Non-Animal Strategies for Toxicity Assessment of Nanoscale Materials: Role of Adverse Outcome Pathways in the Selection of Endpoints
Abstract
Faster, cheaper, sensitive, and mechanisms-based animal alternatives are needed to address the safety assessment needs of the growing number of nanomaterials (NM) and their sophisticated property variants. Specifically, strategies that help identify and prioritize alternative schemes involving individual test models, toxicity endpoints, and assays for the assessment of adverse outcomes, as well as strategies that enable validation and refinement of these schemes for the regulatory acceptance are needed. In this review, two strategies 1) the current nanotoxicology literature review and 2) the adverse outcome pathways (AOPs) framework, a systematic process that allows the assembly of available mechanistic information concerning a toxicological response in a simple modular format, are presented. The review highlights 1) the most frequently assessed and reported ad hoc in vivo and in vitro toxicity measurements in the literature, 2) various AOPs of relevance to inhalation toxicity of NM that are presently under development, and 3) their applicability in identifying key events of toxicity for targeted in vitro assay development. Finally, using an existing AOP for lung fibrosis, the specific combinations of cell types, exposure and test systems, and assays that are experimentally supported and thus, can be used for assessing NM-induced lung fibrosis, are proposed.
1 Introduction
Current chemical hazard and risk assessment, and regulatory decision-making hinges on animal testing, which is both expensive and time intensive. For example, the 2-year rat carcinogenicity study can cost up to $4 million and more than 500 animals for a single chemical,[1, 2] and yet, will require follow-up experiments in a separate set of animals to understand the toxicokinetics of the response and underlying mechanisms of toxicity. With the introduction of thousands of new chemicals into the market every year that require immediate regulatory evaluations, and exuberant costs associated with the traditional animal testing, the path forward for the toxicological community is to find animal alternatives that will reduce or replace animal testing while enabling the generation of high quality scientific information relevant for assessing human health risks associated with chemical exposure.[3]
The situation with respect to assessing hazards and risks of exposure to nanomaterials (NM) is not different and can be perceived as even more complex than for chemicals. NM are engineered materials with a size range in the nanoscale (1 to 100 nanometers). The European Commission defines a NM as “a natural, incidental or manufactured material containing particles, in an unbound state or as an aggregate or as an agglomerate and where, for 50% or more of the particles in the number size distribution, one or more external dimensions is in the size range 1–100 nm”. NM exhibit size associated properties not observed or enhanced properties compared to the same material in its bulk or macro size form.[4, 5] With their small size, particle number density and surface area per unit mass increase, which play an instrumental role in their transport, fate, and reactivity in different biological and environmental surroundings, and so are considered the important attributes of toxicity exerted by some such as insoluble and poorly soluble NM.[6-8] Additional associative physico-chemical properties such as chemical composition, surface coatings, band gap, solubility, surface charge, and geometry influence their behavior in biological systems, resulting in an altered exposure, biokinetics as well as, relatively, a varied biological reactivity and toxicity.[9-11] Thus, in essence, a single NM of a particular chemical composition may be produced in a wide range of sizes with diverse surface properties and shapes, each exhibiting unique abilities that may impact, either negatively or positively, biological and environmental systems. From a toxicological perspective, this poses many challenges as it would mean that each property variant of any NM may require a separate safety assessment unless grouping of NM or feasibility of read-across can be demonstrated. If the existing animal toxicity testing strategies were used to complete the safety evaluations of the first generation NM already in commerce, approximately more than fifty years and billions of dollars will be required.[12] With the increasing sophistication of new generations of NM, and the associated availability of advanced materials,[13] the resources required for traditional safety assessments (i.e., animal testing) will no doubt increase significantly, making it imprudent to embark on such an endeavor.
In the last decade, the regulatory toxicology landscape has witnessed fundamental changes in strategies and approaches to substance safety testing, which promote the adoption of animal alternatives. It is posited that the in vitro and in silico models pose the most advantageous alternatives to the previously renowned “gold standard” animal testing strategies. Considering that the field of nanotoxicology is nascent and new compared to chemical toxicology, it is better placed to embrace the opportunity and root itself predominantly on animal alternatives.[14]
Alternative methods and approaches have always existed and have been successfully applied in drug discovery; however, have witnessed endorsement in toxicology and extensive research investments for evaluating their applications to regulatory assessments only recently, especially following the publication of the National Research Council's “Toxicity Testing in the 21st Century: A Vision and a Strategy” document.[3] Although limited, more than a handful of in vitro alternatives are available that have undergone formal validation, for which internationally harmonized protocols and Organisation for Economic Co-operation and Development (OECD) test guidelines (TGs) are available. These in vitro assays are in use for testing chemical-induced adverse outcomes (AOs) such as genotoxicity/mutagenicity, skin sensitization, skin corrosion, eye irritation, and acute toxicity, among others. Several other in vitro test systems and assays are being evaluated toward acceptance as recognized and validated test methods for use in chemical testing and inclusion in regulatory decision making (https://tsar.jrc.ec.europa.eu/). However, these assays require further modifications before their application to NM testing. Although NM-specific hazards are not anticipated, NM are expected to cause hazards that may not be routinely targeted for regulatory assessments.[14] For example, inhalation toxicity is one of the primary concerns for NM exposure and validated alternatives to in vivo pulmonary toxicity tests are not currently available. Similarly, due to their particulate form, AOs similar to those induced by ambient air particulate matter are anticipated following NM exposure, which mainly involves the cardiopulmonary system.[5, 15] However, cardiovascular toxicity is not routinely assessed in regulatory toxicology. Thus, within the existing framework of toxicity testing, in addition to some modifications to the established strategies, approaches, tools, and assays, and design and development of novel test systems as applicable to NM testing, is also imperative. Yet, it is undecided as to what endpoints and which in vitro assays are relevant to achieve this important step in risk assessment of NM, as well as the progression and adoption of alternative models for toxicology testing.
Previously, in a review by Halappanavar et. al.,[16] a broad overview of the adverse outcome pathways (AOP)/s, a description of why AOPs of relevance to NM are required, and specific AOPs of relevance to inhalation toxicity of NM that are currently under development were described. Furthermore, the authors constructed a network using the five linear AOPs outlining the putative mechanisms of NM-induced pulmonary toxicity and highlighted shared molecular initiating events (MIEs) and key events (KEs) across these AOPs. Authors used the AOP173 for lung fibrosis as a basis to present a putative AOP-informed tiered testing strategy that can be used for screening pro-fibrotic NM and for the prioritization of NM that potentially require further testing by complex in vivo models.
