Optimized Pt Single Atom Harvesting on TiO2 Nanotubes—Towards a Most Efficient Photocatalyst
Abstract
In the present work the authors show that anodic TiO2 nanotubes (NT) show excellent harvesting properties for Pt single atoms (Pt SAs) from highly dilute Pt solutions. The tube walls of anodic nanotubes, after adequate annealing to anatase, provide ample of suitable trapping sites—that is, surface Ti3+-Ov (Ov: oxygen vacancy) defects that are highly effective to extract and accumulate Pt in the form of SAs. A saturated (maximized) SA density can be achieved by an overnight immersion of a TiO2 NT layer to a H2PtCl6 solution with a concentration that is as low as 0.01 mm Pt. Such TiO2 NTs with surface trapped Pt SAs provide a maximized high activity for photocatalytic H2 generation (reaching a turnover frequency (TOF) of 1.24 × 106 h−1 at a density of 1.4 × 105 Pt atoms µm−2)—a higher loading with Pt nanoparticles does not further increase the photocatalytic activity. Overall, these findings show that anodic TiO2 nanotubes provide a remarkable substrate for Pt extraction and recovery from very dilute solutions that directly results in a highly efficient photocatalyst, fabricated by a simple immersion technique.
1 Introduction
Single atom (SA) catalysis, over the last 10 years, has become a forefront in heterogeneous catalysis, electrocatalysis, and most recently also in photocatalysis.[1-4] In photocatalytic reactions, illumination generates in a semiconductor excited mobile charge carriers (electron–hole pairs), that then migrate to the semiconductor surface and react with red-ox species present in the environment. One of the most desired and investigated photocatalytic reactions is the charge transfer to aqueous solutions, namely an electron transfer step from the semiconductor conduction band to water or H+ to produce the fuel of the future, dihydrogen. This step is typically kinetically hampered, and in order to reach reasonable photocatalytic reaction rates, noble metals such as Pt, Pd, Rh, and Au are very widely used as co-catalysts on semiconductor surfaces. These co-catalysts typically act twofold: i) they form a Schottky contact with the semiconductor that aids electron trapping on the surface state; and ii) they promote the recombination reaction of the reduced H0 species to H2 (2 H0 → H2)—the combined effects are generally considered to be key in promoting the photocatalytic H2 formation reaction.[5-10] Metal co-catalysts are typically deposited in the form of nanoparticles (of some few nanometers in diameter) onto the semiconductor surface using a range of deposition techniques, involving chemical and thermal reduction reactions or using photodeposition.[11-14] Over the years, many studies attempted to maximize the reaction efficiency, by optimizing the co-catalytic particle size.[15-17] At the same time, minimization of the precious metal loading is a target to reduce the cost of the final photocatalytic system. Obviously, a single atom state represents a maximized surface to volume ratio. Moreover, SAs can provide not only outstanding reactivity but can also allow for unique reaction pathways.[18]
Pioneering work by Yang et al. showed that Pt SAs can be decorated on anatase powders using a co-precipitation method.[19] The authors showed that single PtO units on TiO2 can lead to a very high activity for photocatalytic H2 generation; yielding turnover frequency (TOFs) of up to >1200 h−1.[19, 20]
In general, a key problem for the exploitation of practical SA catalysts is immobilizing SAs on a suitable substrate and in a suitable surface position.[21, 22] The SA community has discussed some feasible substrates to capture and entrap single atoms on photocatalysts; noteworthy attempts include taking advantage of active traps provided by g-C3N4[23, 24] and semiconductive metal-organic frameworks.[25, 26]
For inorganic semiconductors, however, most efforts to capture SAs rely on substrate defects such as lattice kinks, steps, or more effectively on anion- or cation vacancies in surfaces in combination with adequate surface chemistries. Providing trapping sites is not only key to reactivity but also perceived to be the main factor to provide single atom stability against thermally induced agglomeration.[1, 2, 27-29] Earlier work on creating SA sites on titania in the form of powders used impregnation followed by drying and annealing of a precursor to achieve SA decoration on the TiO2 substrate.[30]
Recently, our group introduced a versatile approach to achieve single atom trapping and to carry out photocatalytic reactions on thin, defect engineered TiO2 layers.[31] Defects were introduced under defined thermal reduction conditions (H2 annealing). More recently, we showed that artificial and native defects on TiO2 nanotubes (NTs) grown by anodization of Ti metal are suitable to trap Ir nanoparticles as well as Ir single atoms.[32, 33] In this work on Ir SA,[33] we introduced a facile but very effective approach to trap and immobilize SAs, that is a simple immersion of annealed TiO2 NTs in Ir solution for extended time.
