced to form the higher-order structures in a pH and Ca2+ concentration-dependent manner.[82]
Amphiphilic block copolypeptoids, composed of three distinct N-substituents—n-decyl (hydrophobic), 2-methoxyethyl (neutral), and 2-carboxyethyl (ionic)—were shown by Sternhagen et al.[86] to form micelles in aqueous solution. Peptoid constructions were synthesized with the same overall length of 25 monomers, with a hydrophobic block of 5 monomers and a hydrophilic block of 20 monomers, constituted by mainly neutral monomers with ionic monomers along the block that differed in number and position. The behavior of these peptoids in aqueous solution was then studied (e.g. Peptoid 7). Properties as critical micelle concentration (CMC), hydrodynamic radius, size polydispersity, and Zeta potential of the aggregates formed by these molecules were investigated by a combination of techniques that confirmed that; 1) all synthesized peptoids formed micelles with radii ranging from 3.4 to 4.2 nm, according to Cryo-TEM data, 2) they all have low size polydispersity, and 3) the Zeta potential proportional to the number of negatively charged side chains was present in each sequence. Micelles-formed peptoids bearing three or one 2-carboxyethyl monomers presented a Zeta potential of, on average, −36 and −27 mV, respectively, and the micelles formed by the peptoid lacking the ionic monomer had a Zeta potential of 2.7 mV. Determined CMC for all the peptoids was in the 10−1 to the 10−2 mg mL−1 range.[86] The radii of the inner core and outside layer, as well as the aggregation number of the micelles, were measured by small-angle neutron scattering (SANS) which allowed a full correlation between the ionic charge density and positioning, micelle size, Zeta potential, and CMC.[86]
3.4 Peptoid Nanotubes
Amphipathic peptides and peptoids can assemble into nanotubes, hollow elongated supramolecular aggregates.[87] Peptide-forming nanotubes have been extensively reported and explored with varied purposes and have been physiochemically designed with multiple architectures that include the stacking of cyclic molecules and the assembly of twisted double-layered membrane-like aggregates.[88-90] More recently, studies on nanotube-forming peptoids have been reported.[91] A series of di-block peptoids was described to form single-walled nanotubes of stacked, porous crystalline rings, in which side chain van der Waals intermolecular interactions were the main energetic components keeping the ensemble. Di-block peptoids composed of hydrophobic pNdc and hydrophilic pNte monomers (Peptoid 8) formed nanotubes, and their properties were analyzed by DSC, SAXS, WAXS, and Cryo-EM as a function of the length of both hydrophobic and hydrophilic blocks (n). The combination of different techniques showed that the measured nanotube diameters ranged from 4.3 to 9.0 nm and did not correlate linearly with the length of the peptoid once the architectural pattern was not kept as the molecule was elongated. The crystalline intermolecular association was investigated by both Cryo-EM and TEM, indicating that peptoid molecules stacked with the same latitudinal periodicity of 2.4 nm, not depending on n, while varying n altered longitudinal periodicity and nanotube overall architecture. Di-block peptoids composed of hydrophilic Nce and hydrophobic Nbpm monomers (Peptoid 9) formed double-layered peptoid nanotubes, according to Jin et al.[83] Peptoid 9 (Nce6-Nbpm6) formed nanotubes with a diameter of ≈35 nm and wall thickness of 3.1 nm, as measured by TEM, Cryo-TEM, and in situ AFM (Figure 3). XRD and molecular modeling allowed the understanding of aggregate organization at the sub-molecular level, confirming the double-layer architecture and highlighting the importance of π-stacking taking place at the hydrophobic layer. Time-dependent TEM images of Peptoid 9 obtained at shifting conditions clarified the assembly mechanism of the nanotube. Peptoid aggregate underwent a series of transformations, starting with nanospheres, that transformed into nanoribbons that had the edges rolled up to form the final nanotube. Nanotubes have also been characterized by Jin et al.[83] Here, wall thickness and nanotube diameter have been shown to vary as a function of the number of Nbmp monomers in the peptoid, and these properties could be tuned by changing the pH; hence, the ionization degree of the hydrophilic portion of the molecules. At high pH conditions (pH > 8), Nce groups were deprotonated, and the high density of negatively charged groups introduced a repulsion component that expanded Peptoid 9-based nanotubes; with height of 24.3 nm, measured by AFM. Decreasing the pH to 3.6 neutralized nanotube surface charge and abolished repulsion. At this condition, the nanotube contracted and presented an AFM height of 13.1 nm. Stepping forward, this same group had described the synthesis of functionalized peptoid nanotubes presenting Peptoid 9 as a scaffold to aggregate assembly, linked to active dansyl (DNS), meso-tetrakis(4-carboxylphenyl)porphyrin (TCPP), and folic acid (FA) groups aiming for the use of chemo–photodynamic therapy.[92] In summary, the studies above had shown that nanotubes could be obtained with excellent yield and similar properties as non-functionalized Peptoid 9 nanotubes.
3.5 Peptoid Vesicles
Amphiphilic di-block copolymers, peptides, and peptoids can assemble into double-layered aggregates, similar to a lipid bilayer, and form planar membrane-like assemblies and vesicles.[93, 94] Di-block copolypeptoid vesicles were first obtained and characterized by Jiang et al.[84] Here, four different peptoids consisting of repetitions of hydrophobic monomer pNeh and hydrophilic phosphonated pNpm combining various lengths of each block in a pNehm–b–pNpmn sequence structure (Peptoid 10) maintaining n + m = 36, were synthesized (Figure 3). The peptoids spontaneously formed vesicles through a solvent switch methodology (tetrahydrofuran, THF, and water). With a combination of MD simulations and cryo-EM, the thickness of the hydrophobic core were measured from 5.4 to 10.0 nm range (MD) for bilayers formed by peptoids containing 18 and 32 hydrophobic pNeh monomers, respectively (Figure 3). Although thickness measurements from MD simulations and cryo-EM did not align, both revealed variation following a power law that accounted for the length of the hydrophobic block, the conformation of the peptoid backbone, and, to some extent, molecular packing.[84] Aiming to develop drug delivery systems, stimuli-responsive peptoid polymersomes were described by Deng et al.[95] A homopolypeptoid block composed of the hydrophobic PMeSPG monomer, containing an oxidation-labile sulfur atom from a methionine derivative, was synthesized in solution through chemical homopolymerization. Following analysis of this initial block, a di-block copolypeptoid, PMeSPG-b-PSar, was then produced by sequentially adding hydrophilic pSar monomers (Peptoid 11). Vesicles were then obtained by nanoprecipitation, a process in which Milli-Q water is slowly added to a DMF solution of the peptoid or by double emulsion method with the formation of intermediary droplets followed by organic solvent evaporation. The resulting vesicles were visualized by Cryo-EM and confocal laser scanning microscopy using Nile red as an intercalating fluorescence probe that confirmed the formation of double-layered membrane-like aggregates and the sensitivity of these aggregates to oxidation with hydrogen peroxide and with TPP, a photoactivated oxidant, and light. Oxidation of the thioether side chains forming hydrophilic sulfoxides was the essential mechanism that changed the hydrophilic/hydrophobic balance of the molecules, and hence of the ensemble, that kept the vesicles thermodynamically viable. Increasing the solubility, the oxidized peptoid monomers were less prompt to form aggregates, and the vesicles were destabilized and disrupted.[95]
4 Peptoid Nanosheets
Peptoid nanosheets are self-assembled ultra-thin 2D aggregates that are potentially significant for many different applications, some already investigated and others yet to be explored (Table 2 and Figure 3). Inspired by the double-layered structure of a phospholipid membrane, peptoid nanosheets have arisen as a robust, easy, and quick way to obtain structures that can be further chemically modified with the addition of biological motifs to serve diverse purposes.[96]
 |
4.1 Peptoid Assembly
The group of Dr. Zuckerman[85] first described the formation of peptoid nanosheets formed from alternating hydrophobic and single-charged blocks. Peptoids were synthesized using positively charged monomer Nae, negatively charged monomer Nce, and hydrophobic monomer Npe; alternating charge sequence was designed with nine repetitions of Nae–Npe–Nce–Npe segment (Peptoid 12), and block charged sequences were designed with the positively charged block composed of nine or eighteen repetitions of the Nae–Npe di-peptoid, followed by nine or eighteen repetitions of Nce–Npe, respectively, forming the negatively charged block, Peptoid 13 (Figure 4A). Electrostatic stabilization energy was calculated for model nanosheets formed by the two peptoids with the same length and alternate or block charge distribution to better understand the possible consequences of charge patterning. Taking into account the two spatially extended dimensions of a sheet and only one of its ionic faces, calculations revealed that a block-charge sheet with fully ionized residues provided greater electrostatic energy stabilization (−38 kBT per peptoid) compared to an alternating charge sheet, which had a calculated energy of −15 kBT per peptoid. Differences were attributed to the number of opposite charges each ionized group experienced around it. Formed sheets were analyzed by a combination of techniques such as fluorescence optical microscopy, scanning electron microscopy (SEM), and powder pattern XRD (Figure 4B,C). XRD data showed peaks at ≈2.7 nm (nanosheet thickness), and of ≈4.5 Å corresponding to the distance between peptoid chains. Here, the sharpness of the peaks indicated that the block-charge chain was more locally ordered than the sheets formed from the alternating charge nanosheet. Microscopy techniques enable the measurement of nanosheet dimensions. Both alternating and block nanosheets exhibited edge lengths ranging from 10 to 100 µm, along with a wide size distribution. (Figure 4B).