The present review article harnesses what was already established in the previous review article by Halappanavar et. al.,[16] and builds on it further. In addition to briefly describing the AOP framework and its role in the design and development of targeted in vitro assays of relevance to the human health risk assessment (HHRA) of NM, the present review article 1) highlights the most commonly assessed and reported in vivo and in vitro ad hoc endpoints in nanotoxicology from the nanotoxicology database consisting of ≈30 000 publications, the discrepancies in what is assessed in vivo versus in vitro, the available OECD TGs for inhalation toxicity and the KEs targeted in these guidelines, and how the ad hoc endpoints reported in the literature can support targeted in vivo response-relevant in vitro assay development and 2) provides specific details on shared KEs derived from the network of inhalation toxicity AOPs, for consideration in in vitro test design. With the continued focus on inhalation toxicity of NM (as inhalation is one of the primary routes of exposure and adverse effects of lung exposure are most frequently assessed and reported for NM),[17] using the AOP network built in Halappanavar et al.,[16] the review will describe in detail the most recurring or shared KEs in the AOPs of inhalation toxicity of NM and summarize alternative assays that are currently available for their measurement. Also, the review describes in detail, the specific cell types, endpoints, assays, exposure systems, and specific biomarkers that can be anchored to the AOP173 for lung fibrosis and used for screening and assessing fibrogenic NM.
While the previous review article focuses on role of AOPs in nanotoxicology broadly, the present article presents select AOP-informed in vitro alternatives and how they can be used in combination to generate the data required for assessing pro-fibrotic NM. Finally, some considerations for validation and refinement of cell based in vitro alternatives are also discussed.
2 Current Nanotoxicology Literature: Most Frequently Reported Biological Endpoints and Their Applicability to In Vitro Cell-Based Assay Development
Most in vitro tests are conducted with an objective of understanding the underlying mechanisms of a substances’ toxicity. Thus, a vast majority of in vitro data available in the literature is not generated, interpreted or reported with a focus of supporting regulatory functions or decision making.[18] The same can be said for the specific in vitro models used in toxicology testing. Nonetheless, some of in vitro methods can serve as targeted approaches for specific toxicity endpoint testing to support the safety assessment of chemicals and NM.
In order to explore what is available in literature, or employed in terms of in vitro tests and identify relevant endpoints for in vitro testing, the nanotoxicology literature landscape was assessed using the Swiss-VCI database (supported by the Swiss FOPH and the German Chemicals Industry Association (VCI, Verband der Chemischen Industrie e.V.). The Swiss-VCI database was primarily established for the purposes of evaluating the potential health, exposure, and environmental effects of NM,[19] consisted of 1878 studies published between 2009 and May 2013 constituting 2598 datasets reporting on both in vivo and in vitro endpoints. The datasets reported on 100 biological endpoints (23 in vivo and 77 in vitro), for 110 different NM including Silver, cerium oxide, copper oxide, multi-walled carbon nanotubes (MWCNT), single-walled carbon nanotubes, titanium dioxide, zinc oxide, and their property variants among others. A total of 203 in vitro cell culture models from nearly 50 tissue types were included and assessed. In vivo studies described different animal species including rats, mice, xenopus, daphnia, and others. Most studies investigated and reported on multiple endpoints. Next, the endpoints as reported by the authors of the specific studies within the publication database were chosen to generate a list of biological endpoints. This list was then then analyzed for the frequency of how often a reported biological endpoint was investigated in all studies, separately for in vitro and in vivo experiments. The results showed that cell death was investigated 1431 times, chemokines/cytokines were investigated 607-times, etc. These specific numbers, that is, the number of times each of the 23 in vivo endpoints and the 77 in vitro endpoints were assessed, were transferred to the freely available program “wordle” (http://www.wordle.net/compose) and each endpoint title was presented in the letter size, which is directly proportional to the frequency of its appearance in the studies. It is important to note that the words chosen for computing were picked directly from the studies in the database and represent the way in which the measured endpoints and results are reported. The results summarized in Figure 1 shows that in vivo, histopathology (192 times assessed) was the most assessed endpoint followed by body weight (78 times), survival (76 times), hematological alterations (61 times), liver toxicity (54 times), development (40 times), embryotoxicity (37 times), and kidney toxicity (30 times). Lung toxicity, heart toxicity, spleen toxicity, and hemolysis were also assessed but in fewer studies (25 times). On the other hand, in studies involving in vitro endpoints (Figure 2), cell death was the most commonly assessed endpoint and reported with 1431 mentions, followed by formation of reactive oxygen/nitrogen species (ROS/RNS, 980 times), cellular membrane integrity (629 times), and altered expression of chemokines/cytokines (607 times). The other frequently assessed in vitro responses or endpoints included DNA strand breaks, formation of oxidative products, cell number of infiltrated immune cells, anti-oxidative response, specific gene expression, depletion of glutathione, cell growth, and micronuclei formation (from 128 times to 274). The results provide a high level summary of what endpoints and how were the endpoints reported in these studies in the database; however, it is acknowledged that granularity with respect to the specific type of measurements were not consistently reported. For example, hematological alterations included several different organ specific toxicity markers which could be associated with different tissues including liver, lung, heart, and kidney. Similarly, in vitro cell death endpoint did not differentiate between the type of death (e.g., apoptosis or necrosis) and different events involved in cell death or different stages of cell death (e.g., mitochondrial dysfunction or increased cellular membrane permeability). This was determined to be the result of inconsistent ontology/terminology used and lack of harmonized reporting standards in nanotoxicoloy. In recent studies by Halappanavar et al., a subset of the Swiss-VCI database consisting of 191 individual publications specifically reporting on NM-induced pro-inflammatory response and inflammation associated events, was analyzed in detail and attempts were made to harmonize the terminology. For example, inflammation response was generically reported in vitro as “cytokines and chemokine expression” or in vivo as “bronchoalveolar lavage fluid cell number” for lung inflammation or “inflammatory cells” for other tissue inflammation, such as liver or simply as “Interleukin (IL)-1”, “IL6”, etc. (the specific pro-inflammatory markers) both in vivo and in vitro studies.[20, 21] Using some level of expert judgement, these reports were reorganized and renamed as “altered expression of pro-inflammatory mediators”, and recruitment of leukocytes', the two generic characteristics of the inflammation process.[22] This allowed categorical grouping of the reported results in a biologically logical manner and allowed identification of each endpoint, assay and specific biomarkers used to assess and report the various inflammation related responses. However, conducting such an analysis for all the publications in the large database of 11 000 publications is beyond the scope of this review.