In the present work, we explore the feasibility to apply this principle to Pt but even more importantly to explore it for harvesting minute amounts of Pt from aqueous solutions in the form of single atoms. After harvesting, we explore their functionality of the Pt SAs as a co-catalyst in photocatalytic H2 generation and we evaluate the conditions for a maximum efficiency with a minimum Pt SA loading on the titania NT layers. This is not only of a high scientific interest in terms of exploring suitable anchoring sites for Pt SA co-catalysts, but also in terms of a most cost-effective use of the precious metal co-catalyst. For this, we first fabricate a series of TiO2 NTs providing different defect levels and signatures and we explore decoration of these defect sites with SA Pt from a H2PtCl6 precursor by a simple immersion reaction relying on a reaction of the precursor with Ti3+-oxygen vacancy (Ti3+-Ov) surface defects. We then extract critical factors for SA Pt trapping on anodic TiO2 NTs and evaluate the conditions for a maximized efficiency of Pt SA use for photocatalytic H2 generation.
2 Results and Discussion
Figure 1a shows self-organized anodic TiO2 nanotubes that were formed on a Ti sheet by a simple but optimized anodization process.[34] In our case, we grew tubes to a length of 7 µm and an individual tube diameter of 110 nm, using the anodization parameters described in the experimental section. As formed, these tubes are amorphous and, in general, they need to be thermally annealed to establish a photocatalytically active crystalline form. Previous work showed that after thermal crystallization such tubes contain ample of intrinsic structural defects.[35-37] Such structural defects are particularly evident from a strong response in electron paramagnetic resonance (EPR) spectra. The EPR spectra in Figure 1b are taken for nanotubes after the tube layers were thermally annealed in air at different temperatures, 350, 550, and 750 °C. In the EPR spectra two distinct signatures from different Ti3+ species can be detected. A first signal, a sharp isotropic resonance just below g = 2.0, corresponds to Ti3+ species in regular lattice positions,[38] while the wide spectral feature at gavg ≈1.93 can be ascribed to surface exposed Ti3+-Ov states in anatase.[38] Such defects emerge from thermal annealing of amorphous TiOx to a crystalline TiO2 lattice. The introduction of oxygen vacancies creates distinct electronic levels below the conduction band of TiO2. On the one hand, such defects can affect the photocatalytic H2 evolution,[38-41] but most importantly for our present work, these Ti3+-Ov states can also act as trapping sites for single atoms. Evidently, for annealing at 350 °C the tubes predominantly contain defects in regular (bulk) lattice positions. In contrast, the tubes annealed at 550 and 750 °C show, instead of an isotropic line, an axial signal with increasing linewidth together with a significant contribution of surface Ti3+-Ov defects. From X-ray diffraction (XRD) in Figure 1c one can see that the tubes annealed at 350 and 550 °C consist exclusively of the anatase phase of a comparable crystallite size (compiled in Table S1, Supporting Information) with characteristic diffraction peaks at 2θ = 25.3° and 48.1°. For the samples annealed at 750 °C, slightly larger anatase crystallites are developed, and additionally a rutile phase with a characteristic peak at 2θ = 27.4° becomes apparent (Figure 1c and Table S1, Supporting Information). This also means that in the interpretation of EPR one has to consider defects in the rutile phase. According to literature, the lattice electron trapping sites in rutile also provide a feature at g ≈1.94,[42] that is, the signal at g = 1.93–1.94 represents a convoluted response from anatase and rutile defects. Most importantly, if these differently annealed tubes are immersed in a Pt solution in dark for 24 h (we call this process “dark deposition” as opposed to “photodeposition”), the tube walls become decorated with ample of single atoms anchored on the nanotube walls. Figure 1d shows a high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) image of anodic TiO2 NTs crystallized to anatase at 550 °C and after Pt loading by immersion in 2 mm H2PtCl6 solution for 24 h. From HAADF-STEM, the Pt atoms are evidently present as individual SAs (circled in yellow) and as multimers (agglomerated to dimers, trimers, etc.). A zoomed out TEM image and its corresponding Pt size distribution chart (Figures S1a and S1b, Supporting Information, respectively) further show that the majority of Pt are in the form of isolated single atoms and a few as multimers. Note that the first bar in Figure S1b, Supporting Information, refers to truly isolated SAs (all agglomerates [dimers, trimers, etc.] are not included in the bar labeled SAs). From these observations one can extract an average spacing of the SAs of ≈3.5 nm—this reflects a SA density of ≈1.4 × 105 µm−2.
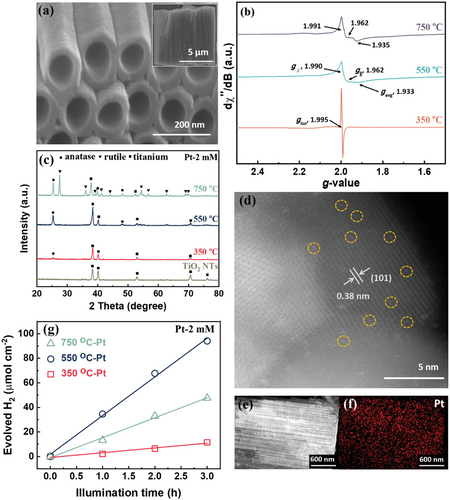
The uniformity of the SA decoration over longer length scales is evident from transmission electron microscopy-energy-dispersive X-ray (TEM-EDX) in Figure 1e,f and further data in Figure S2, Supporting Information. Over the entire tube length, no distinct Pt-aggregates to Pt nanoparticles can be detected, also detailed scanning electron microscopy (SEM) investigations do not reveal any visible particle decoration (with a resolution of SEM of ≈1 nm), see, for example, Figure S3, Supporting Information.
After “dark deposition”—that is, immersion in the H2PtCl6 solution—we tested the tube layers for photocatalytic H2 generation (the details are described in the Experimental Section). After Pt decoration, all annealed tubes show a very high photocatalytic H2 evolution efficiency (Figure 1g). In fact, the highest hydrogen production rate of 31 µmol h−1 cm−2 is achieved with nanotubes annealed at 550 °C. Comparing the defect properties of these champion nanotubes to that of the tubes annealed at 350 °C listed in Table S1, Supporting Information, and their evolved H2, it is apparent that the presence of Pt decorated surface Ti3+-Ov defects is highly beneficial to photocatalytic activity. This can be ascribed most importantly to the ability of Ti3+-Ov defects to trap and immobilize Pt SAs as we will discuss further below.
It should be noted that the H2 evolution performance for the rutile containing tubes is inferior to that of the tubes annealed at 550 °C, even though they also contain surface Ti3+-Ov defects. This can be ascribed to the detrimental effect of rutile on the electron diffusion length in TiO2 nanotubes.[43] Electrons and holes have a considerably shorter life time in rutile, leading to a decrease of the number of charge carriers. Besides, Shi et al. found that the excited trapped electron in Ov of anatase are facilely transferred to Pt, inhibiting carrier recombination, whereas the excited electrons trapped in the intrinsic defects of rutile are hardly transferred to Pt[44] as the trapped states in rutile are very deep.[45]
The photocatalytic H2 evolution of the annealed TiO2 nanotubes (without Pt SA decoration) is also explored (Figure S4, Supporting Information), which suggests the defects alone affect the photocatalytic activity much less than when Pt SAs are present—this further stresses the importance of the Pt SAs decorated on the nanotubes.
