Toward Photosynthetic Mammalian Cells through Artificial Endosymbiosis
Abstract
Photosynthesis in plants occurs within specialized organelles known as chloroplasts, which are postulated to have originated through endosymbiosis with cyanobacteria. In nature, instances are also observed wherein specific invertebrates engage in symbiotic relationships with photosynthetic bacteria, allowing them to subsist as photoautotrophic organisms over extended durations. Consequently, the concept of engineering artificial endosymbiosis between mammalian cells and cyanobacteria represents a promising avenue for enabling photosynthesis in mammals. The study embarked with the identification of Synechocystis PCC 6803 as a suitable candidate for establishing a long-term endosymbiotic relationship with macrophages. The cyanobacteria internalized by macrophages exhibited the capacity to rescue ATP deficiencies within their host cells under conditions of illumination. Following this discovery, a membrane-coating strategy is developed for the intracellular delivery of cyanobacteria into non-macrophage mammalian cells. This pioneering technique led to the identification of human embryonic kidney cells HEK293 as optimal hosts for achieving sustained endosymbiosis with Synechocystis PCC 6803. The study offers valuable insights that may serve as a reference for the eventual achievement of artificial photosynthesis in mammals.
1 Introduction
Natural photosynthesis presently serves as the primary method for harnessing sunlight, which is the most abundant and sustainable energy source on Earth. To enhance the utilization of solar energy, substantial efforts have been directed toward the development of artificial photosynthesis in recent decades. These efforts encompass a range of chemical photocatalysts,[1] such as Sv-ZnIn2S4/MoSe2,[2] ZnIn2S4/SnS2,[3] and TiO2/Bi2O3,[4] as well as hybrid systems that integrate semiconductor nanomaterials with microbial cell factories.[5, 6] Notable examples include cadmium sulfide nanoparticles coupled with Moorella thermoacetica[7] and indium phosphide coatings on Saccharomyces cerevisiae.[8] Now, recent advances in synthetic biology are bringing us closer to the realization of a long-envisioned goal: the direct engineering of animal cells for photosynthetic functions.
There are some precedents within the aquatic realm, where a fascinating class of creatures known as sea slugs has evolved remarkable mechanisms to acquire photosynthetic products, including energy and fixed carbon compounds such as carbohydrates. These sea slugs achieve this by harboring chloroplasts derived from algae, enabling them to sustain their lives for several months solely with access to light, water, and air.[9] Similar forms of photosymbiotic associations are observed in other non-vertebrate aquatic organisms, such as flatworms (Acoelomorpha), nudibranchs (phyla Mollusca), and sponges (Porifera), all of which harbor intact microalgae or cyanobacteria.[10, 11] Inspired by these natural instances of photosymbiosis in non-vertebrates, researchers have injected photosynthetic microalgae Chlamydomonas reinhardtii into the embryos of zebrafish, demonstrating the survival of the microalgae in the embryos for several days without causing significant inflammatory responses.[12] Additionally, engineered cyanobacteria Synechococcuse elongatus PCC 7942, modified with invasin from Yersinia pestis, displayed the ability to invade certain cultured mammalian cells, including hamster ovary cells and mouse macrophages, and survive for several days.[13] More recently, groundbreaking work involved the delivery of thylakoids from spinach into human chondrocytes through membrane fusion. This innovative approach provided light-induced ATP and NADPH to degenerated chondrocytes, effectively restoring cellular metabolism and enhancing cartilage homeostasis.[14] These cutting-edge studies mark significant advances in the field of artificial photosymbiosis.[15] Nevertheless, none of these photosynthetic systems can sustain long-term symbiotic relationships with higher animal cells, primarily due to the non-replicability of the photosynthetic units and the challenges posed by host immune responses.
In this study, we identified Synechocystis PCC 6803 identified as an optimal candidate for establishing endosymbiosis with mammalian cells and developed a membrane-coating delivery strategy that enabled Synechocystis PCC 6803 to be endocytosed by various mammalian cells. Notably, human embryonic kidney cells HEK293 emerged as a suitable host for achieving long-term endosymbiosis with Synechocystis PCC 6803. We believe this represent an important step toward the establishment of sustained artificial photosynthesis in mammals.