Regardless of the issues highlighted above, the results can be used to inform the prioritization or selection of in vivo responses for which in vitro endpoints may be designed. For example, the enriched in vitro endpoints in the database highlight the most acknowledged modes of action for NM, which include oxidative stress paradigm (highlighted by the terms reactive oxygen species/nitrogen reactive species, formation of oxidative products, depletion of glutathione, and anti-oxidative response), inflammation (cytokines and cell number of infiltrated immune cells), genotoxicity (strand breaks), and cell injury (cellular membrane permeability, membrane integrity, and cell death). Thus, in the short term, these individual in vitro endpoints reflecting the toxicity modes of action of NMs can be prioritized and targeted for further development. The in vitro endpoints with an established association to in vivo responses, for example, the endpoints associated with genotoxicity such as DNA strand breaks or mutagenicity should also be prioritized for further development.
However, the issues concerning the relevance and effectiveness of the existing data in predicting in vivo toxicity still remains. The results of the word computing analysis clearly demonstrated a substantial discrepancy between what is assessed in vivo versus in vitro studies included in the database. The discrepancy is probably due to the technical limitation to assess the same endpoints in the two approaches. For instance, in animal experiments the cell specific response, that is, DNA strand break, is difficult to investigate, whereas in cell-based assays body weight or systemic effects can obviously not be assessed. Furthermore, it seemed that most in vivo studies involved organ or system toxicity endpoints (e.g., behavior, development, neuronal damage, toxicity of liver, heart, lung, kidney, and spleen) of relevance to regulatory purposes, whereas, in vitro studies mostly targeted a particular biological process such as inflammation, genotoxicity, or cytotoxicity potentially reflective of organ toxicity in vivo. Although a wide variety of assays were identified for each endpoint or response assessed in in vitro studies, prospectively, the ability to translate the in vitro data collected to understand in vivo toxicity was not evident. For example, the results of cytotoxicity assays were common to most in vitro studies in the database; however, it was not clear if the results can be related to in vivo toxicity. It is also important to note that despite the increased number of studies testing a certain endpoint (e.g., cytotoxicity), that this did not mean that the endpoint was, in fact, a toxicologically positive result (i.e., it caused an effect). At present, cytotoxicity may be regarded as information that may be required for the interpretation of the readouts from other in vitro assays in the same experiment. In a majority of cases, the in vitro test system was not anchored to an in vivo response. Also, what was not clear from the literature findings is what do these in vitro tests predict in terms of the observed toxicity or AO in vivo and how well is the in vivo organ represented by an isolated single cell type or an advanced test system constituting multiple cell types in culture under simulated conditions? Resolution of these issues will require in-depth understanding of the underlying mechanisms of NM-induced toxicity or NM mode-of-action and identification of key biological events involved in the toxicity pathways.
In fact, from the research conducted to-date, the intricate biology, if only partially, of the NM induced response and associated mechanisms of toxicity have been revealed for some NM (e.g., carbon nanotubes (CNT) and metal oxides).[23-26] This complex information can be meaningfully organized into an emerging concept of an AOP framework.[16, 27, 28] Positioned to help identify the most essential components of the NM toxicity mechanisms at the relevant biological organization (molecular, cellular, tissue, etc.), this approach will enable anchoring of the ad hoc in vitro measurements (e.g., inflammation, cytotoxicity, etc. as mentioned above) to an in vivo response that is critical for the occurrence of an adverse effect and thereby guide the design and development of targeted in vitro tools and assays for assessing the NM-induced in vivo response. In the following sections, we describe how mechanistic information organized in line with the AOP framework may be used to identify and define in vitro assays that are potentially useful for generating toxicological data required for NM safety assessment and regulatory decisions.
3 The Adverse Outcome Pathway framework
3.1 What are Adverse Outcome Pathways
AOPs allow systematic collection, organization, and simplistic presentation of the complex toxicological response identified in vivo in a linear and modular format.[29] The components of an AOP include: A MIE, defined as a biological event occurring at the molecular level following exposure to substances; a series of KEs, defined as essential response events spanning the cellular, tissue, and organ levels of biological organization that are sequential, causal, and that connect the MIE with an eventual AO and an AO defined as the disease or the pathological outcome of triggering a toxicity pathway, which are targeted for regulatory decisions.[30] MIE and AO are specialized KEs and serve as anchors; a single MIE can result in multiple AOs or multiple MIEs may be linked to a single AO. There are no restrictions on the number of intermediate KEs involved in any AOP. The other important components or building blocks of an AOP are the key event relationships (KERs) that determine the quantitative aspects, such as toxicologically-orientated thresholds, dose-response of relationships shared between KEs, and the temporal dynamics. In other words, KERs describe the level and duration of engagement of an upstream KE required to trigger the activation of a downstream KE. AOPs allow integration of information from a wide variety of sources, that is, in silico, in vitro, and in vivo (animal and human epidemiological) within a plausible framework, enabling elucidation of the complex mechanisms in simplified details. AOPs also provide a detailed account of the domain of applicability in terms of cross-species conservation of the mechanisms, and gender specific differences in response.[31-33] Another important aspect of AOPs is that they are stressor-agnostic and thus allow for integration of toxicological information derived from a wide variety of diverse chemicals, NM as well as other types of stressors such as radiation. However, although AOPs are not chemical-specific, their application allows consideration of their biological plausibility, which includes elucidation of cause-and-effect relationships, as well as identification of physical and chemical properties of the material that may induce deviations in the toxicity mechanisms. Depending on the quality and quantity of data used to build any AOP, it can be putative, qualitative, semi-quantitative, or quantitative. There are many different approaches to developing AOPs and an AOP development guidance has been established by the OECD[30, 34] and a database of AOPs for various AOs of relevance to human health is available https://aopkb.oecd.org/. Current efforts within the field of nanotoxicology have focused on developing new AOPs or refining existing AOPs based on single or multiple model stressors. In addition, data mining approaches using high-throughput and/or high-content information, such as omics data, for the identification of KEs or to support the development of full AOPs has also been used for NM.[35-38]
3.2 Their Utility in Human Health Risk Assessment of Nanomaterials