In the following, we investigate different concentrations of the Pt-precursor (H2PtCl6) in the solution used for “dark deposition.” Figure 2a shows the photocatalytic H2 evolution performance for TiO2 nanotubes annealed at 550 °C that were treated (dark deposited) using increasingly dilute concentrations of H2PtCl6 solutions, this is from 2 to 0.0005 mm Pt. Clearly, for concentrations from 2 mm down to 0.01 mm, the H2 production rate is virtually constant at ≈31 µmol h−1 cm−2. In other words, the H2 evolution reaction is independent of the precursor concentration until we reach remarkably low contents of 1–2 ppm Pt in the solution.
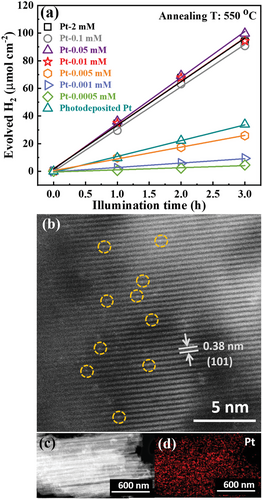
In principle, there may be several reasons for such a finding, such as saturation in the rate determining step (availability of co-catalytic sites, availability of reactant, etc.). In this context, most revealing is a comparison of the HAADF-TEM investigations of TiO2 nanotubes loaded in a 0.01 mm Pt precursor (Figure 2b) with the sample loaded with 2 mm (Figure 1d). An evaluation of such HAADF-TEM images yields a similar SA density of 1.4 × 105 µm−2 for both samples. Also an evolution of size distribution and the average spacing from the TEM images (Figures S1c and S1d, Supporting Information) yields comparable data for the 0.01 mm and the 2 mm concentration. The TEM-EDX in Figure 2c,d confirms also for the dilute sample a uniform Pt distribution with a similar and steady Pt concentration over the entire tube length. In other words, the dark deposition process with a 200 times more dilute solution than used for the data in Figure 1, still provides a maximum co-catalytic efficiency and a very similar SA loading. Only for even lower precursor concentrations (<1 ppm Pt) the photocatalytic activity drops noteworthily with dilution (Figure 2a). This shows that only for these lower concentrations the SA loading is indeed controlled by the Pt concentration in the precursor solution. We ascribe this to a maximum loading in active Pt of the available coupling sites. Only at a precursor concentration <0.01 mm the loading with Pt species becomes overall rate-determining. That is, when Pt precursor concentration decreases to 0.005 mm, there is too little Pt to decorate all potentially active sites. This is further confirmed by atomic absorption spectroscopy (AAS) and X-ray photoelectron spectroscopy (XPS) discussed below. If the TOF is determined for the H2 evolution reaction by considering the H2 evolution rate and the density of single atoms from HAADF-TEM, we obtain a remarkable value of 1.24 × 106 h−1. A value that is close to the record observed for Pt catalysis of the H2 evolution reaction.[46]
To compare the activity of these SA loaded NTs with a classic co-catalytic approach using Pt nanoparticles, we decorated TiO2 nanotubes with Pt nanoparticles by reductive deposition and photodeposition using a 2 mm H2PtCl6 solution (see Figure S5 for further SEM characterization, Table S2 for SEM-EDX, Figure S6, Supporting Information, for XPS). The photocatalytic performance for these tubes (annotated “Photodeposited Pt” in Figure 2a) shows clearly a lower activity (11 µmol h−1 cm−2) than the maximized activity (≈31 µmol h−1 cm−2) observed for the SA decorated samples (even if the SAs are decorated from a 0.01 mm Pt solution). In any case, the presence of Pt cannot be detected by XRD for any sample investigated here (Figure 1c). However, direct