2 Results
2.1 Identification of Synechocystis PCC 6803 as a Promising Candidate for Endosymbiosis
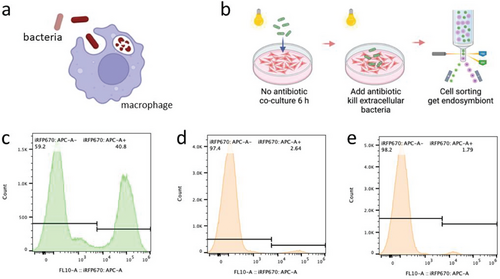
To identify a suitable photosynthetic bacterium for establishing endosymbiosis with mammalian cells, several model cyanobacteria in the field of synthetic biology were selected: Synechocystis PCC 6803, S. elongatus UTEX 2973 and S. elongatus PCC 7942. These three cyanobacterial species exhibited comparable sensitivity to DMEM as regards growth (Figure S1, Supporting Information). Then, they were co-cultured with J774A.1 macrophages, and phagocytosis of the cyanobacteria was examined (Figure 1a). The presence of phycocyanin in the cyanobacteria enabled us to rapidly identify potential endosymbionts as red-fluorescent cells through fluorescence-activated cell sorting (FACS) (Figure 1b; Figure S2, Supporting Information). There were marked variations in the efficiency of phagocytosis among the cyanobacteria species. After 6 h of co-culture, approximately 40% of the macrophages exhibited red channel autofluorescence due to phagocytosis of Synechocystis PCC 6803 (Figure 1c). The corresponding values for S. elongatus UTEX 2973 and S. elongatus PCC 7942 were much lower, at 2.6% and 1.8%, respectively (Figure 1d,e).
Next, the FACS-isolated red-fluorescent macrophages were cultured under both light and dark conditions. Microscopic observations showed that the duration of red fluorescence within macrophages was markedly longer in the case of Synechocystis PCC 6803 than that observed for S. elongatus UTEX 2973 or S. elongatus PCC 7942, both under illumination and in the dark (Figure 2). Indeed, Flow-cytometric analysis showed that 60% of endosymbiont J774A.1-PCC 6803 cells retained red fluorescence after 3 days (Figure 3b), whereas the population of endosymbiont J774A.1-PCC 7942 retaining red fluorescence decreased to 14% after the same period (Figure 3a). Approximately 18% of J774A.1-PCC 6803 cells still exhibited red fluorescence even after 10 days of culture (Figure 3c). Remarkably, after 4 weeks of culture, 5% of the J774A.1-PCC 6803 cells continued to exhibit fluorescence (Figure 3d).
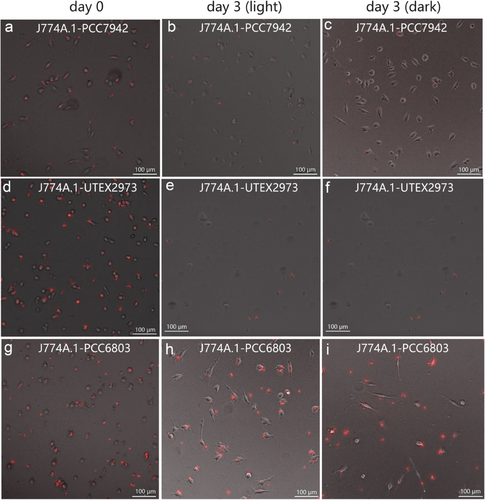
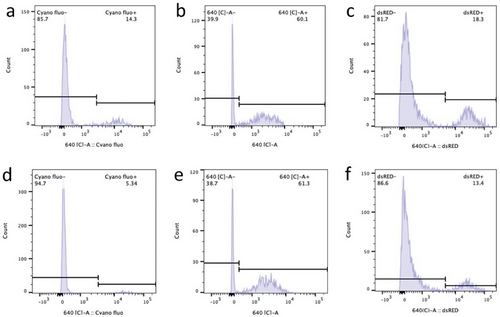
Furthermore, Synechocystis PCC 6803 demonstrated superior metabolic adaptability within the cytoplasm of J774A.1 macrophages. During culture in the dark, the remaining proportion of endosymbiont J774A.1-PCC 6803 cells displaying red fluorescence was 61% and 13.4% after 3 and 10 days, respectively (Figure 3e,f), which was comparable to the value observed under illumination. However, the fluorescence of J774A.1-PCC 7942 cells vanished completely when they were cultured in darkness for 3 days (Figure 2c). This phenomenon may be attributed to the superior metabolic adaptability of Synechocystis PCC 6803 to the dark condition. Previous research has indicated that Synechocystis PCC 6803 can exhibit both autotrophic and heterotrophic growth.[16] Therefore, Synechocystis PCC 6803 is likely to adopt a similar mechanism to Rickettsia or Shigella flexneri, utilizing pyruvate or hexose phosphates within the host cell to sustain cellular viability in the dark.[17] Host cells can sustain themselves by continuously accessing nutrients from the culture medium. Consequently, the organic carbon sources within their cytoplasm are replenished, thereby facilitating the prolonged survival of endosymbiotic Synechocystis PCC 6803 in a heterotrophic mode, even under dark conditions. These findings collectively indicate that Synechocystis PCC 6803 is the most promising candidate among those examined for achieving photosynthetic endosymbiosis with mammalian cells.