Depending on the quality and quantity of data used in constructing AOPs, AOPs can have far-reaching application in the formal NM risk assessment process.[39, 40] As stated above, the AOP framework allows collation and logical organization of a battery of experimental data from different sources and identification of essential biological events that are both physiologically and chemically plausible. By that, AOPs can help identify key data gaps concerning a stressor, an AO, or the associated mechanism, and thereby focus research priorities and resource investments. The putative, qualitative, or semi-quantitative AOPs can be used to identify available alternative methods, selection, and design and development of novel in vitro alternatives and, to support integrated approaches to testing and assessment (IATA) principles[41-45]–—the guidance published by OECD[46] to standardize and evaluate IATA mentions six general principles that all IATA's constitute, which include a defined endpoint, a defined purpose, a description of the underlying rationale, a description of the individual information sources used, a description of how data from individual sources are processed, and a consideration of the known uncertainties along with a description of the magnitude of each source of uncertainty.[47] When the available data for quantitative risk assessment is insufficient, AOPs help identify the type of new data required. AOPs provide biological context for the selection of in vitro assays and inclusion of in vitro data derived from such assays in decision making.[48] MIE describes an initial point of interaction between stressors and the biomolecule, and thereby, link the structural features and chemistry of a stressor to a response. MIEs allow the development of predictive methods such as quantitative structure-activity relationship (QSAR) and, support chemical categorization and read across strategies. The intermediate KEs are as important as MIEs and used to identify and prioritize measurement endpoints and assays, as relevant to the biological organization, and in early screening tier assessments. Assays based on KEs have been successfully applied in early identification of hazards during the drug development and research, and in prioritization of chemicals for further regulatory assessments. Quantitative AOPs, which mathematically describe the complex dose, time, and response–response relationships shared between KEs and the AO, and the factors that modulate these relationships, enable quantitative prediction of probability of the AO occurrence or expected extent of the AO manifestation at a specified exposure level. Where exposure is characterized and the physico-chemical properties of the substance are identified, the quantitative AOPs facilitate formal and effective HHRA, aiding regulatory decision making.[40]
4 Strategic Use of Adverse Outcome Pathways in the Design and Development of In Vitro Cell-Based Alternatives Relevant to Safety Evaluation of Nanomaterials
4.1 A Case Study of Inhalation Toxicity for Focused In Vitro Assay Development
- Lung fibrosis (including inflammation) (AOP173 (https://aopwiki.org/aops/173): Substance interaction with lung resident cell membrane components leading to fibrosis, and NO ID: Substance interaction with lung epithelial and macrophage cell membrane leading to lung fibrosis)
- Lung emphysema (AOP1.25: Increased substance interaction with alveolar cell membrane leading to lung emphysema)
- Acute lung toxicity (AOP302: Lung surfactant function disruption leading to acute lung toxicity)
- Lung cancer (AOP303: Frustrated phagocytosis leading to lung cancer)
- Atherosclerotic plaque formation (AOP237: Cellular sensing of stressor leading to plaque progression)
Of these, only the AOP for lung fibrosis is fully developed and has undergone both internal and external reviews organized by the OECD EAGMST committee. It is important to note that as AOPs are developed according to the principles and guidance published by OECD,[30] the individual AOP titles are subjected to change; however, they can be looked up in AOPwiki using the unique AOP identification number associated with them (https://aopwiki.org/). The individual KEs identified in these AOPs can serve as the basis for the targeted in vitro assay development.
In a recent review published by Halappanavar et al.,[16] each of these linear AOP roadmaps were examined for their interconnectivity and a network of AOPs representing the complexity of the respiratory biology perturbed by NM was described. Although none of the AOPs have been formally validated or endorsed by the OECD,[16] they can be used to inform the research and development priorities. The shared, or commonly occurring KEs in the network can be prioritized for the development of in vitro cell-based bioassays that can be used in tier-1 screening and assessment of NM.
Figure 3 is a modified AOP network presented in Halappanavar et al.,[16] and shows how individual KEs in a single, linear AOP interact with KEs in other AOPs. A detailed analysis of the network showed a significant overlap between the KEs across the AOPs; however, the KE (biological events) titles were described using different words and terms, resulting in redundant KEs. The MIEs were largely non-specific and did not identify a specific NM target at the molecular level, rather described the NM-bio interactions in a broader sense involving different lung cell types, lung cell membrane components, and other biomolecules of the lung microenvironment. The NM-bio interaction could be physical, mechanical, receptor-mediated, and/or binding to proteins. Even though NM may be in the molecular size range and capable of significantly influencing the functions of biomolecules in the surrounding environment, the major toxicity mechanisms identified to date do not specify the type of interactions or specific biomolecules involved in the nano-bio interaction. Among the KEs, KEs of inflammation process, including altered expression of (pro-)inflammatory mediators and increased recruitment of leukocytes, were common to greater than three AOPs in the network (Figure 3), respectively. However, similar to the MIE, inflammatory KEs were broadly described and did not specify the main actors such as the specific (pro-)inflammatory mediators or inflammatory cell types involved. There was also a high degree of overlap between the KEs describing loss of alveolar capillary membrane integrity (common to three AOPs), and activation of T helper type 2 type response, fibroblast/myofibroblast proliferation and extracellular matrix (ECM) deposition (common to two AOPs). In addition, events such as oxidative stress and cytotoxicity were common to two AOPs. Oxidative stress was defined as an associative event (responsible for the exacerbation of a response but may not be deemed essential to be listed as a KE in the pathway) in two AOPs—AOP173 lung fibrosis and AOP1.25 lung emphysema, and was positioned as a KE in the AOP303 for lung cancer. The most highly connected KE was the “Increased, recruitment of (pro-)inflammatory cells” related to inflammation and the most central KE was “Loss of alveolar membrane integrity”, a tissue level KE reflecting tissue injury. From the network derived, it may be stated that NM-induced AOs have a similar origin, involve common KEs and that the eventual AO trajectory may be influenced by the NM property variations and, duration and time of exposure. The network analysis also suggested that certain NM may have the capacity to induce multiple AOs in a single exposure setting.
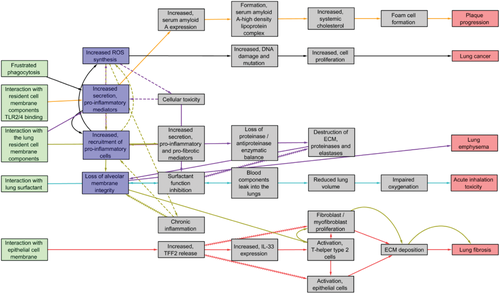
Albeit the lack of specificity, the shared KEs from the network can be used for identification and selection of in vitro endpoints for further development to support the first-tier testing strategies for the purposes of screening and prioritization of NM. In fact, the individual AOP roadmaps and shared KEs can be used to anchor the ad hoc in vitro endpoints identified from the literature (Section 2), thereby enabling mechanistic interpretations of the data generated in the context of in vivo toxicity relevance. The other considerations for selection and prioritization may involve targeting of KEs, which have demonstrated in vivo relevance, which can be easily measured, and for which well established in vitro assays and methodologies already exist. Below, each shared KE from the network (Figure 3) are described in detail along with the endpoints available for measuring them.