information on the Pt loading of the different structures after immersion can be obtained from SEM-EDX—the data is summarized in Table S2, Supporting Information. If one compares the Pt loading from energy dispersive X-ray (EDX) in Table S2, Supporting Information, for the SA samples (Pt-2 mm, Pt-0.1 mm, Pt-0.05 mm, and Pt-0.01 mm) and the tubes photodeposited with Pt nanoparticles, we find that the latter has a loading that is 7 times higher than for the SAs. This means that SA loaded tubes show a Pt mass normalized activity that is >20 times higher than for classic Pt nanoparticles. Remarkably, immersion of the TiO2 nanotubes in the different concentrations from 2 mm down to 0.01 mm leads to a steady Pt loading of ≈0.2 at% detected by EDX (Table S2, Supporting Information). Only for lower concentrations, steadily a decrease in the Pt concentration on the tubes (below 0.11 at%) is found in line with a limitation by available active sites. This similar Pt loading for dark deposition from the Pt-2 mm and the Pt-0.01 mm precursor is further confirmed by TEM-EDX (see Figure S7 and Table S3, Supporting Information) and by chemical analysis of the entire tube layers performed by using AAS—see Table 1. The results show a similar picture as obtained from EDX. For the large variations in concentrations of 2 and 0.01 mm, similar Pt loadings of 0.5–0.8 wt% are obtained and only for lower concentrations the Pt loading drops significantly (e.g., 0.001 mm precursor leads to a 0.12 wt% concentration). EDX and AAS thus show that also on a macroscopic scale, Pt SA loading is already saturated at a precursor concentration of 0.01 mm [PtCl6]2−.
| Photocatalyst | Pt (wt%)-AAS | Pt (at%)-XPS | Pt (at%)-SEM-EDX | Pt (at%)-TEM-EDX |
|---|---|---|---|---|
| 350-Pt (2 mm) | 0.5609 | 0.50 | 0.13 | \ |
| 550-Pt (2 mm) | 0.8946 | 0.79 | 0.21 | 0.09 |
| 550-Pt (0.01 mm) | 0.5202 | 0.59 | 0.16 | 0.09 |
| 550-Pt (0.001 mm) | 0.1199 | 0.16 | 0.08 | \ |
| 750-Pt (2 mm) | 0.6974 | 0.45 | 0.15 | \ |
Information of the Pt-state and surface concentration was obtained from XPS. Figure 3a–c shows XPS spectra for the Pt 4f and the Cl 2p regions after “dark deposition” of Pt from the 2 mm and the 0.01 mm PtCl62− solution (further data are given in Figure S8, Supporting Information). An evaluation of the relative concentrations from XPS yields for the 2 mm samples 0.79 at% Pt and 1.07 at% Cl and for the 0.01 mm sample 0.59 at% Pt and ≈0% Cl species., that is, also from XPS the resulting surface concentration of Pt either from exposure to the 2 mm or the 0.01 mm solution is very similar. The peak positions for Pt of ≈72.5 eV for the immersion deposited samples (72.5 eV for 2 mm and 72.3 eV for 0.01 mm), correspond well to single atom Ptδ+ with δ ≈2 in literature.[31] For reference, Pt nanoparticles deposited by photodeposition in the Pt 4f region is shown in Figure S6, Supporting Information. In this case, the Pt4f7/2 position is located at 70.4 eV, which corresponds well to literature on metallic Pt0 nanoparticles.[47] Interestingly, for the “dark deposition” using the higher precursor concentration, a small Cl peak (Figure 3c) can be detected whereas for the lower concentration this peak is absent. Additionally, for the higher concentration (2 mm) the peak deconvolution shows a small contribution from Pt4+ (Pt4f7/2 at ≈74.1 eV). This suggests that for the higher concentration still some minor amount of Cl-coordinated precursor is present.