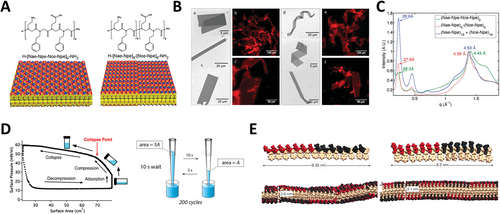
Nanosheets formed by Peptoid 14, a slightly shorter version of Peptoid 13, were also investigated by MD simulations by Mannige et al.[100] and their atomic-resolution structure have been reported. Nanosheet properties such as a thickness of 28.2 Å, polymer spacing of 4.7 Å, and sidechain spacing of 3.5 Å agreed with previous and new experimental data obtained. Other structural in silico studies were able to reveal how the interplay between cis and trans conformation determined overall packing; and thus, other nanosheet features such as thickness and spacing between strands (Figure 4E).[99]
4.2 Nanosheet Assembly Mechanism
4.2.1 Mechanical Nanosheet Assembly
Vial Rocking Method
Following their discovery, the nanosheet assembly mechanism was extensively investigated by the Zuckermann research group[101, 102] (Figure 4D). Nanosheets can be obtained by a vial rocking methodology. During this process, peptoid nanosheets are formed through the spontaneous arrangement of amphiphilic peptoid molecules at the air–water interface, forming a monolayer intermediate. When submitted to lateral compression due to area decrease, the monolayer intermediate collapses, favoring hydrophobic interactions between non-polar groups at the aqueous environment, originating a double-layered aggregate and resulting in the irreversible production of free-floating nanosheets. Following the same thermodynamic principles, (Nae–Npe)7–(Nce–Npe)7 Peptoid 14 (B28) accommodates other hydrophilic/hydrophobic interfaces between water and organic solvents such as hexane or tetrachloromethane in a highly organized fashion to make the common B28 polymer that nowadays serves as a golden standard.[103] Lateral compression of the peptoid monolayer also yields peptoid nanosheets with a bilayer structure, thickness, and bi-dimensional organization similar to the ones formed in the air–water interface, as characterized by XRD, AFM, and optical microscopy. More recently, other techniques such as liquid transmission electron microscopy and in situ AFM have been used to deepen our understanding of the assembly mechanism. Different from cryo-TEM and dry AFM that can capture details of the assembled supramolecular structures, these techniques can capture features of the assembly process taking place in liquid environment.[104, 105] Ex situ and in situ TEM (characterization in response to external stimuli such as for example, temperature, stress, and so on) have also been used to further characterize the physical behavior of peptoid nanomaterials, more specifically peptoid Au nanostars. Here, Jin et al. have used liquid TEM to understand how the fivefold peptoid nanostars formation occurred and monitored the growth of the particles as well as Au nanoparticle attachment to the peptoid.[106]
Pipetting Method
Kim et al. recently reported a new method for nanosheet loop formation using standard pipetting equipment.[98] During suction, pipetting cycles increase the air–water interface area, allowing molecules to organize at the surface (Figure 4D). After some time, the liquid is withdrawn from the pipette tip, triggering an interfacial area reduction and monolayer collapse to form the nanosheets, in analogy to what happens during cycles of the vial rocking methodology. Producing nanosheets with this method has shown not only to be viable but also very adequate to the large number of tested molecules, consisting of a simple non-expansive way to obtain the nanosheets that can easily be automated. In addition, in comparison with the vial-rocking methodology, which requires milliliters of sample to properly obtain the bidimensional aggregates and can hardly be automated to deal with many samples, forming nanosheets by repeated pipetting cycles can be achieved with samples in the microliter range.[98]
4.2.2 Factors Affecting the Nanosheet Assembly Mechanism
Role of Solvents and pH
An energetic balance between intermolecular side chain–side chain interactions and secondary structure acquisition plays an important role in defining aggregates’ properties and functions. As expected, solvent properties also play a major role in peptoid interaction and the morphology of each aggregate at defined conditions. As an example, a multidisciplinary investigation from Zhao et al.[77] on the molecular self-assembly pathways of an amphiphilic diblock peptoid comprising a block of six hydrophobic N-((4-bromophenyl)ethyl)glycine residues conjugated to a polar 6-aminohexanoic acid tail (Peptoid 1), described how changes in solvent characteristics can dictate an ordered monomer assembly, leading to different supramolecular structures such as 1D helical rods and 2D crystalline sheets. The influence of the ionization state of side chains along the peptoid during sheet formation and their properties has been investigated by varying the pH of the aqueous solution in which sheets were obtained. Nanosheet formation yield showed stronger dependence on pH when constituted of the alternating charged (Nae–Npe–Nce–Npe)9 Peptoid 12, with an optimum pH of 6.[85] Block charge nanosheets could be formed at all studied pH, ranging from 5 to 10, highlighting a lesser dependence on pH and an optimum pH of 8. Stability tests also pointed toward previous observations; while alternate block charge nanosheets decreased in size and number when pH was varied from 6 to 5, 9, or 10; block charge nanosheets remained essentially stable at this entire pH range. Considering that, alongside electrostatic interactions, the hydrophobic effect contribution also played an important role in sheet formation and stability; nanosheets formed at optimum conditions were titrated with acetonitrile and visualized with a fluorescence microscope. No apparent degradation was observed at 10% acetonitrile; while increasing the organic solvent amount to 20% or 30%, some sheets of the alternate or the block charged presented loss of integrity, respectively, and no sheet-like structures were observed at > 50% of acetonitrile.[85] When comparing organic solvents with the same dielectric constant, authors showed that diluents with high viscosity hindered nanosheet formation.[103] The assembly of nanosheets at the water–oil interface opened several possibilities regarding incorporation of hydrophobic molecules or hydrophobic coated nanoparticles previously solubilized in the organic solvent. Nanosheets of (Nae–Nmbu)7–(Nae–Nmbu)7, Peptoid 15, formed at the water–oil interface could incorporate gold nanoparticles coated with aliphatic dodecanethiol inside their hydrophobic core to generate free-floating nanosheets with plasmonic properties.[107]
Surface Area
Surface area measurements are critical for understanding the formation and assembly processes of peptoid nanosheets as the high surface-to-volume ratio significantly influences molecular interactions, stability, and overall nanosheet architecture, impacting their functional properties and potential applications in nanomaterial design. To exemplify, Robertson et al., looked into nanoparticle incorporation and nanosheet formation by following surface pressure, followed by plasmonic properties measurements of the aggregates monitored by UV–visible spectroscopy. In both cases, the analysis was performed as a function of gold nanoparticle concentration on the organic phase.[107] Cycles of compression and decompression taking place with vial rocking were carefully examined by measuring how lateral pressure varied as available air–water interfacial area increased and decreased in a Langmuir-trough setup (Figure 4D). With precise control over the surface area and the possibility to evaluate how molecules pack at the available interface, Sanii et al. captured important details of nanosheet formation and how peptoid concentration, molecular depletion through cycles, rate of compression, and other parameters influence the process.[101] The authors explored the nanosheet formation mechanism of an equimolar mixture of the sheet-forming peptoids, (Nae–Npe)18 (Peptoid 16) and (Nce–Npe)18 (Peptoid 17), positively and negatively charged, respectively, considering that these molecules spontaneously arrange at the air–water interface in an intercalated fashion to form a bidimensional monolayer. Correlating surface pressure with the interfacial area, Langmuir isotherms presented four distinct regions corresponding to i) compression, ii) collapse, iii) expansion, and iv) adsorption. These events can be translated to the vial rocking method as vial repositioning from a horizontal position to a standing one (i. compression followed by ii. collapse) and returning to the horizontal position (iii. expansion and iv. adsorption) (Figure 4D).[101, 102] One important factor for nanosheet formation is the time between expansion and the next cycle of compression, in which molecules adsorb and organize at the interface. Peptoid adsorption at the interface occurs up to a so-called equilibrium monolayer pressure. By monitoring the pressure after decompression or after interface aspiration, authors determined that for Peptoid 16 and 17 concentrations of 10 µm each at room-temperature (RT) and pH 9 buffer, the equilibrium pressure is 30 mN m−1, and it takes 450 s for a clean interface to reach 90% of the equilibrium pressure (Figure 3D). The long time necessary to recover the interfacial monolayer after decompression and to reach equilibrium monolayer pressure may be due to slow adsorption or ongoing reorganization and, allowing an adequate monolayer equilibration time is crucial to nanosheet formation and yield. Repeated cycles of nanosheet formation deplete the solution of free soluble molecules available to adsorb at the interface to form new nanosheets. During the first cycles, the shape of the compression isotherms is very similar, but, after a sufficient number of cycles, solubilized peptoid is consumed, and the compression ratio required to reach collapse pressure increases. The authors showed that controlling adsorption time and establishing an adequate number of cycles are crucial for nanosheet formation, in terms of both qualitative and quantitative outcomes.[101] In addition, by investigating nanosheet formation over several cycles, authors can better understand nanosheet stability and whether it can be releasing or nucleating monomers through time and confirm that during the formation cycles of Peptoid 16 and 17 nanosheets, none of these phenomena take place.[101] More detailed rheological studies aim to further understand the phenomena dictating nanosheet formation; correlate them with physicochemical properties of different di-block peptoids composed of Nae, Nce, and varied hydrophobic side chains; and predict the ability of a determined peptoid to form a nanosheet based on its sequence and the hydrophobic side chain properties.[97, 108] The incorporation of hydrophilic sections in the middle of the structure of nanosheet-forming peptoid was also investigated (see Section 5.2).[109] Surface-pressure isotherms of this peptoid confirmed that a monolayer similar to the one observed for Peptoids 16 and 17 was formed, giving origin to nanosheets characterized by hydrophilic loops displayed on the nanosheet surface.
Peptoid Chain Length
The length of the peptoid chain has been shown to play a crucial role in influencing nanosheet formation, as demonstrated through studies correlating this parameter with various physicochemical properties. Combining two published works, for all eighteen peptoids studied, authors correlated nanosheet formation outcome with the following properties: peptoid length, calculated logP of chosen hydrophobic residues, the retention time of each synthesized peptoid in a C18 column, the equilibrium surface pressure achieved after 900 s of adsorption time (SP900), and the residence time of the peptoid in the monolayer (τD). In general, peptoid monolayers that present τD > 5000 s and higher SP900 values consistently formed nanosheets. A comparison among Peptoids 18, 19, and 14 showed that, for short peptoids, length can be critical for nanosheet formation. (Nae–Npe)3–(Nce–Npe)3 presented SP900 of 14.8 ± 3.2 mN m−1 and tD of 44 ± 1 s and did not form nanosheets. Longer versions with four or seven repetitions of each hydrophobic–hydrophilic pair presented SP900 of 21.8 ± 3.3 and 22.5 ± 2.2 mN m−1 and tD of 5000 ± 2000 s and infinite, respectively; conditions in which they formed nanosheets with good yield.[97] Inter-chain interactions were also seen as decisive in determining both the monolayer fluidity and the residence time of the peptoid in the monolayer; hence, predicting whether a peptoid monolayer would, upon compression, form or not a nanosheet.