2.2 Synechocystis PCC 6803 Relieves Macrophage ATP Deficiency under Illumination
Following the choice of Synechocystis PCC 6803 as the preferred cyanobacterium for establishing endosymbiosis, we next focused on its capacity to provide energy for the host organism. Previous research has noted the presence of uncharacterized nucleotide transport fusion proteins in S. elongatus PCC 7942, which act as ADP/ATP translocases, potentially facilitating ATP secretion when extracellular ADP is present.[18] We first assessed the ATP secretion capability of Synechocystis PCC 6803. As depicted in Figure 4a, when the test buffer contained 80 µM ADP, Synechocystis PCC 6803 exhibited a 1.5-fold higher ATP secretion within 30 minutes compared to S. elongatus PCC 7942 at an equivalent cell density. This observation indicates that Synechocystis PCC 6803 possesses a more robust ATP secretion ability. In view of the previous results showing that S. elongatus UTEX 2973 was the least successful candidate, we did not include it in the present comparison.
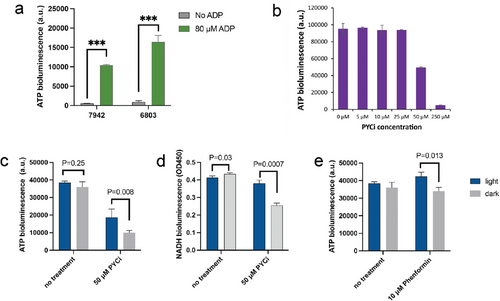
The next objective was to assess whether the secreted ATP could mitigate the energy-deficient phenotype of the host cell. To induce an energy-deficient state in macrophages, we selected the mitochondrial pyruvate carrier inhibitor (PYCi) as an inducer. This chemical effectively prevents the transport of cytoplasmic pyruvate into mitochondria, resulting in a reduced flux within the tricarboxylic acid (TCA) cycle, decreased NADH regeneration, and consequently, a reduction of ATP production via respiration. For J774A.1 macrophages, the introduction of 50 µM PYCi led to a significant 47% reduction in ATP bioluminescence (Figure 4b). We subsequently employed this concentration of PYCi to treat the endosymbiont J774A.1-PCC 6803. As depicted in Figure 4c, ATP bioluminescence of J774A.1-PCC 6803 decreased by a remarkable 3.5-fold after a 10-day treatment with 50 µM PYCi in the dark. Notably, this ATP deficit was partially compensated under illumination, where ATP bioluminescence reached twice the level observed in the dark (Figure 4c).
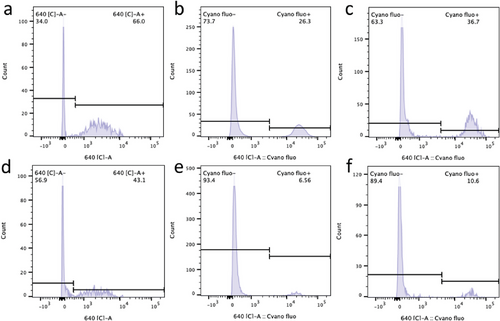
Similar outcomes were observed when assessing intracellular NADH concentration as an indicator of cell viability (Figure 4d). Cell viability was also analyzed through flow cytometry. After three days of culture with 50 µM PYCi, the proportion of endosymbiont J774A.1-PCC 6803 cells exhibiting red fluorescence was 66% under illumination, which was 53% higher than the proportion observed in the dark (Figure 5a,d). This difference became more pronounced after 10 days, with the proportion of cells exhibiting red fluorescence under illumination being four times higher than in the dark (Figure 5b,e). Furthermore, we utilized phenformin, which directly inactivates complex I in the respiratory chain,[19] to induce ATP deficiency in macrophages. The results indicated that intracellular Synechocystis PCC 6803 partially rescues the phenformin-induced ATP deficiency in macrophages (Figure 4e), thereby enhancing their survival under illumination (Figure 5c,f).