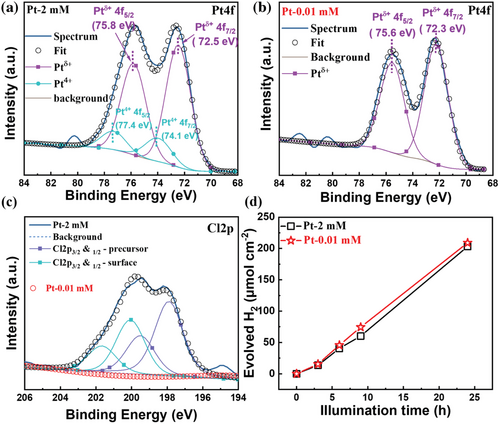
This is evident from the fact that the surface that is fully decorated with Ptδ+ immediately acts as a catalyst—this without catalytic loss over time (Figure 3d). Ptδ+ (approximately Pt2+) can also act as an electron sink (see Figure S10, Supporting Information: note that intermediate Pt red-ox states can act as electron sink in relation to the TiO2 conduction band).
Finally, we explored if the high activity provided from the higher and lower concentration precursor is similarly stable over time. Figure 3d shows the activity of the SA samples (Pt-2 mm and Pt-0.01 mm) measured over illumination time of 24 h. Not only is the photocatalytic activity of these SA decorated nanotubes steady over time, but also the Pt-0.01 mm sample provides virtually the same activity as the Pt-2 mm sample over extended illumination times.
3 Conclusion
In conclusion, our work shows that anodic TiO2 NTs are highly effective for harvesting Pt in the form of single atom species from very dilute solutions. Optimally annealed anatase nanotubes provide surface Ti3+ that are suitable for anchoring SA species directly from the solution. Tubes can be loaded by a simple immersion into very dilute Pt-solutions in the parts per million range. The resulting Pt SA decorated TiO2 nanotubes provide an outstanding co-catalytic performance with a maximum TOF for H2 generation of 1.24 × 106 h−1 at a density of 1.4 × 105 Pt atoms µm−2. Most remarkable is that an optimized performance can be reached by extraction from a very dilute Pt solution. Overnight immersion into a 0.01 mmol Pt solution is sufficient to reach a maximized SA decoration and in turn a maximized photocatalytic H2 evolution efficiency. In comparison, the resulting Pt SA decorated TiO2 nanotubes at this low level of Pt SA decoration outperform the H2 evolution efficiency observed for tubes decorated with classic Pt nanoparticles. As the SA trapping mechanism of the tubes is based on a galvanic displacement with surface defects, we believe that the approach presented here (i.e., the use of anodic nanotubes) for harvesting Pt SAs can find applications for trapping other noble metal SAs and this wide range of photocatalytic reactions becomes accessible for SA co-catalyzed applications. In view of applications such nanotube layers fixed on their Ti-substrate represent highly promising features for the harvesting of Pt from waste streams,[48] and thus turning the Pt waste directly in a valuable photoelectrode enhancement.
4 Experimental Section
TiO2 Nanotubes Preparation
TiO2 nanotubes were grown on Ti foil (0.125 mm, Advent, 99.6+%) via electrochemical anodization using a typical organic electrolyte (Ethylene Glycol (Carl Roth), 3 vol% DI water, and 0.15 m ammonium fluoride (NH4F, Carl Roth). Ti foil was anodized at 60 V for 20 min at room temperature. Before anodization the Ti foil was degreased by sonication in EtOH, acetone, and DI water sequentially, and then dried in a N2 stream. TiO2 nanotubes were then air annealed at different temperatures (350, 550, and 750 °C) for 1 h.