Intermolecular Interactions
Intermolecular interactions between sidechains have been extensively investigated regarding nanosheet formation. Comparing Peptoid 18 with the other two 12mer, characterized by the presence of either N-2-((4-biphenyl)ethyl)glycine (Nbpe) (Peptoid 20) or N-2-(4-(trifluoromethyl)-phenylethyl)glycine (Nfpe) monomers (Peptoid 21) into the peptoid, peptoids with the same length but bearing hydrophobic groups with chemical structure more prompt to interact could form nanosheets. Nbpe and Nfpe residues are more hydrophobic in comparison to Npe and able to increase the establishment of π–π interactions between molecules at the interface, increasing SP900 and decreasing both monolayer fluidity and the rate of solution-interface exchange, requirements for nanosheet formation (Figure 4D).[97, 108] Distance between the nitrogen atom and the aromatic group is also varied, indicating that, in general, longer distances increase the degree of freedom of the end of the side chain allowing aromatic rings to be better positioned to establish π–π interactions. 16mer with Npe (Peptoid 19) or N-(4-methylbenzyl)glycine (Nmb) (Peptoid 22) can form nanosheets, while peptoid with N-(4-ethylphenyl)glycine (Neph) (Peptoid 23), due to the proximity between the aromatic ring and the nitrogen atom, is not able to establish intermolecular π–π interaction and does not form nanosheets.[97, 108] Aliphatic hydrophobic sidechains are also investigated, and result pointed out that, in this case, packing potential determines nanosheet formation. Peptoids composed of N-cyclopentylglycine (Ncp) (Peptoid 24) and N-cyclohexylglycine (Nch) (Peptoid 25) do not form nanosheets due to the low packing promptness of these side chains; and the peptoid with leucine-like analog N-(3-methylbutyl)glycine (Nmbu) (Peptoid 15), because its side chain is branched and known to pack with itself in leucine zipper protein, is able to form nanosheets with satisfactory yield. In all studies, nanosheet formation correlated with higher SP900 and tD values as mentioned before.[97, 108] Intermolecular interaction can also determine the pathway of nanosheet formation as shown by Ma et al.[104] By synthesizing two peptoid sequences, Peptoid 26 and 27, and investigating assembly process using in situ AFM, authors showed that Peptoid 27 formed disordered clusters before crystallization, while Peptoid 26 assumed a crystalline form directly, displaying a number of features characteristic of nucleation. In addition, correlating peptoid structure and intermolecular interactions taking place with the observed assembly pathways, authors suggested the possibility to design tuned sequences to select nanosheet formation pathway.[104]
4.3 Nanosheet Secondary Structure Stability
Nanosheet chemical and mechanical stability can be drastically improved through photo-crosslinking of the hydrophobic core, as demonstrated by Flood et al.[110] When exposed to ultraviolet radiation (254 nm), nanosheets formed using Peptoid 28, characterized by the presence of N-2-(4-chlorophenyl)ethyl) (N4Clpe) monomer, had the hydrophobic core of each monolayer covalently linked, becoming more stable under a variety of rather harsh physicochemical conditions. N4Clpe monomer, when under ultraviolet radiation, simultaneously with the leaving of a chlorine anion, became a radical cation that readily reacted with a phenyl group of a Npe sidechain on the other nanosheet leaflet, resulting in a biphenyl group structure covalently connecting the monolayers. Besides proving the formation of the covalent bond using Raman spectroscopy, the authors have characterized the nanosheets before and after the treatment with ultraviolet light. When comparing nanosheets formed with Peptoid 14 to non-irradiated Peptoid 28 nanosheets, it is possible, through XRD and AFM data, to note an increase in thickness corresponding to the volume of the chlorine atom. After irradiation, Peptoid 28 nanosheet thickness decreased, indicating the leaving of the chloride anion and formation of the biphenyl bridge in the interior of the nanosheet. XRD analysis also showed that the inter-strand space between the peptoids was the same for Peptoid 14 and both non-crosslinked and crosslinked Peptoid 28 nanosheets.[110] Crosslinked and non-crosslinked Peptoid 28 nanosheets were subjected to sonication for 30 min, lyophilization and resuspension, centrifugation followed by pellet resuspension, or to a 20-min exposure to a 50% acetonitrile solvent to test their stability. For all four different treatments, fluorescence microscopy of the nanosheets showed that crosslinked aggregates were exceptionally robust, being able to resist without significant variation in the number and size of the bi-dimensional aggregates. At these same conditions, non-crosslinked counterparts were either destroyed or had the number and average size considerably decreased.[110]
4.4 Nanosheets Molecular Packing and 3D High-Resolution Structures
More recently, peptoid conformation and molecular packing effect over nanosheet formation were extensively investigated at the atomic level using MD simulation of peptoid nanosheets also in combination with experimental data, such as XRD, NMR, and cryo-EM.[111-113] Considering the high complexity and the massive number of atoms composing a single nanosheet formed by thousands of peptoid molecules, coarse-grained models were developed to study nanosheets in a more simplified but still powerful and accurate manner.[111] With this method, a group of atoms was simplified as being a single spherical coarse grain encompassing the relevant properties of the group being represented. In addition, authors considered anisotropic effects of the atom's spatial distribution within the group when attributing the properties to the coarse-grain to be part of the calculations. Authors simulated peptoid nanosheets formed by (Nae–Npe)–(Nce–Npe)n and, initially considering nanosheets formed by the peptoid with n = 7 (Peptoid 14), basic properties as thickness and interstrand spacing were confirmed to match the experimental data available and to validate further observations of the system. From the coarse-grained simulated nanosheets, a series of theoretical XRD scattering curves was generated and shown to agree with the experimental XRD data.[111] These coarse-grained simulations served as a base for interfacing the data with all-atom simulations, laying the foundation for future multiscale simulations that take advantage of both strategies. On the one hand, it was possible to optimize computing time in terms of computing capacity, overall size of the box simulated (number of atoms or groups), and simulation time duration, and, on the other hand, it was still possible to obtain relevant information on an all-atom scale and detail level.[103] To determine the atomic-resolution structure of Peptoid 14 nanosheets, Mannige et al.[100] used atomistic MD simulations, together with experimental XRD and microscopy data. MD simulations of nanosheet properties, such as a thickness of 28.2 Å, polymer spacing of 4.7 Å, and sidechain spacing of 3.5 Å, agreed with experimental data obtained that indicated these dimensions as being 27.6, 4.5, and 3.6 Å, respectively. Analysis of the torsion angles φ and ψ of the peptoid backbone forming the nanosheets confirmed how aggregation restrained peptoids and conformation and allowed the observation of a novel secondary structure motif defined by a very specific and narrow angles combination and distribution, which the authors named as Σ-sheet conformation resembling a zig-zag pattern.[100] Peptoid nanosheets formed by molecules with the same di-block construction but varying in length from 4 to 28-mer were also investigated aiming to understand how sheet formation, intermolecular packing, and stability can be determined by the length of the polymers forming it. The generated data unambiguously showed that, as polymer length decreased, it was less energetically favorable for each residue to form a nanosheet because the ensemble possessed fewer favorable electrostatic and aromatic interactions. Peptoid length could be decreased down to the point where polymers were not stable in nanosheet form. In addition, authors showed that nanosheets are structurally and dynamically heterogeneous, and are in theory, porous to water and ions, relevant features considering potential applications of these bidimensional aggregates. The molecular structure of the peptoid strands once assembled on nanosheets was also investigated using solid-state NMR.[113] Labeling adjacent α-carbons on specific positions on the peptoids, the authors showed that the amide bonds favored cis over trans configuration when assembled into nanosheets. On the contrary, trans configuration was favored in non-assembled peptoid. To shed more light on the effect of peptoid backbone conformation over the molecular packing on nanosheets, molecular dynamics and quantum mechanical simulations were used in combination with experimental data (Figure 4E).[99]According to calculated data, the peptoid backbone adopting cis conformation was 8.2 nm long and packed into a nanosheet with 3.1 nm of thickness, and the trans conformer was 9.35 nm long and packed into a nanosheet 2.6 nm thick. Analysis of the orientation adopted by the phenyl groups inside the hydrophobic core showed a narrower distribution of angles in the cis peptoid nanosheet in comparison with the trans (Figure 4E). The pattern in which the linear peptoid backbones of the cis conformer was aligned suggested a greater degree of intermolecular interaction, and the calculated area density showed that this nanosheet was more densely packed, with 31% more chains per unit area, confirming that the nanosheet formed by the cis peptoid was more packed and organized than the one formed by the trans counterpart.[99] Calculated properties such as nanosheet thickness, spacing between the strands, and lamellar spacing between sheets were compared with experimental data obtained by AFM and XRD, and the results indicated that the dimensions of the cis nanosheet were in better agreement with experimental data than those of the trans nanosheet. Additionally, X-ray diffraction scattering spectra of nanosheets were simulated considering the dimension and other calculated properties, and again, results point to the cis nanosheet model as the more adequate to interpret and understand the conformations adopted by the peptoids inside the nanosheets and how packing and conformation interplay. Precise synthetic control over the peptoid structure at the atomic level and the possibility to predict some molecular properties enabled a fine tuning of the conformation adopted by the peptoids when forming the nanosheets; hence, allowing tuning of the nanosheet itself. Xuan et al.[112] studied a class of diblock copolypeptoids (Peptoid 29, as an example) characterized by a poly(N-2(2-(2-methoxyethoxy)ethoxy)ethylglycine) (pNte) block and different Npe-based hydrophobic blocks bearing different functional groups on the aromatic rings with electron withdrawing or donating character (e.g., fluoride, chloride, bromide, nitro, and methyl). Cryo-TEM, X-ray scattering, and MD simulations were used in combination to thoroughly characterize the crystal structures of the nanosheets. It was possible to directly superimpose MD simulated peptoid structures with the crystal structures observed by Cryo-TEM, resulting in a very good depiction of how the nanosheets were organized at the molecular level. MD simulations indicate that all studied peptoids conserved the cis conformation, with dihedral angles occupying the regions corresponding to the energy's minima at the Ramachandran plot, corroborating previously discussed observations. Although always displaying a stacked “V” shaped organization lattice, depending on the para-substituents, peptoids can adopt either a parallel or anti-parallel relative orientation.[114] Individual peptoid chains and their relative packing orientations were directly observed using Cryo-TEM with a 1.5 Å resolution indicating that peptoid organization within the nanosheet crystalline structure was very similar for all different molecules considered. Comparing para-substituents with different sizes (and volumes), it was possible to show that increasingly larger groups determined a slightly lengthier space between adjacent rows of peptoids; for example, a change from a hydrogen to a bromine atom increased the distance from 16.2 to 18.2 Å.[112]
5 Peptoid Nanosheets with Biological Activity
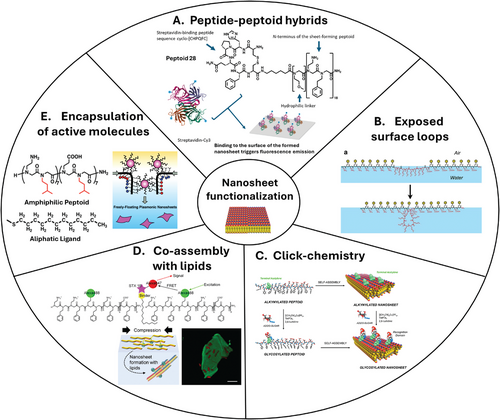
Nanosheet surfaces can be chemically controlled or modified to display a large variety of other chemical active motifs, including bio-derived molecules, paving the way to a diverse range of potential biological applications. Nanosheet functionalization can be undertaken at different stages of the nanosheet rational design and obtention. Insertion of functional groups can take place during peptoid (or peptoid/peptide hybrids) synthesis, during nanosheet assembly at the air–water interface, and after nanosheets if formed by a post-chemical surface functionalization. Figure 5 highlights the five up-to-date reported modes of nanosheet functionalization (chemical structures of functionalized peptoids are depicted in Tables 3 and 4).