4.2 Shared Key Events as the Basis for Potential Development of Animal Alternatives
Table 1 shows the most overlapping KEs from the network (common to more than two linear AOPs), the existing OECD[49] approved in vivo methods available (TGs 403, 412, and 413) for measuring these KEs, potential in vitro systems available, as well as listing ad hoc endpoints that are commonly measured in vitro to assess these KEs. It is evident that only a small fraction of the toxicity response pathway induced by NM, targeting mainly one of the early KEs and the end disease KE, is assessed in vivo and currently, there are no validated cell-based alternatives for the same.
| Overlapping KEs | OECD in vivo inhalation toxicity assaysTG 403, 412, and 413 | Potential animal alternativesNon-validated | |||
|---|---|---|---|---|---|
| Endpoints | Systems/models | ||||
| MIE—Substance interaction with the resident cell membrane components. | Expression changes in alarmins, lysosomal enzymes, receptor pathway activation, cellular uptake, and lysosomal destabilization assays NM–protein, NM–lipid interactions, and surfactant functionality assay |
Statistically determined gene signature(s) targeting multiple KEs | Ex vivo Precision cut lung slice model |
In vitro EpiAlveolar constructs, co-culture models, and single cell models |
|
| KE—Increased, proinflammatory and profibrotic mediators | BALF parameters— –Lactate dehydrogenase –Total protein or albumin –Total leukocyte count, absolute cell counts, and calculated differentials for alveolar macrophages, lymphocytes, neutrophils, and eosinophils. Histology |
Resident immune cell mobilization—histology Ex Vivo | |||
| KE—Increased, recruitment of proinflammatory cells | Expression changes in proinflammatory and profibrotic mediators | ||||
KE—Loss of alveolar capillary membrane Integrity |
BALF parameters— –Lactate dehydrogenase –Total protein or albumin Lung burden—persistence of NM |
TEER Cell death/cytotoxicity |
|||
| AE/KE—ROS synthesis | ROS levels | ||||
4.2.1 Molecular Initiating Events Highlighting Types of Nanomaterials–Cell Interaction
The MIEs described in linear AOPs involve the interaction of NM with the outer cell membrane components or interfere with the biomolecules in the surrounding medium (e.g., physiological fluids, cell culture medium) in a way which has a direct impact on the tissue/cell response. Thus, for NM-relevant AOs, the term MIE may not always reflect a molecular level interaction as required by its formal definition.[36] In the human lung, inhaled NM are first received in the bronchoalveolar lining fluid (BALF), where they are exposed to a complex mixture of lipids and proteins, known as lung surfactant. The secretions protect the host from pathogens and environmental threats, as well as contribute to the normal functioning of the lung. The binding of secretory proteins, for example, surfactant proteins, to NM results in their displacement via wetting forces, altering their function, and can impact the surfactant layer required for the maintenance of surface tension in the alveolar region,[50-52] ultimately resulting in an acute respiratory distress and inhalation toxicity (AOP302). Mechanical interaction of high aspect ratio NM (HARN) such as CNTs with cells is shown to pierce the cell membrane resulting in frustrated phagocytosis, cellular injury, and death,[53, 54] which is associated with multiple AOs including lung fibrosis (AOP173, AOP NO ID), lung cancer (AOP303), lung emphysema (AOP1.25), and even a systemic AO involving the cardiovascular system[55] (AOP237, atherosclerotic plaque formation).[5, 56] The NM are also suggested to induce foreign body reaction in the host cells. The danger associated molecular patterns (DAMPs) such as the cellular debris and cytokines or alarmins released from the injured or dying cells act as ligands to the pattern recognition receptors present on the surface of the resident epithelial, alveolar macrophage, and other lung immune cells, and consequently, activating them.[57] Insoluble NM adsorbed with opsonins including immunoglobulins and serum proteins also serve as ligands to the receptors on the macrophage cell surface, triggering the immune response cascade. In many instances, multiple types of interactions can be initiated simultaneously by a single NM. Thus, the broad MIE involving NM-bio interaction at large is critical for governing the cellular response to NM and it is the determinant of the toxicity trajectory toward an AO; however, the MIE is not targeted for assessment in vivo in acute, sub-acute, or sub-chronic inhalation exposure studies and not captured in the OECD TGs 403, 412, and 413.[58]
As such, NM-bio interaction per se is scarcely investigated in nanotoxicological studies and some examples of endpoints used for the measurement include the assessment of lipid-NM and protein-NM corona[59, 60] which does, however, not consider the direct nano-bio interaction at the molecular level. However, the MIE is routinely measured indirectly by assessing the downstream consequences of NM-bio interactions. For example, the release of DAMPs (alarmins, IL1α, and IL1β) in the extracellular medium, expression changes in effector genes and proteins downstream of an activated receptor pathway, and cellular uptake of NM, are some of the endpoints employed to indirectly measure the MIE activation. Although not routinely used, sophisticated cell-free microscopic methods such as fluorescent resonance energy transfer microspectroscopy are employed for detecting NM interacting with lipid layers in real time. The justification for selecting specific endpoints can be derived from the NM physical-chemical property alerts. For example, for soluble NM or HARN, in addition to DAMP release, the specific measurements could include lysosomal enzyme release (cathepsin B), lysosomal membrane destabilization, and release of (pro-)inflammatory cytokines. Some endpoints such as lysosomal destabilization and cellular uptake may be considered as MIEs for certain NM. Another possibility to study NM-cell interaction is to assess visually or quantitatively the interaction of NM with cells and cellular internalization. A battery of techniques is available to detect and to quantify metals, metal oxides, magnetic, fluorescent, as well as electron dense NM, including inductively coupled plasma atomic emission spectroscopy, flow cytometry, light, and electron microscopy.[61, 62] Thus, a number of endpoints are available that assess indirectly the MIE of the NM-bio interaction. In a recent study by Jagiello and colleagues,[63] transcriptomic responses following exposure to MWCNTs that corresponded with the inflammatory KEs of the AOP173, were employed to build a QSAR model to identify the structural aspects of MWCNTs that may be responsible for initiating the lung inflammatory response. The study identified several individual gene (molecular) targets for MWCNT interaction, which may be used to refine endpoints and specific assays for the MIE measurement.
4.2.2 Key Events of Inflammatory Process in General
Inflammation is the organism's response to threats and insults such as pathogens, (bacterial) infection, trauma, and hypersensitivity. Inflammation is a complex and broad act involving several events at the molecular, cellular, and tissue levels, played by different actors in a substance, cell, tissue, or otherwise species-specific manner. However, the process of inflammation is characterized by the three cardinal events “Tissue resident cell activation, ‘Increased pro-inflammatory mediators”, and “Leukocytes recruitment/activation”, of which the latter two are frequently reported events following NM exposure.[64, 22]
Altered Expression of (Pro-)Inflammatory Mediators
(Pro-)inflammatory mediators are the chemical and biological molecules that initiate and regulate inflammatory reactions. They include cytokines, chemokines, and growth factors (cell-derived), and vasoactive amines, complement activation products, and others (blood derived). A variety of them are secreted during innate and adaptive responses to inflammogens, independent of gender or developmental stage influences but in a species, tissue, or the exposure specific manner. A large number of NMs induce expression of cytokines and chemokines in the lungs of rodents exposed via inhalation[65-68] or in vitro in lung cells exposed under submerged conditions.[69, 70] Inflammation induced by many NMs such as metal oxide nanoparticles and CNTs is characterized by continual release of (pro-)inflammatory cytokines and ROS, which is attributed to their pathogenicity potential.[71] These modulators can be grouped based on the cell type that secrete them and also based on the type of immune response they trigger, which is one of the criteria used in their selection for the KE measurement. The other factors that influence specific selection of mediators for investigation vary based on the expertise of the lab, cell types studied, and the availability of resources. Expression changes in single or select set of (pro-)inflammatory mediators is the routine endpoint used to measure this KE both in vitro and in vivo, and involves simple assays such as the single or multiplex quantitative real time polymerase chain reaction (PCR) and enzyme linked immunosorbent assay (ELISA) or flow cytometry techniques that enable detection of intracellular mediator proteins. This KE is not specified in the OECD TGs for inhalation toxicity assessment.