2.3 Construction of Photosynthetic Endosymbionts with Non-Macrophage Cells
While macrophages have a natural ability to phagocytose cyanobacteria, time-lapse microscopy revealed that they were unable to replicate after the phagocytosis of cyanobacteria (Figure 7a–d; Video S1, Supporting Information). Besides, this would be a significant impediment to achieving long-term endosymbiosis. Consequently, we sought to introduce cyanobacteria into other types of mammalian cells. Employing the same co-culture method (Figure 6a), we tested non-macrophage mammalian cells, including HEK293, Jurkat, and 3T3. However, Flow-cytometric analysis revealed that none of these non-macrophage cells exhibited a natural propensity to form endosymbionts with cyanobacteria (Figure 6b–d).
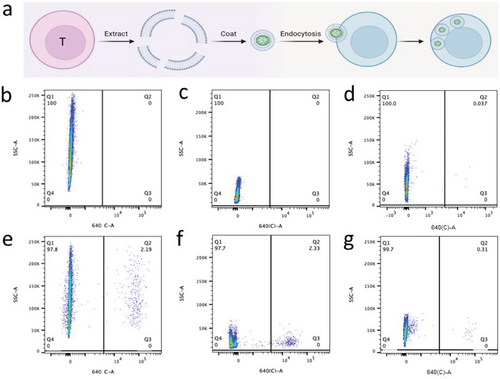
Motivated by a recent study that utilized a membrane-coating strategy to deliver spinach thylakoids into mammalian cells, we endeavored to develop a similar approach for delivering cyanobacteria into mammalian cells. We began with the extraction of the cell membranes from Jurkat cells. These were employed to coat Synechocystis PCC 6803 using a Mini-Extruder device, and the resulting membrane-coated Synechocystis PCC 6803 was then co-cultured with Jurkat cells. Following this co-culture, approximately 2.3% of Jurkat cells exhibited red fluorescence (Figure 6f). Similarly, we extracted cell membranes from HEK293 and 3T3 cells and used them to coat Synechocystis PCC 6803. In these cases, we observed phagocytosis efficiencies of ≈2.2% and 0.4% for HEK293 and 3T3 cells, respectively (Figure 6e,g).
Subsequently, we collected these endosymbionts for time-lapse microscopy. As depicted in Figure 7e–h and Video S2 (Supporting Information), the proportion of Jurkat-PCC 6803 cells displaying red fluorescence exhibited a sharp decline after just 2 days. A similar phenomenon was observed for 3T3-PCC 6803 endosymbionts (Figure 7i–l; Video S3, Supporting Information). Concurrently, both Jurkat and 3T3 cells exhibited a significant reduction in replication and cellular activity. These results collectively indicate that these two types of mammalian cells are ill-suited as host cells for endosymbiosis with cyanobacteria.
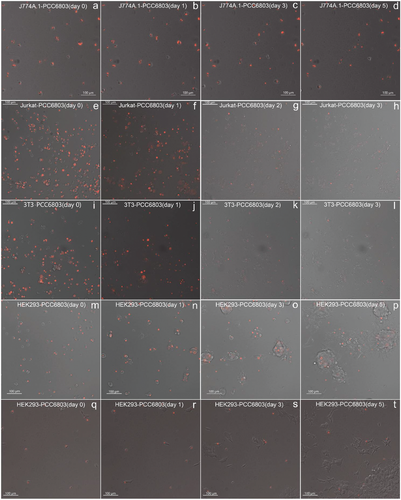
Intriguingly, however, the red fluorescence of Synechocystis PCC 6803 within HEK293 cells remained remarkably stable, and the host HEK293 cells continued to divide and replicate normally despite the presence of phagocytosed Synechocystis PCC 6803 (Figure 7m-p; Video S4, Supporting Information). This phenomenon persisted when we utilized FACS to re-sort the duplicated cell populations for further examination (Figure 7q-t; Video S5, Supporting Information).