 |
 |
5.1 Peptide and Peptoid Hybrid Molecules
Following conventional solid-phase synthesis approaches, peptide–peptoid hybrid molecules that offer a wide variety of biologically active segments can be synthesized.[115, 116] These can be made with full precise control over the chain length, side-chain chemistry, and monomer sequence of the hybrid molecules being generated. Hybrids with different designs have been synthesized and tailored for various biological applications such as surface-binding ligands, active site ligands, and antimicrobial agents.[116-119] In general, these molecules present varied short segments of each chemical character sited at different positions along the polymer sequences. In agreement with the physicochemical aspects of nanosheet formation and their desired functionality, hybrid constructions with a more defined peptoid–peptide–peptoid architecture are more widely explored in the literature for that matter.[96] In these constructions, peptoid segments work as nanosheet scaffolds; while, the peptide segment is presented at the nanosheet surface or borders acting as a functional motif. Nanosheet-forming amphiphilic peptoids appended to hydrophilic peptide or peptoid sequences can retain their nanosheet-forming ability and have these hydrophilic motifs exposed to the aqueous solution on the nanosheet surface. For example, Nam et al.[69] have incorporated a short hydrophilic linker followed by the streptavidin-binding peptide sequence cyclo-[CHPQFC] to the N-terminus of (Nae-Npe)18 Peptoid 16 to form Peptoid 30. Mixing this hybrid construction with (Nce–Npe)18 Peptoid 17, nanosheets are formed with a good yield (Figure 5A). Although not extensively characterized, authors proved through fluorescence microscopy and fluorescence resonance energy transfer (FRET) experiments with a Cy3-streptavidin that the nanosheet surface exposing cyclo-[CHPQFC] bound the fluorescently labeled ligand with good efficiency. With that, authors not only achieved a proof of concept regarding the formation of nanosheets with exposed functional hydrophilic motifs but also retained the proposed biological function, opening the field for a variety of new applications.
5.2 Exposed Surface Loops with Biological Functionality
Olivier et al.[109] reported, for the first time, that peptoid polymers with a specific sequence and architecture can “fold” or self-assemble into a peptoid nanosheet scaffold embellished with surface-exposed loop domains (Figure 5B). By inserting a linear segment of hydrophilic residues in the middle of the linear sheet-forming strand, small loops can be selectively displayed on the water-exposed face of the nanosheet (Table 4). In general, peptoid sequences that give rise to nanosheets with surface exposed loops display the following architecture: amphiphilic nanosheet forming segment–hydrophilic peptide/peptoid loop segment–amphiphilic nanosheet forming segment. By placing the loop sequence in the middle of the strand, between two amphiphilic sheet-forming segments, the ends of the loop sequence are anchored to the air–water interface. The amphiphilic segments of the polymer are intended to line up and pack closely with surrounding strands during compression of the peptoid monolayer, drawing the hydrophilic insert into the shape of a loop at the nanosheet surface (Figure 5B). Initially, hydrophilic peptoid sequences of varied lengths were tested as loops; segments of N-(2-methoxyethyl)glycine (Nme) with 4, 8, or 12 residues were inserted between Nae, Nce, and Npe amphiphilic sequences according to the following design: (Nae–Npe)4–(Nme)X–(Nce–Npe)4. All four peptoids, including a control without the Nme loop sequence, formed nanosheets with good yield, and AFM imaging and measuring confirmed the presence of the expected loops at the surface of the nanosheet. Both nanosheet height and roughness increased as a function of the size of the Nme stretch. Comparing the nanosheet without the loop with the one containing 12 Nme monomers, height varied from 3 to 8 nm and surface roughness from 0.3 to 1.4 nm. XRD analysis of the formed nanosheets showed that the inter-backbone spacing between polymer chain of 4.6 Å was conserved in all four studied nanosheets, indicating that, in general, the loop insertion does disturb the molecular packing of the peptoid portions forming the nanosheet to a large extent. Langmuir isotherms showed that nanosheets are formed following the same pathway whether having or not having hydrophilic loop-forming segments. Using a Langmuir trough equipped with an X-ray reflectivity (XR) setup, the electron density of the air–water interfacial arrangement was measured at different depths at a fixed surface pressure. Results indicated that, up to 20 Å of depth, all nanosheets presented similar electron density distribution and that a distinct, third region of electron density appeared in the profile of the peptoid containing Nme8, attributed to the exposed hydrophilic loops.
Peptide loops were inserted in between similar peptoid scaffolds with various degrees of success regarding nanosheet formation. A nanosheet formed with peptoid–peptide hybrid with a hydrophilic loop sequence composed of -Nme-βAla-Lys-Thr-Gln-Ala-Ser-βAla-(Nme)2- (Peptoid 36) was imaged and measured by AFM and presented the expected height and roughness as for a nanosheet displaying loops at the surface. Treatment with proteases followed by imaging by AFM revealed that the enzymes are capable of binding and cleaving the peptide exposed at the nanosheet surface. After treatment, AFM imaging of the nanosheets showed that both thickness and roughness were reduced; measured height profiles indicated that after treatment, the nanosheets formed by the polymer containing the loop sequence became similar to the nanosheet formed by the control peptoid without the loop. Advances in automated synthesis allowed the screening of peptoid sequences forming loops aiming to develop nanosheets displaying antibody mimetic motifs.[98] Starting from a set of 20 chemically diverse peptoid monomers, authors produced a library of 256 individual loopoid strands with sequences based on a 36mer nanosheet forming peptoid with a 6mer variable segment positioned in the middle of the polymer. Insert sequences were designed from 20 monomers with properties like the natural amino acids and combinatorial libraries were planned to avoid extremes of hydrophobicity or charge density. Alternating hydrophobic and hydrophilic insert sequences were also avoided as this property would prompt the 6mer intended loop to anchor at the air–water interface and assemble as a part of the scaffold nanosheet. Functionalization via loop exposing method was performed not with vial rocking methodology but with the use of the pipetting technique explained in Section 4.2.1 and illustrated in Figure 4E.
5.3 Click-Chemistry and Covalent Bonding of Active Motifs (Minerals, Sugars, and Proteins) for Biological Recognition
Due to the peptoid solid-phase synthesis advantages, the insertion of active peptide segments can be achieved in very convenient ways as discussed. However, more complex and diverse biological motifs cannot be easily incorporated into peptoid polymers due to several reasons such as incompatible covalent appendix chemistry or lack of molecular chemical or folding stability during synthesis, cleavage, and purification steps (Figure 5C). To circumvent these limitations and develop nanosheets displaying biologically relevant sugar motifs at their surfaces, Battigelli et al.[70] synthesized peptoids bearing alkyne functional units for the conjugation of carbohydrates through copper-catalyzed azide–alkyne cycloaddition (CuAAC) (Figure 5C). This reactive group was placed at different positions of the sequence depending on the peptoid. In one group of molecules, the reactive alkyne side chain was positioned at the positive block, close to the N-terminal end of the polymer; while, another group of molecules presented this same reactive moiety in a loop-forming segment of the molecule flanked by hydrophilic trimers of Nme on both sides. Carbohydrate conjugation was tested before or after nanosheet assembly (Figure 5C). Carbohydrate conjugation before nanosheet assembly was achieved by first reacting the azido-sugar with the peptoid polymer soluble in water, followed by HPLC purification, and then, through the vial rocking method, assembling the nanosheets. The authors made use of a recently disclosed technique created by the Fairbanks group to synthesize peptide glycoconjugates from native sugars.[120] Azido derivatives of mannose, galactose, globotriose, and N-acetylglucosamine were successfully obtained and used to glycosylate the peptoids (Peptoids 38–41). Glycosylated peptoid nanosheets were then characterized by fluorescence microscopy, which showed the assembly of the expected structures with a good yield for peptoids containing the chemically modified loops. XRD data confirmed that the peptoids formed nanosheet packing similar to the unfunctionalized peptoids, as measured peptoid interstrand spacing of 4.6 Å were conserved over all controls and functionalized samples. Glycosylated peptoid nanosheets without loops were 3.1 nm thick, and the corresponding control measured thickness was 2.8 nm. As also in agreement with the modifications accomplished, nanosheets with glycosylated or non-modified loop thicknesses were on average 3.5 and 3.2 nm, respectively. XRD data and AFM data indicated regular and ordered structures of the assemblies, consistent with the presence of sugars on the surface.[70] More recently, Ma et al.[121] reported the covalent binding of a super folder green fluorescent protein (sGFP) conjugated with a Car9 carbon binder peptide to a peptoid nanosheet of Nbpe6–Nce6 (Peptoid 31) Appendix of the protein to the nanosheet surface was achieved through a thiol-based reaction of a Cys residue in the protein, which had its position varied to investigate how binding varies in accordance, and a maleimide group at the N-terminal of the peptoid sequence MAL-Nbpe6-Nce6 (Peptoid 32). Nanosheets were formed either using only Peptoid 31 or a 1:1 mixture of Peptoids 31 and 32, and their properties were investigated by XRD, AFM, and fluorescence microscopy. In combination, the techniques showed that the nanosheets with the reactive maleimide group formed with a good yield and had the same characteristics as its control, with a thickness of 3.6 nm and interstrand space of 4.5 Å. sGFP-Car9 with the Cys residue at position 51 of the sequence was appended to the nanosheet with reactive maleimide and showed increased reactivity when compared to the mutants with Cys residue at positions 2 or 225. Greater G51C mutant reactivity was confirmed by both SDS-PAGE and by evaluating the colocalization of sGFP and Nile Red stained nanosheets by fluorescence microscopy.[121]
Reactant stoichiometry was also investigated and correlated with relative surface occupancy determined by AFM imaging and measuring, indicating that it was possible to tune the surface by manipulating the ratio between reactants. Comparing non-reacted nanosheet with the same scaffold reacted with 1 or 10 µm of the protein conjugate, it was clear how occupancy was proportional to the amount of available protein. While non reacted peptoid nanosheet surface was completely flat, with 1 µm of protein, it was possible to observe discrete elevation corresponding to folded sGFP-Car9, and with 10 µm of the protein nanosheet surface being essentially completely covered. Calculated coverage was 26 ± 4% and 90 ± 5% for 1 and 10 µm of reacted protein, respectively.[121]
5.4 Co-Assembly with Other Amphiphilic Molecules (Lipids) for Biological Application
Nanosheets can also be functionalized by incorporating amphiphilic molecules during their assembly at the air–water interface. Amphiphilic molecules like single-chain detergents and double-chain phospholipids, in the same way as amphiphilic peptoids, due to their character, accumulate and organize at the air–water interface, making them liable to co-assemble and form mixed peptoid: lipid nanosheets. Currently, a variety of lipid conjugates is commercially available and can present motifs such as fluorophores, peptides, and carbohydrates with a wide range of biological activity.[122, 123] Kim et al.[71] reported the co-assembly of nanosheets formed by Peptoid 13, incorporating single or double-chain lipids displaying different chemical groups appended to their polar head groups (Figure 4D). Octadecyl hydrocarbon chain was conjugated with either rhodamine B or acridine orange fluorophores; while a double chain glycero–phosphoethanolamine served as amphiphilic moiety for the attachment of Oregon green or rhodamine B fluorophores, or biologically active N-Biotynoil, globotrialosyl, or mannose structures. As lipid incorporation takes place during the formation of the nanosheets, Langmuir isotherms of the peptoid and peptoid: lipid mixtures were obtained to understand how the presence of the lipids influences the parameter of nanosheet formation. In general, the presence of the labeled lipids at the interface during nanosheet assembly does not largely alter the compression and expansion patterns in comparison with the nanosheets being formed only by the scaffold peptoid. XRD data indicates that, in agreement with the idea that the nanosheets are being formed and organized through the same mechanism as suggested by the Langmuir isotherms, an interstrand distance of 4.5 Å is conserved among all samples. In addition, thickness measurements performed by both XRD and AFM indicate an increase from 2.8 nm to ≈5.0 nm as lipids become incorporated into the macromolecular structure. Conservation of the Langmuir isotherms patterns and the distance between peptoid strands support low interference of the lipids upon the overall organization of the peptoids, suggesting the lipids can sit immobilized inside pockets formed at the termini of the peptoid strands. Combining MD simulations and FRET, a consistent distance of 4.9 to 5.1 nm between each pocket or between the two immobilized lipids was determined, reinforcing this idea of packing.[71] As incorporated lipids are thermodynamically compatible with the nanosheets up to a certain extent, the desorption of the fluorescently labeled lipids overnight was evaluated by dialysis. A reduction of nanosheet fluorescence of less than 5% confirmed that the lipids were tightly anchored to the peptoid bilayer sheet. With that, the authors established a simple, modular, and efficient functionalization strategy for peptoid nanosheets via the co-assembly of peptoid strands with lipids at the air–water interface.