Increased Recruitment of Leukocytes
(Pro-)inflammatory leukocytes (dendritic cells, monocytes/macrophages, and neutrophils) are circulating white blood cells, derived from the bone marrow, that are recruited to the site of infection in lungs, upon sensing or recognition of danger, to clear the invading threats, pathogens, or toxic substances.[72] They differentiate into mature immune cells and are categorized based on their phenotype and activation status, and subsequently their size, the type of receptors they carry on their surface, and their ability to differentiate following external or internal stimulus such as increased expression of cytokines. Activated immune cells secrete a variety of (pro-)inflammatory mediators, which propagate the immune signaling and response, which when not controlled, leads to chronic inflammation and tissue injury. Thus, the two shared KEs of inflammation act in a positive feedback loop mechanism and fuel the (pro-)inflammatory environment. Increased neutrophil number in BALF is the predominantly assessed and reported endpoint for NM exposure via inhalation in vivo. All known inflammogenic NM induce the recruitment of inflammatory cells to the site of injury in lungs. The extent of this recruitment depends on the exposure doses, duration, and the properties of the specific substance, and thus, is an excellent endpoint to determine the potency of NM. This endpoint is routinely measured in vivo and a detailed guidance on how to measure this endpoint is outlined in TGs 412, 403, and 413; however, it cannot be assessed in vitro. Instead, the KE “Altered expression of pro-inflammatory mediators” is used as a proxy in vitro. A suit of (pro-)inflammatory mediators specific to cell types are assessed using the same techniques mentioned above (qRT-PCR, ELISA, flow cytometry, and immunohistochemistry) in different cell culture models. In vivo measurement of leukocyte influx is a signatory of the clinical manifestation of inflammation, it lacks specificity in discriminating the material or mode-of-action specific responses. The expression, abundance, and activity measurements of specific pro-inflammatory mediators enable identification of specific pro-inflammatory mechanisms involved in the response.[5]
4.2.3 Key Event of Tissue Injury, Loss of Alveolar-Capillary Tissue Membrane Integrity
The alveolar-capillary tissue membrane (ACM) is the gas exchange surface of the lungs acting as a barrier between air and the blood circulation. The alveoli are lined by squamous cells, the alveolar type I epithelial cells which cover about 95% of the surface and share a basement membrane with the endothelial cells covering the pulmonary capillaries, and also contain alveolar type II epithelial cells, which secrete lung surfactant (surface active agent) to prevent alveolar collapse.[73] The structural barrier between air and blood is reduced to a mean arithmetic thickness of 2.2 µm or thinner tissue layers in the alveoli.[74] ACM is the primary target for all inhalation toxicants and is subjected to injury constantly. Extensive injury to ACM resulting in the loss of its barrier integrity presents a “point of no return” situation for the tissue. The loss of ACM integrity and increased membrane permeability leads to efflux of protein-rich fluid into the peribronchovascular interstitium and the distal airspaces of the lung, altered fluid transport via disturbances caused in Na channels or malfunctioning Na+/K+ATPase pumps, loss of the surfactant layer (particularly surfactant protein D), as well as altered expression of adhesion molecule-1 (ICAM-1) and many more markers of decreasing lung compliance arising from the lost integrity of ACM.[75] Chronic inflammatory conditions and oxidative stress contribute to ACM loss, which is further influenced by the physico-chemical properties of NM and their exposure regime. The compromised ACM integrity in vivo is measured by measuring total protein or total albumin content in BALF fluid such as recommended in the OECD TGs 403, 412, and 413. In the case of NM, the lung burden of NM is suggested to assist in determining the potential extent of injury.
In vitro, studies often assess the altered expression of (pro-)inflammatory mediators, increased ROS synthesis, or oxidative stress and cytotoxicity, interplay between which is suggested to induce cell injury, reflective of tissue injury, or loss of ACM in vivo (Halappanavar[20]). As such, cellular viability or cytotoxicity assays are the most commonly used endpoints to assess the leaky or compromised cell membrane. The tissue barrier integrity in lung cell models can be assessed if the lung cells are grown on permeable supports enabling the culture medium to be separated on either side of the cultured epithelium.[76] Trans-epithelial electrical resistance (TEER) is an accepted quantitative technique that measures the barrier integrity in cell culture models of endothelial and epithelial cell monolayers. They are based on measuring ohmic resistance or measuring impedance across a wide range of frequencies. Another method to test the epithelial integrity is to measure the permeation of the lung cell barrier by a paracellular marker (e.g., 14C-mannitol, fluoresceine, or different dextrans) which can be characterized by the apparent permeation co-efficient. Finally, epithelial/endothelial cultures can be screened for tight junction or adherens junction proteins using a variety of methods, such as real-time PCR, western blot, immune histochemistry, and immunofluorescence (for a review see Rothen-Rutishauser et al.).[77]
4.2.4 Key Event Representing Oxidative Stress, Formation of Reactive Oxygen Species
Oxidative stress mainly assessed by measuring the increase in total intracellular ROS levels is associated with cellular response to interaction of NM with surrounding cells. Acute increase in ROS, which is mainly benign, is generated by the surface reactivity of NM. The endogenous anti-oxidant capacity of cells renders protection from ROS induced acutely after exposure to NM, which is a DAMP that participates in the host defense mechanisms by sensing and signaling the presence of danger, and activating redox-sensitive processes. ROS is also generated by the metabolic activity of inflammatory phagocytes (commonly known as an “oxidative burst”) during the process of phagocytosis, which also serves to protect the host. However, repeated or persistent exposure to ROS-inducing NM results in persistent ROS generation, quickly depleting the cellular anti-oxidant capacity and induction of oxidative damage to cells. If not resolved timely, ROS generated can contribute to other downstream events such as inflammation, cell death, and tissue injury, all of which can act in a positive feedback loop exacerbating the response toward pathological or toxicological consequences. In the AOP for lung cancer (AOP303), ROS synthesis is associated with oxidative DNA damage leading to DNA mutations and carcinogenic transformations.