Moreover, we compared the impact of engulfing Synechocystis PCC 6803 on the viability of both macrophages and HEK293 cells. As shown in Figure S3 (Supporting Information), macrophages and HEK293 cells without cyanobacteria showed higher viability compared to those containing cyanobacteria, indicating that engulfing cyanobacteria imposes a metabolic burden on mammalian cells. Furthermore, this burden has a more pronounced impact on the viability of macrophages compared to HEK293 cells, indirectly suggesting that HEK293 is a more suitable host for Synechocystis PCC 6803. Finally, macrophages engulfing Synechocystis PCC 6803 exhibited a slight decrease in overall viability within 3 days, whereas HEK293 cells engulfing Synechocystis PCC 6803 showed a slight increase in overall viability. These findings are consistent with observations made during time-lapse microscopy. Taken together, these findings strongly suggest that HEK293 is a favorable candidate host for endosymbiosis, and HEK293-PCC 6803 appears to have the potential to establish long-lived photosynthetic life-forms.
3 Discussion
Compatibility between invading cells and host cells is a pivotal factor in achieving endosymbiosis. In 2018, thiamin auxotrophic Escherichia coli was equipped with a functional ADP/ATP translocase and introduced into the cytosol of respiration-deficient Saccharomyces cerevisiae. In this model system, yeast and bacteria mutually rectified each other's physiological deficiencies, resulting in a stable symbiotic relationship that endured for more than 40 cell doublings.[20, 21] However, when E. coli was introduced into the cytosol of hamster ovary cells, it precipitated the death of the mammalian cells.[13] Similarly, cyanobacterium S. elongatus PCC 7942 was capable of forming a symbiotic chimera with S. cerevisiae, persisting for 15 to 20 generations, yet it could only survive within mouse macrophages for 0.5 to 3 days.[18] These studies highlight the substantial immune response between microorganisms and animal cells, which is markedly more robust than the response among microorganisms.[22] Therefore, it becomes imperative to identify cyanobacterial strains better suited for adaptation to animal cells to realize endosymbiosis.[23-25] In this study, we initially observed that Synechocystis PCC6803 can be more efficiently engulfed by macrophages compared to S. elongatus PCC 7942 (this is a commonly used strain in symbiosis research), possibly due to its spherical cell morphology[26] or to the presence of cell-surface proteins, such as lipopolysaccharides, that stimulate macrophage signaling pathways.[27] Besides, Synechocystis PCC 6803 was found to show higher compatibility with mouse macrophages compared to S. elongatus PCC 7942 or S. elongatus UTEX 2973. Furthermore, Synechocystis PCC6803 could partially ameliorate respiratory deficiency in macrophages, which would be facilitated by the ability to perform ADP/ATP exchange. This ability may have evolved in Synechocystis PCC6803 as a quorum feature to provide energy to cells that have lower exposure to sunlight.[28] In summary, we believe that identifying the enhanced compatibility of Synechocystis PCC 6803 with mammalian cells represents a critical step toward the eventual establishment of permanent photosynthetic endosymbiosis in mammalian cells (Table S1, Supporting Information).
Another key factor for realizing endosymbiosis is the characteristics of the host cell. Until now, the majority of documented natural endosymbiotic relationships have been observed in invertebrates.[29] However, establishing long-term symbiotic relationships between microorganisms and mammalian cells has proven challenging due to the high degree of evolution and exclusivity characterizing mammalian cells. While mammalian macrophages are capable of spontaneously phagocytosing cyanobacteria, it has been demonstrated that they are ill-suited to function as host cells for endosymbiosis with cyanobacteria, primarily due to their robust immune responses.[22, 30] Notably, however, evidence has recently emerged suggesting that mouse tumor cells and bacteria may form an endosymbiotic relationship, with the bacteria facilitating the spread of tumor cells within the host.