5.5 Encapsulation of Active Molecules in Peptoid Nanosheets
Apart from peptoid nanosheet surface functionalization and co-assembly, studies about encapsulation of active molecules inside peptoid nanosheets have been reported as well. Robertson group has done some extensive studies on gold nanoparticle (AuNP) encapsulation to synthesize plasmonic peptoid nanosheets.[124] In this work, the authors used an amphiphilic peptoid sequence (Nae–Nmbu)7–(Nce-Nmbu)7 (Peptoid 15) constituted by N-(3-methylbutyl) glycine instead of phenylethyl glycine normally used in B28 and 5 nm dodecanethiol-functionalized AuNPs. The rationale behind this work was based on the fact that the assembly and subsequent collapse of peptoid monolayers at the interface between liquid phases offered means of incorporating hydrophobic species into the nanosheet interior, thereby enabling encapsulation. During the process of nanosheet formation at the interface between toluene and water, the aliphatic hydrophobic segments of the peptoid monolayer could interact with the aliphatic dodecanethiol ligands coating gold nanoparticles (AuNPs) suspended in toluene, forming a composite layer at the toluene–water interface. The amphiphilic nature of the peptoid allowed hydrophobically modified AuNPs to assemble onto it from the toluene phase. Compression of the toluene–water interface resulted in the collapse of this composite monolayer, forming freely floating nanosheets in which the AuNPs were encapsulated between two peptoid monolayers. This assembly protocol had a wide applicability because it created a path to any solute in the toluene phase that adsorbed to the peptoid monolayer to get encapsulated into the peptoid nanosheet interior, opening new possibilities for nanocomposite design and fabrication. The same group then studied the packing density of AuNPs within the nanosheets.[107] By varying the bulk concentration of the NPs during the nanosheet formation, the interparticle distance was tuned in the nanosheet interior, as calculated using localized surface plasmon resonance. The authors showed that being able to tune the NP packing on the nanosheet core allowed them to also tune and control the optoelectronic properties of the composite material. These novel 2D plasmonic peptoid nanosheets could be of particular importance in surface enhanced Raman scattering (SERS) sensing application, serving as substrate for solution-based biosensors.
6 Biological Applications
Due to their biological compatibility, resistance to proteolysis, and self-aggregating properties, peptoids have been explored to serve many biological applications as molecular transporters,[125-128] antimicrobial agents,[63, 129-134] toxin neutralizers,[135] gene delivering,[136] and so on.[137, 138] Inside the wide range of possibilities concerning peptoid uses, functionalized peptoid nanosheets with tunable properties have emerged as a potential alternative to several other more specific biological applications such as sensors, biomolecular recognition, catalysis, cell recognition and adhesion, and antibody mimicking. Rational design, functionalization, and tuning of other types of peptoid aggregates aiming for specific biological applications have also been successfully explored. For example, using the nanotube-forming Peptoid 9 as a scaffold, Jin et al.[83] and Cai et al.[139] conjugated biological active moieties such as RGD peptide for cellular adhesion and uptake, or IR780 for chemophototherapy of malignant glioma, respectively, both achieving substantial success. More recently, Jian et al.[140] developed peptoid nanotubes functionalized by co-assembly with the Hemin group that mimicked the peroxidase activity with high catalytic efficiency. The main attraction regarding the use of peptoid nanosheets was that they form highly stable scaffolds with large surface areas that can be tunable and designed to present a variety of active biological motifs with the desired functionality.[96] In addition, the work with functionalized nanotubes indicated the possibility of even broader biological applications of peptoid nanosheets considering the monomer packing similarities between these two types of aggregates.
6.1 Protein-Specific Binding and Recognition
Biological interactions that govern a protein binding to other proteins, peptides, sugars, lipid polar headgroups, or other derived templates can be explored by funtionalizing peptoid nanosheets. Nanosheets functionalized through co-assembly of a peptoid scaffold packed alongside glycosphingolipid molecules of globotriaosylsphingosine were designed and tested regarding the interaction and binding to Shiga toxin 1 subunit B (STX 1B) protein.[71] STX 1B forms a homopentamer that displays in each subunit a globotriaose binding site able to strongly interact with this carbohydrate displayed over the nanosheet surface. To confirm the interaction between the sugar motif displayed at the nanosheet surface and the toxin, peptoid strands were conjugated to FRET donor Alexa Fluor 488 at the fifth position along the 36mer peptoid polymer (Nae–Npe)9–(Nce–Npe)9 Peptoid 13, and STX 1B was conjugated with an Alexa Fluor 647 FRET acceptor fluorophore (Figure 6A,B). FRET emission signal at 675 nm increased, while the emission signal at 520 nm decreased, as nanosheets of B36 Peptoid 13 co-assembled with globotriaosylsphingosine were incubated with 250 nm of STX 1B. The result indicated a proximity between the FRET acceptor and donor, confirming that STX 1B bound to the carbohydrate on the nanosheets. The ratio between emission intensities at 675 and 520 nm was analyzed as a function of the amount of the glycosphingolipid incorporated into the nanosheet and as a function of STX 1B concentration. Assays generated a good response when nanosheet samples were prepared, incorporating 15 or 20 µm of globotriaosylsphingosine, yielding aggregates with 1:0.75 or 1:1 peptoid: lipid ratios, respectively. These preparations responded to the presence of STX 1B in a concentration ranging from 10 to 250 nm (Figure 6B). Nanosheets formed only by B36 Peptoid 13 conjugated with the fluorophore in absence of glycosphingolipid, did not exhibit the FRET phenomenon attesting the selectivity of the functionalized nanosheets. Taken together, peptoid/lipid hybrid nanosheets can serve as multivalent scaffolds to capture target proteins with high selectivity and sensitivity.[71]
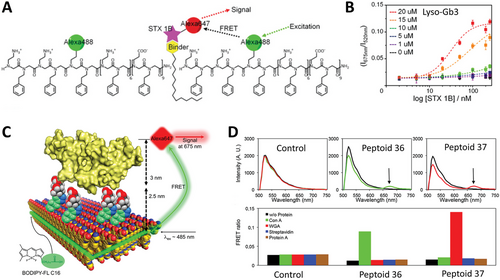
A more complex sugar-protein binding interaction was investigated using FRET and fluorescence microscopy.[70] Dimeric Concanavalin A (ConA) and trimeric Wheat Germ Agglutinin (WGA) proteins displayed binding sites to mannose or N-acetylglucosamine, respectively.[70] Both proteins have multiple binding sites for their respective carbohydrates, that are separated from each other by 7.0 nm in ConA and 1.1 nm in WGA. These proteins were conjugated with fluorescein isothiocyanate (FITC) fluorophore that served as report label to both FRET and microscopy assays. Colocalization by fluorescence microscopy confirmed the binding of FITC-ConA and FITC-WGA to nanosheets functionalized to present either mannose or N-acetylglucosamine epitope appended to hydrophilic loops on its surface. Fluorescence emission from the FITC label moiety was colocalized with the nanosheets stained with Nile red. Binding was also analyzed as a function of the amount of sugar displayed on the surface of the nanosheet and the extent of colocalized FITC fluorescence varied accordingly. FRET between FITC moiety and hydrophobic BODIPY incorporated inside the non-polar core of the nanosheet was used to further investigate protein–nanosheet interaction (Figure 5C,D). Results corroborate the general qualitative observation from fluorescence microscopy: first, no FRET was observed in samples with nanosheets with non-functionalized loops; second, ConA and WGA bound to nanosheet with mannose and N-acetylglucosamine exposed at the hydrophilic functionalized loops, giving rise to observed FRET phenomenon. As a control for binding specificity, ConA and WGA were incubated with nanosheets displaying N-acetylglucosamine and mannose, respectively, and two other FITC conjugated proteins, streptavidin and protein A, were incubated with these same nanosheets. No FRET or colocalization was observed for these samples attesting that only the correct protein–substrate pair could interact. By forming nanosheets with different architectures in regard to how the carbohydrate is presented on the surface, authors also investigated how binding was influenced by the distance between the exposed carbohydrate and the surface of the nanosheet and by the distance between each carbohydrate. Peptoids were synthesized with the reactive group either as the sidechain at the third position of the nanosheet forming segment or, also as a sidechain, situated at a hydrophilic loop forming segment. After reaction with the azido-sugar, carbohydrates were displayed rather differently on the nanosheet surface, and no significant interaction was detected between the proteins and the nanosheets functionalized at position three of the amphiphilic segment. In this case, the lack of binding was caused by the inaccessibility of the carbohydrates on the material surface. By varying the amounts in the mixture between scaffold and functionalized peptoids, it was possible to play with the distances between each exposed sugar and, briefly, results made clear the idea that sugar functionalities need to be displayed at the right distance to simultaneously interact with multiple binding sites on the protein of interest.