Oxidative potential of the particle surface is measured using acellular assays including 2′,7′dichlorofluorescin diacetate which has been widely used as a marker for oxidative stress[78] or use of carboxy-H2DCFDA oxidation inside cells as a fluorescence marker for ROS production. The latter can be used in in vivo (BALF) as well as in vitro experiments.[79] For metal oxide NM, energy levels of the conduction band (band gap) is used to identify the redox potential of the material. Measurement of increased ROS synthesis in cells, expression changes in genes related to pro-/anti-oxidative stress, activation of anti-oxidant canonical pathways, and oxidative damage of DNA, proteins, and lipids, are some of the endpoints routinely measured to assess this event in vitro.
Thus, there are several endpoints and specific assays currently available for investigating the occurrence of shared KEs. These measurements of shared KEs observed early in the AOPs can serve in tier-1 screening and guide the endpoint selection for investigation in the next tier. Thus, the shared KEs can be prioritized for the design and development of fit for purpose in vitro models and assays.
4.3 Advanced In Vitro Systems for Inhalation Toxicity Testing
It is important to note that none of the existing in vitro model or systems, in isolation or in combination, are complete in terms of their effectiveness in accurately recapitulating the complex human or animal lung responses to xenobiotic exposure. The whole lung response includes proximal and distal or alveolar airway components, and signaling from mesenchymal, vascular, immune, neural, and other components of the tissue, which are not entirely mirrored in the available 2D or 3D cell culture models. However, the combination of in vitro models and systems proposed below offer real time monitoring of pathological responses under controlled environments, enable higher-throughput assessment capacity and can be reproduced, which is a critical advantage over clinical or animal studies. As the field ploughs through with advancements in technology and deeper knowledge of the biology, more complexity is expected to be added to these systems to bring them as close to the lung environment as possible.
4.3.1 Exposure Models
One of the important considerations for design and development of an optimal inhalation toxicity testing system for NM is the mode of exposure. Historically, lung toxicity models including single cell type cultures, advanced in vitro culture models of multi-cell types, as well as that of precision cut tissue slices due to the cost of their resource as well as the expertise and time, have been exposed under submerged conditions; That is, where cells are exposed to NM pre-suspended in relevant exposure medium. However, in order for bio-NM interaction to occur, for some NM, lung cells may have to be exposed to an aerosol. The air–liquid interface (ALI) culture method has seen significant developments as is recommended from many experts for this purpose.[80-82] In addition to the possibility to culture lung cells at ALI a number of different (commercially) available ALI exposure systems are available.[83] Such ALI exposure systems also have the advantages that the cells are directly exposed to the NM aerosol allowing the assessment of the MIE. Since most ALI systems work with integrated quartz microbalances, they enable quantitative assessment of the deposited dose, which is an important aspect of the hazard and risk assessment. Currently, literature is beginning to become increasingly focused upon this research approach, yet there are physiological aspects missing from this in vitro next-generation testing strategy that are highly pertinent toward their successful combination with AOP approaches. Specifically, the addition of lung lining fluid compartment, that is, mucus or aqueous lining layer covered with surfactant, should be emphasized to mimic the interaction of the NM aerosol with those first barrier. In addition, some of the exposure systems are technically challenging and time consuming, requiring specific expertise for the production of a reproducible aerosol. Also, each cell type and cell culture model requires extensive optimization and validation under ALI before application for the specific assessment. This makes the system low throughput, making the screening scenario much less possible. However, exposure using ALI may only be required to evaluate the NM-bio interaction and not for the measurement of other KEs in the AOP, which can be assessed in submerged conditions.
4.3.2 Cell Type Considerations
There are many characterized primary or immortalized cell types derived from lung tissues of rodents and humans, the building blocks of the in vitro cell culture systems, available (https://www.epithelix.com, https://www.mattek.com/products/epiairway/) and routinely used to construct various in vitro models from simple monolayer and advanced multi-cell co-culture models to artificial organ constructs that reflect different physiological and anatomical complexity across the different regions of the respiratory system.[84, 85] Single cell culture models are the most commonly used as they are easy and cheap, ready, and ease of access to a variety of cell types from different species, including humans, and simplicity of the system.[86] These include rat alveolar macrophages (NR8383), SV40 immortalized mouse alveolar macrophage cell line (MH-S), mouse alveolar epithelial adenoma cells (LA-4), human lung epithelial type II (“like”) cells (A549), human leukemia monocytic cells (THP-1), and a plethora of others such as the lung epithelial cell-line from transgenic MutaMouse (FE-1) that is specifically tailored to assess the mutagenic potential of a range of chemical substances. Simple co-cultures involving epithelial cells and alveolar macrophages[87] or epithelial and endothelial cells have become a routine.[88] Adding a layer of complexity, more recent models have involved epithelial cells, macrophages, and dendritic cells in a triple cell[89] or epithelial cells, macrophages, dendritic cells, and mast cells in a quadruple cell type co cultures.[90]
While such models have been useful in assessing the acute effects of lung exposure to NM, such as acute oxidative stress and inflammation, they are not positioned to assess complex pathogenicity such as lung fibrosis, a widely assessed and reported AO for NM. The lung fibrosis mechanism described in AOP173 involves multiple components including an early inflammatory component, a tissue injury component, and the tissue regeneration component, all of which involve multiple cell types in the lung. Thus, an ideal in vitro lung model must include immune cells (e.g., macrophages, dendritic cells) covering the inflammatory response, type 1 and 2 pneumocytes to reproduce damage to the epithelium and fibroblasts to represent the initiation of proliferation and migration of epithelial cells and differentiation of fibroblasts into myofibroblasts, a critical KE that results in ECM components to repair the damaged ACM. The use of fibroblast cultures to assess the release of pro-fibrotic mediators and ECM components such as fibronectin and collagen has been investigated. A study by Vietti and colleagues demonstrated the applicability of various fibroblast monocultures to induce fibroblast proliferation upon exposure to MWCNTs; however, the release of collagen, the building blocks of fibrotic lesions, could not be assessed in these mono culture systems.[91] More recently, a study by Barosova et al.,[92] established a novel tissue construct, that is, the EpiAlveolar model, mimicking the lower respiratory tract involving alveolar epithelial type I cells, fibroblasts, and endothelial cells that in addition to enabling investigation of the acute effects such as inflammation, also permit the assessment of chronic effects such as barrier integrity and the biological indicators associated with the onset of lung fibrotic disease. While more simple mono-culture systems provide an interesting and easy avenue to rapidly assess the toxicity induced by thousands of NM variants, the more complex models still need a lot of technical skills, time, and money to test a variety of NMs and endpoints.