[31] These findings prompted our efforts to engineer photosymbiosis with non-macrophage cells, highlighting the need to develop effective strategies for delivering cyanobacteria into other types of mammalian cells. In this study, we developed a strategy involving the encapsulation of cyanobacteria with mammalian cell membranes, thereby facilitating the introduction of cyanobacteria into a diverse range of non-macrophage cells. Although this approach draws parallels with the technique used to deliver spinach thylakoids into human chondrocytes, it is pertinent to acknowledge that the delivery of living cells, as distinct from sub-organelles, poses inherent challenges due to their larger size and inherent replication potential.[14] The membrane-coating strategy has previously found application in safeguarding therapeutically engineered probiotics from immune cell clearance.[32] However, our study represents the first utilization of this method for the intracellular delivery of bacteria into mammalian cells. We anticipate that this technique can be extended to deliver various bacterial species into the cytoplasm of animal cells. An illustrative application of this extension could involve delivering magnetically responsive bacteria to achieve magnetic regulation of animal cell movement.[33]
Our research revealed that the endosymbiotic cyanobacteria imposed a metabolic burden on mammalian host cells during the initial stages (macrophage viability decreased by 48% at day 0 after engulfing Synechocystis PCC 6803, and the corresponding value for HEK293 cells was 26%). However, this phenomenon is similar to the evolutionary process observed in nature, where the first encounter between two different species may be lethal, but symbiosis may eventually develop over time.[34] This is also common in diseases, where bacteria may initially be toxic to the host, but eventually establish a mutually beneficial symbiotic relationship with the host.[35] Therefore, future endeavors should focus on achieving synchronized replication of Synechocystis PCC 6803 and HEK293 cells (Table S2, Supporting Information). One potential approach would involve the genetic engineering of Synechocystis PCC 6803 to express listeriolysin O from Listeria monocytogenes, a protein known to aid Bacillus subtilis in escaping vacuoles and replicating intracellularly in macrophages.[36] Another promising avenue of investigation would be precise regulation of the circadian rhythm inherent to Synechocystis PCC 6803, given its notably swifter replication rate in comparison to HEK293 cells.[37, 38] The successful achievement of synchronized replication is anticipated to culminate in the evolution of Synechocystis PCC 6803 into a synthetic chloroplast within HEK293 cells, thus resulting in photosynthetic mammalian cells. The prospective ability of mammalian cells to engage in artificial photosynthesis holds the promise of hastening the realization of light-driven energy acquisition in mammals. Such an advancement is poised to instigate profound transformations within the energy and food sectors.
4 Experimental Section
Cell Culture
Mouse macrophages (J774A.1, ATCC, TIB-67), human embryonic kidney cells (HEK293, ATCC, CRL-11268) and mouse fibroblasts (3T3-L1, ATCC, CL-173) were cultivated in Dulbecco's modified Eagle's medium (DMEM, Catalog number: 52100–39, Thermo Fisher Scientific) supplemented with 10% fetal bovine serum (FBS, Catalog number: F7524, Sigma-Aldrich) and 1% (v/v) streptomycin/penicillin (Catalog number: L0022, Biowest). Human T lymphocytes (Jurkat, ATCC, TIB-152) were cultured in RPMI 1640 Medium (Catalog number: 72 400 021, Thermo Fisher Scientific). Normally, mammalian cells were grown at 37 °C in a humidified atmosphere containing 5% CO2. Cyanobacteria was cultured in BG-11 medium (Sigma-Aldrich C3061) with 50 mm NaHCO3 at 30 °C (for S. elongatus PCC 7942 and Synechocystis PCC 6803) or 37 °C (for S. elongatus UTEX 2973) under white-light illumination (200 µmol photons m−2 s−1).[39]
Chemical Reagents
Mitochondrial pyruvate carrier inhibitor (CAS number: 56396-35-1), phenformin hydrochloride (CAS number: 834-28-6), hexokinase (CAS number: 9001-51-8), adenosine 5’-diphosphate sodium salt (CAS number: 20398-34-9), adenosine 5’-triphosphate magnesium salt (CAS number: 74804-12-9), gentamicin solution (CAS number: 1405-41-0), DAPI nucleic acid stain (CAS number: 28718-90-3) and Cell Counting Kit-8 (Catalog number: 96992-100TESTS-F) were purchased from Sigma-Aldrich. CellTiter-Glo 2.0 Cell Viability Assay Kit (Catalog number: G9241) was purchased from Promega.