6.2 Antibody Mimetics Nanosheets
One step forward regarding protein recognition and binding to functionalized nanosheets[109] tested the interaction of peptoid nanosheets presenting antibody mimetic peptide loops that specifically fold and recognize the enzyme casein kinase II (CK2α). Peptide sequences that form the loop and specifically interact with CK2a were selected because it is short (heptamer) and highly hydrophilic, properties that, as discussed, are relevant for the correct assembly and function of the nanosheets. After proving that the peptide loop sequence can be presented at the nanosheet surface to be processed by proteases (Section 5.2), authors demonstrated that the loop peptide also folded as designed to be recognized and serve as a substrate for casein kinase II alpha subunit (CK2α) that could phosphorylate the Serine residue displayed on the loop of nanosheets formed by Peptoid 35.[109] Recognition of CK2α was confirmed through the analysis of the chemical modification exerted by the enzyme on the loop. Immunofluorescence imaging of the nanosheets stained with Nile Red and FITC-labeled anti-phosphoserine antibody demonstrated high degree of colocalization; FITC fluorescence at the nanosheet increased as the percentage of the loop containing peptoid–peptide hybrid increased from 0 to 50%, also suggesting that both enzyme and antibody bound specifically to the peptide loop, rather than nonspecifically to the surface of nanosheets (Figure 7).
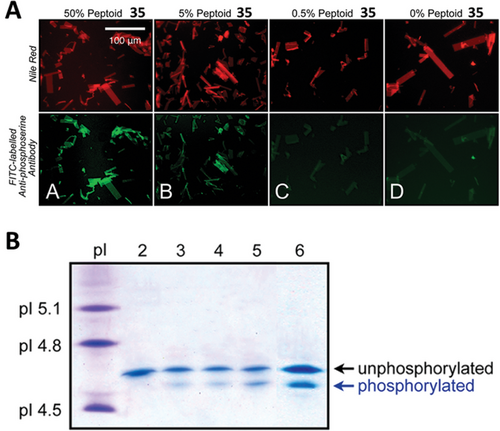
Nanosheets incubated with CK2α and undone using sodium dodecyl sulfate were analyzed by isoelectric focusing gel electrophoresis (Figure 7). Quantitative analysis of the gel revealed 20% phosphorylation of the loops, against 30% conversion on solubilized peptoid strands incubated with the enzyme at similar conditions, confirming that the loops are rather accessible on the nanosheet surface. Authors attribute the observed gap in conversion to the slower diffusion of the nanosheet assembly rather than any limited accessibility. More recently, nanosheets displaying peptoid loops were proven to have a potential use as antibody mimetics.[98] A library of 256 individual loopoid strands, consisting of a 36mer nanosheet forming peptoid with a 6mer variable segment insert, was created starting from a set of 20 chemically diverse peptoid monomers, and nanosheets produced by repeated pipetting cycles. The affinity and selectivity of the formed nanosheets displaying peptoid loops to anthrax protective antigen (PA63)7, a homoheptameric toxin-related protein, was quantitively evaluated by homogeneous binding FRET assay. For that matter, nanosheet functionalization through co-assembly of the peptoid scaffold and labeled amphiphilic molecules becomes a powerful tool of investigation (Section 5.4) as peptoid nanosheets were co-assembled octadecyl rhodamine, an amphiphilic FRET donor fluorophore that is anchored to the nanosheet hydrophobic core by its aliphatic hydrocarbon chain. As FRET acceptor, Alexa Fluor 647 was conjugated to (PA63)7 and, as control, to STX 1B and streptavidin. From FRET quantitative analysis of affinity and specificity toward (PA63)7, a loopoid strand L034 (Peptoid 42) was selected from 60 variants as having the highest score. Peptoid 42 and other high score candidate had their synthesis scaled up to further structural and binding characterization, validation, and comparison with a peptide/peptoid hybrid nanosheet (Peptoid 43) displaying a loop containing a peptide hexamer, TYWWLD, a known (PA63)7 binding motif (Figure 7). Fluorescence microscopy allowed visualization of the octadecyl rhodamine co-assembled nanosheets formed by Peptoid 42 or 43, and colocalization of (PA63)7 conjugated with Alexa Fluor 647. Controls behaved as expected and no colocalization was observed between the nanosheets and STX 1B, streptavidin, or Protein A (Figure 8). Validation of specificity shows that combinatorial libraries can be quickly screened at the microscale to find antibody mimetics and that positive hits can be scaled up to the milligram scale for further testing and application. The loop of Peptoid 42 is composed of two small polar carboxamidomethyl (Namd) side chains, and four aromatic hydroxyphenethyl (Ntyr) side chains displayed in the following sequence: Namd-Ntyr-Ntyr-Ntyr-Ntyr-Namd. In comparison with the control peptide loop consisting of TYWWLD (Figure 8), it is noticeable that both follow the same pattern distribution between the polar hydrophilic and hydrophobic group, with four hydrophobic residues flanked by two hydrophilic ones and that both have at least three aromatic residues.[98]
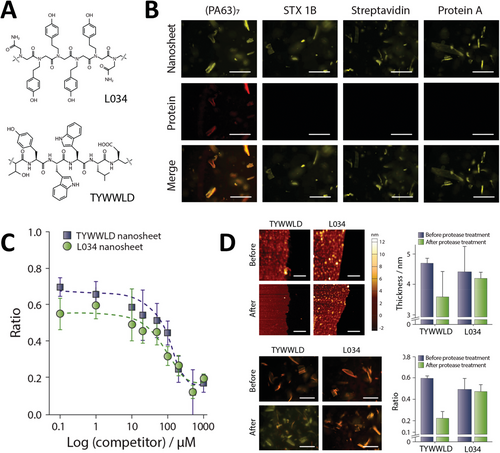
Given the chemical similarities between the loop-inserts of Peptoid 42 and 3 components of the library displaying the same hydrophilic/hydrophobic pattern were more carefully analyzed regarding their specificity and no major specificity or correlation among the other five peptoids was determined. The high density of aromatic sidechains is another noticeable feature of the loops on Peptoid 42 and 43 that other similar peptoids of the library possessed. The analysis of these peptoids regarding their specificity confirmed that such characteristic played a major role in (PA63)7 binding, revealing that three or more aromatic monomers are required for binding the substrate in the presence of a hydrogen bond donor.
The binding affinity of Peptoid 42 and 43 to (PA63)7 are also similar and suggest, alongside other evidence, that both loops interact with the same region of the toxin. The half-maximal inhibitory concentration (IC50) values of the L034 and TYWWLD nanosheets was calculated to be 78 and 96 µM, respectively (Figure 8).[98] Computational docking study of TYWWLD indicates that the peptide in a loop conformation interacts with a hydrophobic pocket of (PA63)7 displaying Phenylalanine residues, compatible with other aromatics through π-π pairing, and Proline residues that associate preferably with electron-rich side chains as Tryptophan and Tyrosine. Because (PA63)7 is a homoheptamer, it presents the possibility of multivalent binding sites that can interact in a cooperative manner increasing the overall affinity. Computational simulations showed that the toxin covers several loops on the nanosheet's surface, indicating multiple binding events. Multivalent binding was also investigated experimentally by playing with the distances between loops on the surface of the nanosheets and by confirming the positioning of (PA63)7 toxin bound to the loops on the nanosheet using Cryo-EM. The need for an adequate distance between the loops and the fact that the central pore of the heptameric toxin is aligned perpendicularly to the surface strongly supports the multivalent cooperative binding. The proteolytic stability of Peptoid 42 and 43 nanosheets were compared using both AFM and fluorescence microscopy (Figure 8). Samples of Peptoid 42 nanosheets were imaged before and after being treated with proteases and showed no major change in thickness, which remained at approximately 4.3 nm, or in the degree of colocalization with the toxin, confirming it also remains functional. Submitted to the same protease treatment, the thicknesses of Peptoid 43 nanosheets was reduced from 4.7 to 3.4 nm and a significant drop in colocalization between fluorescent (PA63)7 and the nanosheets was observed (Figure 8). These results confirmed peptoid nanosheets stability against protease degradation and their robustness, highlighting significant advantages of the synthetic construct.[98]
6.3 Peptoid Nanosheet for Eukaryotic and Prokaryotic Cells and Virus Recognition
Peptoid nanosheets' large dimensions and the potential to expose peptide or peptoid motifs that interact with a specific biological molecular structure open many possibilities for interacting and capturing larger assemblies such as liposomes, viruses, and entire prokaryotic and eukaryotic living cells. For that, molecular recognition of the external structural features of these larger assemblies is key. Nanosheets functionalized by co-assembly of a Peptoid 13 scaffold (B36) with a lipid binder (e.i., 1,2-dipalmitoyl-sn-glycero-3-phospho((ethyl-1′,2′,3′-triazole)triethyleneglycolmannose)), composed of a phospholipid bearing a hydrophilic spacer, appended to the third carbon atom of the glycerol, and a mannose functional group were used to capture E. coli cells under physiological conditions.[71] The authors investigated the interaction of the mannose functionalized nanosheets with a K-12 derivative strain that expresses mannose-specific adhesin FimH of type 1 pili on its surface, E. coli ORN178. As a negative control, E. coli ORN208, which does not express the FimH receptor was used. Confocal fluorescence microscopy confirmed that the peptoid nanosheet co-assembled with mannose phospholipid can capture bacterial cells of E. coli ORN178 (Figure 9A,B) but does not bind and capture the strain lacking the required receptor. Overall binding decreased to a very low level as the amount of mannosylated lipid was lowered from 20 to 10 µM, demonstrating how a high density of exposed sugar eases the efficient and selective binding affinity for E. coli ORN178 via multivalent interactions and, likely, a cooperative effect.