Dark Deposition
Annealed TiO2 nanotubes were immersed in 10 mL MeOH (50 vol%) solution containing diluted H2PtCl6·6H2O (Metakem) solution in a container that can be sealed (in the authors’ case, a quartz cell) for 24 h in dark. Before sealing, the solution was purged with Ar to remove residual gases, such as oxygen and nitrogen. After staying in dark for 24 h, the tubes were then soaked in EtOH and DI water for 15 min each. Subsequently, the samples were dried in a N2 stream.
Photodeposition
The reference sample was decorated with Pt nanoparticles by chemical reduction and photodeposition. 2 mm of metal precursor (H2PtCl6) solution was dissolved in 50 mL ethylene glycol and ultrasonicated till homogeneous. The prepared TiO2 nanotubes were added into the solution and 30 mm NaBH4 was slowly dropped into the mixture. The pH of the solution was adjusted to 9 using 1 m NaOH along with vigorously stirring for 2 h. Subsequently, the TiO2 nanotubes were washed with EtOH and DI water and dried in a N2 stream. Finally, the tubes were illuminated for 3 h with a 365 nm LED.
Characterization
The morphology and chemical composition of the tubes were investigated by field-emission SEM (S-4800, Hitachi, Japan) and EDX, respectively. Chemical state of Ti was studied by a JEOL continuous wave EPR spectrometer, equipped with an Xband Gunn oscillator bridge, a cylindric mode cavity, and a N2 cryostat. All the samples were measured with similar weight (≈5 mg). The EPR spectra were measured with the following parameters: microwave frequency = 9.08 GHz, microwave power = 1.0 mW, modulation width = 1.0 mT, modulation frequency = 100 Hz, time constant = 30 ms, and temperature = 77 K. There was a constant flow of nitrogen in the EPR cavity to eliminate the formation of ice. In all measurements, EPR tubes were kept at the same position in the cavity for comparison. Crystallinity of the samples was characterized by X-ray diffraction (XRD, X'pert Philips PMD diffractometer) operating with graphite monochromatized Cu irradiation (wavelength: 0.154056 nm). High-angle annular dark-filed scanning transmission electron microscopy (HAADF-STEM) and EDX mapping were obtained by a high resolution transmission electron microscope (HRTEM, FEI Titan G2 60–300). Chemical composition of the sample surfaces was studied by XPS (PHI5600); all XPS spectra were calibrated with the Ti2p peak to 458.5 eV. Peak deconvolution was carried out by MultiPak software. Pt loading was further determined by electro thermal atomization-atomic absorption spectroscopy (ETA-AAS) using a graphite furnace with a ContrAA 600 Spectrometer (Analytik Jena AG) equipped with a high resolution Echelle double monochromator and a continuum radiation source (Xe lamp).
Photocatalytic H2 Generation
Prepared photocatalysts were irradiated with a 365 nm LED (power intensity: 65 mW cm−2) in a 10 mL 50 vol% MeOH solution in a sealed quartz reactor. Evolved H2 was determined by a gas chromatograph (GCMS-QO2010SE, SHIMADZU) with a thermal conductivity detector (TCD).
Calculation
The illuminated surface area in this case was ≈1 cm2 and the density of Pt SAs was determined directly from HAADF-TEM images (Figure S1, Supporting Information). Please note that this definition deviates from other approaches used in photocatalysis.[49]
Acknowledgements
The authors would like to acknowledge the DFG (EXC315) and the Operational research program, Development and Education (European Regional Development Fund, Project No. CZ.02.1.01/0.0/0.0/15_003/0000416 of the Ministry of Education, Youth and Sports of the Czech Republic) for financial support. They also thank Dr. Anca Maraze for her contributions to this work. Jan Kolarik is greatly appreciated for his help with quantitative determination using atomic absorption spectroscopy.
Open access funding enabled and organized by Projekt DEAL.
Conflict of Interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
Research data are not shared.