Mammalian Cell Membrane Extraction
Cultured cells (≈108 cells) were dissociated with 0.25% trypsin-EDTA solution for 1–3 min, then an equal volume of DMEM medium supplemented with 10% FBS was added, and the cells were collected by centrifugation at 1000 g for 3 min, and washed twice with cold 2 mm EDTA-PBS (10 mL). The washed cells were incubated in 20 mM Tris-HCl (10 mL) at 4 °C for 40–60 min (with shaking every 15 min) and sonicated for 90 s (nine 10-sec cycles at 10% power with 5-s intervals). The supernatant obtained by centrifugation at 15 000 g per 20 min at 4 °C was further centrifuged at 50 000 g for 2 h at 4 °C. The obtained pellet was resuspended in 1 mm EDTA-10 mm Tris-HCl (2 mL), which was then stored at −80 °C.[40]
Introduction of Cyanobacteria into Mammalian Cells
Cyanobacteria were introduced into the cytoplasm of macrophages according to the following steps.[41] On day 1, J774A.1 macrophages were seeded at a density of 300 000 cells per well in 6-well plates in 2 mL DMEM medium containing 10% FBS and 1% (v/v) streptomycin/penicillin for 24 h. On day 2, 1 mL of cyanobacteria suspension (OD730 = 0.5) was centrifuged at 6000 g for 1 min. The pellet was washed twice with DPBS buffer (Catalog number: 14 190 144, Thermo Fisher Scientific) and resuspended in 200 µL DPBS buffer. Next, the J774A.1 cells were washed with pure DMEM (without FBS and antibiotics), and 10 µL of the cyanobacteria suspension was added to each well in the 6-well plates. The plates were incubated for 6 hours under illumination (200 µmol photons m−2 s−1) at 37 °C in a humidified atmosphere containing 5% CO2. The medium of each well was then replaced with DMEM containing 100 mg mL−1 gentamicin and the plates were incubated under illumination at 37 °C, 5% CO2 for 12 h. On day 3, the co-cultured cells were collected and subjected to fluorescence-activated cell sorting. The same procedures were used to deliver cyanobacteria into non-macrophage cells, except that the cyanobacteria were replaced by membrane-coated cyanobacteria, which were prepared as follows. Cyanobacteria suspension (OD730 = 0.5, 2 mL) was centrifuged at 6000 g for 1 min. The pellet was washed twice with DPBS buffer, resuspended with 500 µL DPBS buffer and mixed with 500 µL the prepared cell membranes (2 mg mL−1) for 5 min. The mixture was then extruded 13 times through a polycarbonate porous membrane (pore size: 5 µm) with a Mini-Extruder (Avavti Polar Lipids, USA). The resulting suspension was centrifuged at 6000 g for 1 min, and the pellet was resuspended in 200 µL DPBS buffer.
Analytic Methods
Images and videos were obtained with a Nikon Eclipse Ti2-E (inverted) microscope equipped with an Andor Sona 4.2B-11 camera, under full environmental control for live cell support (temperature, CO2, humidity), and with high-precision motorized XYZ positioning. Bright-field images (exposure, 100 ms), DsRed fluorescence images (632 nm, exposure, 30 ms) and DAPI fluorescence images (438 nm, exposure, 30 ms) were analysed using Image J software. The time interval for time-lapse photography was set at 1 h. For flow-cytometric analysis, cells were collected by centrifugation (300 g, 2 min), washed and resuspended in DPBS. Then, 9 µm DAPI nucleic acid stain was added to the cell suspension. The assays were performed using the 405(E) (DAPI) and 640(C) (phycocyanin) channels of a BD LSRFortessa instrument. The voltage was set to 350 and 295 V on the 405(E) and 640(C) channels, respectively. The data were exported in FCS3 format and processed using FlowJo software (FlowJo, LLC). Intracellular ATP and NADH were determined using a CellTiter-Glo 2.0 Cell Viability Assay Kit[42] and Cell Counting Kit-8,[43] respectively.
Acknowledgements
The authors thank Professor Kirstin Gutekunst for providing Synechocystis PCC 6803, Professor Yin Li for providing S. elongatus UTEX 2973, and Professor Khaled Selim for providing S. elongatus PCC 7942. The authors also thank Yuqing Xie, Chiara Cavallini, Mariangela Di Tacchio and Aleksandra Gumienny for assistance with flow cytometry, and Zhihua Lin and Tom Lummen for help with time-lapse microscopy. This work was supported by a European Research Council (ERC) advanced grant (ElectroGene; grant no. 785800) and in part by the Swiss National Science Foundation NCCR Molecular Systems Engineering.
Open access funding provided by Eidgenossische Technische Hochschule Zurich.
Conflict of Interest
The authors declare no conflict of interest.
Author Contributions
G.H. and M.F designed the project. G.H. and J. H. performed the experiments. G.H. and M.F. analyzed the data and wrote the manuscript.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