The interaction of E. coli ORB178 to B36 Peptoid 13 nanosheets without any modified lipids was tested as the control for nonspecific binding and no cell capturing was observed. By checking the lack of interaction between ORN208 and functionalized nanosheets, and between ORN178 and nonfunctionalized nanosheets, it was possible to confirm the desired specificity.[71] Confocal fluorescence microscopy imaging shows many bacteria cells settled and immobilized at the nanosheet surface (Figure 9A), a condition in which bacteria proliferation is inhibited. Authors then measured the agglutination index and overall proliferation of E. coli ORN178 in the presence of either mannose functionalized or non-functionalized nanosheets and confirmed the high number of bacteria cells captured per area (A.I. = 198), and approximately 50% of growth inhibition (Figure 9B). These observations lead one to recognize how can lipid-anchored peptoid nanosheets be considered as candidate materials to neutralize pathogens by direct binding. Other peptoid aggregates can serve as scaffolds to bind and capture a variety of large assemblies. Jin et al.[83] used peptoid nanotubes displaying a peptide RGDG binding motif to capture live A549 cancer cells. At first, glass slides were coated with an RGDG functionalized nanotube, and cell attachment was visualized by optical microscopy. No significant attachment was observed when glass slides received no treatment or were treated with nanotubes formed by the peptoid lacking the RGDG motif. The calculated cell density is approximately 800 cells/cm2 in the Peptoid 9-RGDG-treated glasses and approximately 50 and 200 cells/cm2 in the non-treated and Peptoid 9-treated glasses, respectively (Figures 8C,D). Peptoid nanotubes co-assembled from Peptoid 9, RGDG- Peptoid 9, and rhodamine B- Peptoid 9 with a molar ratio of 8:1:1, previously cut by sonication, were also used to capture live A549 cancer cells. Both cells and nanotubes were imaged by fluorescence confocal microscopy using stained cytoskeleton actin and using DAPI to stain cells nuclei enabling the visualization of the cells densely bound to the nanotubes. Again, the peptoid nanotube control lacking the RGDG motif demonstrated considerably less cell attachment. Nanosheets formed by peptides can also be used to bind and capture viruses. Dai et al.[141] have formed and characterized nanosheets composed of a peptide heptamer (KLVFFAK) the sequence corresponds to the segment 16–22 of the αβ-amyloid forming protein bearing and Italian familial mutation. After a complete biophysical characterization of the nanosheets, the attachment of lentivirus on the surface of an amyloid-like nanosheet was directed and visualized by electron microscopy (Figure 9E,F). In this case, binding is driven not by specific antibody-like or protein-protein interactions but by the electrostatic attraction between both positively charged Lysine residues at each end of the peptide and the virus particle that is negatively charged. While substituting a terminal Lysine to Arginine does not virus binding to a large extent, function was abolished when an Aspartic acid was the substituent at the C-terminal position of the peptide, confirming the electrostatic attraction as the driving force behind virus binding to the nanosheets. The binding of either viruses or cells to supramolecular ensembles as peptide nanosheets or peptoid nanotubes suggests that exploring peptoid nanosheets to these applications can be extremely advantageous. Peptide nanosheets able to bind viruses could be easily substituted by its more easily obtained, proteolytic resistant, and robust peptoid counterpart. As for peptoid nanotubes, substituting them as glass coating material for peptoid nanosheets may consist of a gain regarding how much area can be covered by a limited amount of material.
6.4 Peptoid Nanosheets for Biological Sensors and Sensing Applications
The numerous possibilities to chemically derivatize the poly-glycine backbone of peptoids appear to have infinite potential, opening prospects to develop novel, useful sensors for a variety of applications. Many peptoid sensors able to sense light, cyanide, mercury, solvent, and cations have been reported in the literature.[142] In this regard, nanosheets have also emerged as an interesting possibility due to their bi-dimensional profile that suits the usual need to uniformly cover larger surfaces in many sensor setups. In addition, nanosheet robustness, biocompatibility, and tunability, as discussed, have developed significantly, broadening the potential for the design of biosensors. Aiming to develop a sensor to detect in serum the αβ-42 amyloid-forming peptide, responsible for Alzheimer's Disease (AD), Zhu et al.[143] have synthesized nanosheet forming di-block peptoids containing or not containing a hydrophilic loop insert (Peptoid 37). The insert sequence forms a loop that is conformationally restricted and mimics an anti-αβ-42 antibody (Alzheimer's Disease Peptoid 3, ADP3) (Figure 10). Nanosheets are formed using peptoids without and with the loop (Peptoid 37), mixed at different ratios: 100:0, 90:10, and 0:100, respectively. The authors evaluated nanosheet formation as a function of the proportion between each peptoid molecule and compared manual and automated vial tilting procedures to obtain the nanosheets. Sensor functionalities were based on surface plasmon resonance imaging (SPRi), which required the immobilization of the nanosheets, prepared in aqueous solution, on the chip sensor (Figure 10). The gold surface of the sensor was fabricated with a coat of poly-(OEGMA-co-HEMA) through a surface-induced polymerization process that resulted in a polymeric layer that was carboxylated by succinic anhydride and 4-dimethylaminopyridine. Carboxylated groups were then covalently linked to the amino group of the nanosheets immobilizing them on the chip sensor.
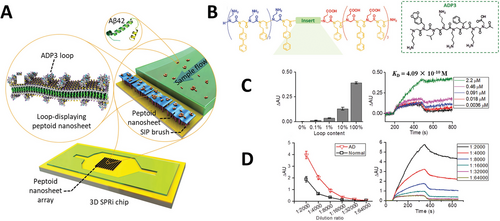
As a positive control and to attest to sensor binding and detection of Aβ-42, a flow of the peptide solution was passed through the chamber of the sensor, and the SPRi signal was registered. No significant signal was detected using the sensor with nanosheets without an ADP3 loop; while testing the functionalized sensor with Peptoid 37 nanosheets retrieved a detection signal that was proportional to the loop content of the nanosheets (Figure 10). The equilibrium dissociation constant (KD) of the interaction between the nanosheet formed by 100% of the ADP3-containing construct and αβ-42 was 4.09 × 10−10 m−1 (Figure 10), ten times lower when compared with the linear water-soluble ADP3 peptide. The result suggests that loop density and restricted conformation increased binding affinity.[143] To further confirm selectivity, the affinity between the 100% ADP3 containing nanosheet and αβ-40, an abundant isoform of αβ-42, was evaluated and yielded a KD of 1.34 ×10−7 m−1, 1000 times lower than the desired αβ-42-ADP3-nanosheet interaction. The next step was to confirm that the chip with peptoid nanosheets displaying ADP3 loops (Peptoid 37) could detect Alzheimer's Disease through the analysis and comparison of human serum that was obtained from sick and healthy donors. The registered signal, in ΔAU, for the interaction between AD sera and non-functionalized nanosheets was 1.4. Interaction between 100% ADP3 functionalized nanosheets and AD or normal sera retrieved ≈3.9 or 1.9, respectively, allowing one to distinguish each case for diagnostic purposes (Figure 10). Sera were diluted from 2000 to 64 000 times to establish sensor sensitivity, and it was possible to distinguish between healthy and AD samples, up to a 1:8000 dilution (Figure 10). An alternative for coating sensors with functional peptoid nanosheets was explored in the work from Murray et al.,[144] in which a surficial monolayered or bilayered ensemble of the amphiphilic peptoids was transferred to a solid support by Langmuir–Blodgett or Langmuir–Schaefer techniques. B28 loopless Peptoid 14 monolayers were deposited onto hydrophilic or hydrophobic substrates composed of Si/SiO2 and 2-phenylethyl-functionalized Si/SiO2, respectively. For hydrophilic substrate coating, after peptoid surface adsorption/desorption equilibrium was reached, at ≈22 mN m−1, surface pressure was increased and fixed to 30 mN m−1 to ensure that the peptoid molecules were tightly packed; while the Si/SiO2 substrate, previously submerged, was lifted from the liquid at a rate of 1 mm min−1 (Figure 11). At these same conditions, hydrophobic substrate 2-phenylethyl-functionalized Si/SiO2 coating was accomplished by lowering the support into the solution and aspirating the surface to remove any remaining peptoid before withdrawing it from the liquid.
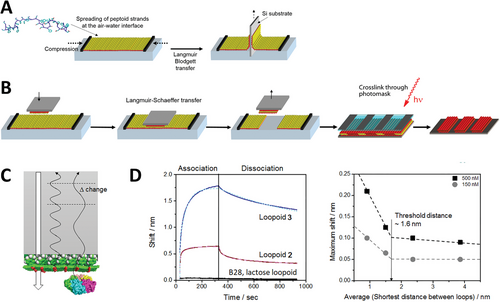
Substrates were analyzed by reflectance optical imaging and AFM showing that both were coated uniformly, with no observable defects and an average thickness of 1.2 ± 0.2 nm. In addition, the decrease in the area of the surface in the Langmuir trough setup matched the area of the substrates, confirming a transfer ratio of 1 and the uniform coating. The possibility to coat hydrophilic and hydrophobic surfaces opened opportunities to exploit peptoid functionalization with loops displaying different chemical properties, considering that these loops can protrude to either the hydrophobic or hydrophilic phase and, after coating, be exposed to the surface of the transferred monolayer. Monolayers formed by peptoids containing loop segments were deposited onto hydrophobic support through the same Langmuir–Blodgett method and analyzed by AFM to evaluate its thickness, roughness, and uniformity. The measured thickness of the loop containing monolayer was measured as 2.5 ± 0.3, indicating a satisfactory uniform coating and that the loops protrude from the surface as expected. Loopless B28 Peptoid 14 bilayer deposition was achieved by the Langmuir–Schaefer method and the transfer of a second monolayer onto the previously dried first peptoid layer (Figure 10). In this setup, the monolayer-coated substrate was horizontally lowered to gently contact the air–water interface, allowing the hydrophobic face of each monolayer to interact forming the core of the immobilized nanosheet. Staining of the deposited material with Nile Red, known to selectively incorporate into the hydrophobic core of the nanosheets, suggests the assembly of the desired bilayered supramolecular aggregate. By AFM, a bilayer thickness of 3.0 ± 0.2 nm was measured, further confirming the material morphology, and roughness was estimated to be the same as the Si/SiO2 support. Using grazing-incidence wide-angle X-Ray scattering (GIWAXS) and selected area electron diffraction (SAED) by transmission electron microscopy, a space between each peptoid molecule of 4.6 Å was measured, further confirming that the packing of the peptoid bilayer coating was similar to that observed for water floating nanosheets obtained by the vial-rocking method. Coating long-range order was improved by performing an annealing step before transferring the monolayer from the air–water interface to the silica substrate. While being compressed, the monolayer was annealed at 50 °C for 3 h, and SAED data of Langmuir–Schaefer transferred bilayers indicates a considerable gain in long-range order, a significant advantage considering the importance of the high molecular organization within the nanosheets for its sensing application. Bilayered films were irradiated with 254 nm UV light to covalently crosslink the hydrophobic core of each monolayer of the peptoid nanosheets (Figure 11).[144] Cross-linking rendered the nanosheet film insoluble in organic solvents and further reinforced the molecular structure of the construct. The irradiated surface could be controlled using photomasks to crosslink the nanosheet to a determined area and pattern (Figure 11) as non-crosslinked areas of the film could be removed using organic solvents. With that, it was possible to create different patterns of nanosheet coating on the substrate surface and to precisely control the coated area, a very relevant property considering that the overall reproducibility of coating regulated sensing properties. Langmuir–Schaefer methodology was used to coat the tip of glass optical fibers with nanosheets, displaying loops that could specifically detect analytes using a bio-layer interferometry technique (BLI) (Figure 11). Peptoid nanosheet constructs with loops displaying either a globotriose (Peptoid 40)[70] or the hexamer peptide TYWWLD (Peptoid 43)[98] were immobilized at the tip of the glass optical fiber and employed to detect protein macromolecules as Stx 1B toxin or (PA63)7 through BLI, respectively. Based on interferometry signals, association and dissociation rates and binding constant of each sensor-analyte pair were studied; measured KD between Peptoid 40 nanosheet and STX 1B was 15.5 nm, and, between Peptoid 43 nanosheets and (PA63)7 was 31.1 nm, indicating high affinity and specificity even in comparison with the free forms of globotriose and the peptide TYWWLD. Measured high affinity arose from the multivalent interactions that could take place when binding motifs were exposed at the sensor surface, adequately spaced from each other, inducing a cooperative binding behavior.[70] The possibility of reusing BLI sensors was evaluated by immersion of the tips in 10 mm HCl, for 10 s, at room temperature, to remove bound proteins. Binding efficacy and other association parameters were maintained after three cycles of using and cleaning, indicating the possibility of reusing the assembled sensor tips. In addition, long-term stability was verified by storing the tips for 9 days at 4 °C, and similar binding signals were obtained. The two different approaches to assembling and applying peptoid nanosheets as sensors showed promising results with both setups retrieving high specificity and sensitivity, crucial characteristics regarding sensor development. Wang et al.[86] developed a rather different approach to making use of bi-dimensional peptoid nanosheets in sensing applications aiming to detect and quantify intracellular H2S. For that purpose, nanosheets were formed by co-assembly of different ratios between a scaffold peptoid containing six hydrophobic N-[2-(4-chlorophenyl)ethyl]glycine (N4Cle) residues and a polar block having six N-(2-carboxyethyl)-glycine (Nce) groups (Peptoid 33), and a functionalized peptoid with this same scaffold sequence and an H2S-responsive 3,5-dinitrophenol (NDP)-modified 1,8-naphthalimide (NI) conjugated at the N-terminus (Peptoid 34) (Figure 12). This group, in the presence of H2S, underwent a thiolysis reaction that resulted in the formation of a fluorescent substituted naphthalimide species that emitted light when excited at 420 nm and could be used to monitor reaction as well as the polarity of the environment (Figure 12A).[122, 145] Nanosheets formed by a 2–3 days crystallization process were visualized by TEM and AFM and showed the presence of uniform bidimensional aggregates with straight edges and a thickness of 3.8 nm, as measured in the AFM image. XRD curves presented peaks at 3.8 nm, 4.5 Å, 1.8 nm, 3.0 A, and 6.0 A, corresponding to the previously measured thickness, the ordered alignment of peptoid chains (interstrand distances), the peptoid backbone–backbone distance along the y direction, the distance between two adjacent residues of a cis conformation, and the length of the N4Clpe side chain, respectively.[145] Detection of H2S in vitro was investigated by incubating the nanosheets or their solubilized peptoid components, with different concentrations of Na2S and registering both absorbance and fluorescence emission of the samples, when exciting the fluorophore at 420 nm. Emission from samples containing the nanosheet aggregates increased to a large extent as Na2S concentration was varied from 0 up to 5 mm; while no major increase in emission was registered in the samples containing the corresponding solubilized peptoids (Figure 12B). These observations highlighted the importance of the high crystallinity in the fluorescence performance of nanosheets to the desired application.[145] By varying Na2S concentration, authors also calculated the detection limit, this time focusing on nanosheets formed by the peptoid bearing the reactive group and its matching scaffold sequence in the following ratios: 1:9, 2:8, 3:7, and 4:6. The best performance was achieved by nanosheets formed by the 3:7 ratio between functionalized and non-functionalized molecules, NDP-NI-2DNM-37, with a detection limit of 0.53 µm (Figure 12C). By incubating H1299 cells with a suspension of NDP-NI-2DNM-37 and 0, 50, or 100 µm of Na2S, endogenous and exogenous intracellular detection of H2S was performed using confocal fluorescence microscopy. Images of the cell and nanosheet sample incubated without the addition of Na2S displayed a low but significant red color fluorescence emission, indicating that the setup was sensitive enough to detect intracellular endogenous H2S (Figure 12D).[145] Samples with 50 and 100 µm of Na2S, in agreement with in vitro observations, clearly showed an increase in the emission of the fluorophore (Figure 12D),[145] confirming that the setup could successfully respond and detect intracellular H2S.
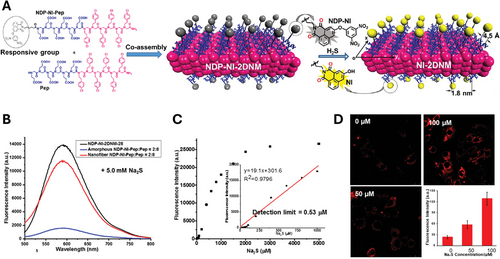
7 Summary and Future Perspectives
The literature contains a vast array of nanomaterials collectively referred to as “nanosheets.” In this review, we emphasize the most commonly utilized materials that exist in a 2D nanosheet form, with a particular focus on peptoid nanosheets, which present themselves as promising and intriguing materials. Over the past 11 years, significant progress has positioned peptoid nanosheets at the forefront of 2D nanomaterials, particularly for a wide range of biological applications. These novel 2D nanomaterials are distinguished by their robustness and high flexibility, setting them apart from traditional self-assembly systems. In this review, we introduce peptoid nanosheets and summarize various theoretical, computational, and experimental efforts to elaborate on multiple factors that guide the synthesis, formation, and application of peptoid 2D nanomaterial. The formation process, from synthesis to their self-assembly or co-assembly into desired 2D nanostructures, is both rapid and efficient. To synthesize this material, one advantage lies in the fact that the synthesis process is compatible with bio-sustainable solvents, making the production of these innovative nanostructures environmentally friendly and suitable for physiological conditions, such as buffers and specific pH levels. Moreover, the assembly principles are also highly versatile as from one material, one can make nanotubes, nanosheets, bundles, vesicles, cargo flowers, and so on, and this highlights an advantage over other nanomaterials such as titanium surfaces and carbon nanomaterial. Another advantage of peptoid assembly into 2D nanostructures is the modulation. Functional groups (e.g., sugars, peptides, and small molecules [e.g., biotin[71]]) can be precisely incorporated as peptoid side chains, for example, nanoparticles can be encapsulated via various flexible modes of assembly, and as such, be used for a wide variety of biological interaction studies. The modulation of the various biological functions onto peptoid nanosheets can be easily quantified as the molarity of the building blocks and bioactive molecules can be monitored in a controlled amount, when compared with the functionalizations available on other types of nano surfaces (e.g. Ti, carbon nanosheets). It is pivotal to highlight that peptoid nanosheet research area is highly interdisciplinary and requires theoretical, computational, and experimental approaches to generate understanding about the peptoid organization and shape-shifting behavior. To exemplify, molecular dynamic simulations can predict folding and assembly on an atomic level. Our review highlights the potential and effectiveness of peptoid nanosheets, underscoring their utility in various applications. While their use is gradually expanding, advances in nanotechnology and new methods for characterizing biological activities have facilitated their broader application in microbiology and molecular biology. Current research demonstrates that peptoid nanosheets are a promising class of nanomaterials, with designs that can be tailored for self-assembly and co-assembly into multiple 2D nanostructures. In the upcoming years, we believe peptoid nanosheets will serve as a foundation for the development of novel medical diagnostic tools, although, further progress and extensive research are needed to fully realize their potential.
Acknowledgements
The work is funded by a grant from the Villum Foundation, grant number 4095.
Conflict of Interest
The authors declare no conflict of interest.
Author Contributions
G.C. and B.M. contributed to the manuscript's initial draft writing and conceptualizing. G.C., H.K.S., A.B., and B.M. contributed to the final manuscript writing.
Biographies

Gustavo Penteado Battesini Carretero is a postdoctoral research fellow with Assoc.Prof.Biljana Mojsoska at Roskilde University. He received his Ph.D. in Biology in 2016 from the University of São Paulo, Brazil under the supervision of Professor Shirley Schreier. Following his Ph.D., he worked as a postdoctoral fellow researcher with Professor Iolanda Cuccovia and Emeritus Professor Hernan Chaimovich in the Department of Biochemistry at the Institute of Chemistry of the University of São Paulo, Brazil (2021). He is mainly engaged in the design and synthesis of long peptoid polymers that assemble into 2D nanosheets with antimicrobial properties, and the use of these nanosheets to coat the surface of medical devices such as catheters and contact lenses.

H. Kalani Samarasekara is a graduate student in the Department of Chemistry of the University of Maine working on the synthesis of novel peptoid nanostructures in the group of Dr. Battigelli. Her current research interests include the development of peptoid nanomaterials for cancer therapy and tissue engineering.

Alessia Battigelli received her Ph.D. in chemistry and pharmaceutical sciences from the University of Trieste and the University of Strasbourg in 2012. In 2014, she joined the group of Dr. Ronald N. Zuckermann at Lawrence Berkeley National Laboratory where she worked on peptoid nanosheets for biosensing. She was then a postdoctoral research associate at Brown University working on hydrogels for stem cell adhesion. She is currently an assistant professor in the Department of Chemistry at the University of Maine, and her research interests include the design of peptoid-base nanomaterials and their biological applications.

Biljana Mojsoska received her Ph.D. degree in Chemistry and Microbiology from Roskilde University in 2015 working on design and discovery of antimicrobial peptoids with Prof. Håvard Jenssen, and Dr. Ronald N. Zuckermann at the Molecular Foundry at Lawerence Berkeley National Laboratories. She then joined Prof. Søren Molins’ lab at the Novo Nordisk Center for Biosustain, where she worked on dissecting the persister phenotype of P. aeruginosa clinical isolates. Her current research at Roskilde University focuses on bridging disciplines within chemistry, biology, and nanomaterials. She specializes in peptoid-based research, from nanomaterials to proteomic investigations, exploring innovative solutions for antibiotic resistance.