Whilst long-term efforts continue to close the divide between in vivo and in vitro in order to obtain completely replacement orientated testing strategies,[93] in the short-term cultures of tissues, such as the precision cut lung slice (PCLS) culture reflect a good example of a model with next level complexity and mimicking an organ response in its entirety.[94] Although a considered “refinement” approach to in vivo experimentation, since tissue slices can be harvested from any tissue and species including humans, the ability to translate data to understanding human responses will become more evident. In another recent study by Rahman et al.,[95] a lung organ mimic involving PCLS culture model, which allows the intricate cellular signaling occurring after NM exposure and measurement of various KEs along the path of lung fibrosis including the histopathological manifestation of the fibrotic disease, was established. Conditional to further optimization, this easy to operate and resource effective organoid model offers a promising alternative to whole animal testing, significantly reducing the number of animals required for testing. It enables generation of quality data relevant for regulatory assessments. However, they lack a systemic response component that is responsible for recruitment of inflammatory cells to the lungs after exposure to NM, which is the prime KE node linking all AOPs of relevance of NM inhalation exposure (Figure 3). There have been attempts to bridge this gap by constructing an organ-on-a-chip or lung-on-a-chip model, which not only constitutes essential cell types that are involved in orchestrating lung responses to stressors but also accurately incorporates the systemic components required for the lung response.[96] Whilst efforts continue to bioengineer the human lung in this manner, systems currently available omit the important immune cells within the lung,[97] for example, macrophages in the alveolar region. Although attempts are underway to amend this issue,[98] until it is possible to incorporate these inflammatory cells, such systems remain unavailable for absolute incorporation toward AOP approaches.
5 Putative Adverse Outcome Pathway-Informed Animal Reduction and Replacement Strategies for Lung Fibrosis
While many in vitro model options are available to choose from, it is also realized that no single model is sufficient to use in isolation. Thus, a test system that constitutes a combination of single cell culture models, co-cultures, tissue constructs, and even tissue culture models, is highly recommended to accurately predict an in vivo response as relevant to humans. The schematic presented in Figure 4 lists three strategies involving an ex vivo test system or model constituting the complex lung tissue architecture and all lung cell types, an advance in vitro model constituting multiple lung cell types chosen to represent the important aspects of lung injury following NM exposure and a model constituting different cell types that specifically allow separate measurement of early or late KEs of the AOP173. All model systems including specific measurements are anchored to AOP173 on lung fibrosis. The strategy also highlights that NM deposition techniques or mode of exposure can vary across different strategies or within a single strategy.
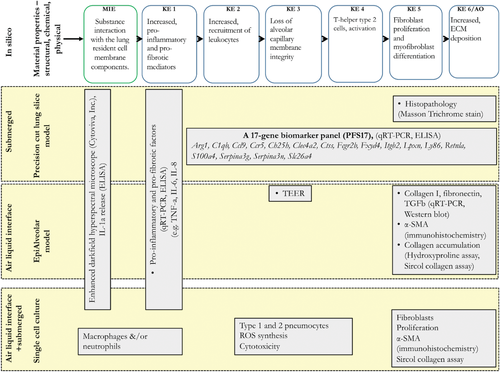
Most test systems, endpoints, and assays identified in this review as well as those presented in Figure 5 are well described, peer-reviewed, easily transferable, and have experimentally demonstrated level of confidence in the results generated, and thus are recommended. An ex vivo PCLS method is included in this alternative testing strategy even though it involves animal experimentation.[95] This is because 1) PCLS uses fewer animals (four times fewer) to achieve what is traditionally achieved in animal experiments. While animal alternatives refer to the complete replacement of animals in testing, the PCLS method allows significant reduction in the total number of animals used, thereby conforms to the reduction philosophy of the 3Rs principle. 2) The current regulatory decision making relies on animal tissue histopathology results, which establish clinical manifestation of a disease that cannot be recapitulated using the available in vitro (2D or 3D) techniques. Moreover, in vitro cell based models of exposure and assessment are still under development, efficiency of which to predict the occurrence of an adverse event in vivo is yet to be established. Until that time, PCLS technique can play an important role in bridging in vitro results with in vivo responses. 3) PCLS technique allows potential assessment of responses in human tissues, and thus will enable establishment of human relevance. For these reasons, PCLS method has been included in the strategies shown in Figure 5. However, none of the assays are validated formally by the prescribed national or international validation standards.
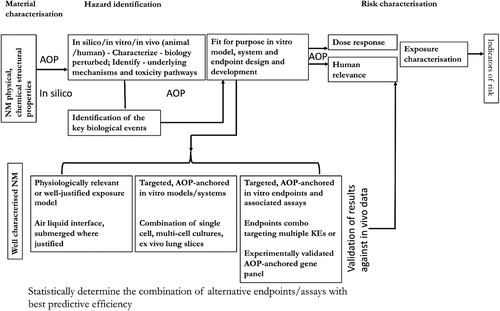
Thus, for now, these assays can be used to serve in an early screening tier of toxicity testing. However, to be useful in regulatory decision making, in addition to being anchored to a mechanism or an AOP, the in vitro assays should also be in alignment with the traditional components of the risk assessment framework that include hazard identification, dose-response characterization, and exposure characterization (Figure 5). The selected assays must have been validated at the national or international level, which includes evaluation of assays and methods for relevance, reliability, specificity. and method transferability. However, the caveat for validation is that human exposure and effects data are non-existent for NM. For example, there is no experimental evidence available to suggest that inhalation exposure to NM induces lung fibrosis or respiratory diseases in humans. Previously, onsite studies carried out in USA, Republic of Korea, Japan, and Europe involving research laboratories and both small and large scale production facilities, suggested that exposure to NM during their synthesis or during accidental spills can occur, especially if exposure-control measures or safety protocols are not implemented or followed. Consumer exposure, although possible, has not been well documented (reviewed in Guseva et al.)[99]. Epidemiological studies involving workers exposed to CNT have found increased levels of markers of fibrosis in serum and sputum of exposed workers, suggesting that pulmonary inflammation and fibrosis to be the likely outcomes in these workers if exposed to certain levels and duration. In a recent review article published by Schulte et al.,[100] human epidemiological studies of workers exposed to nine most widely used NM, including carbon black; synthetic amorphous silica; aluminum oxide; barium titanate; titanium dioxide, cerium dioxide; zinc oxide; CNT and carbon nanofibers; and silver nanoparticles, were systematically evaluated to identify human health effects of exposures to NM. The results showed that there is limited evidence to suggest pathological manifestation; however, indicators of adverse effects in workers exposed to specific NM (from the nine reviewed in this article) have been reported. However, the data on levels of NM in environment and biological exposure data, that is, biologically available or internal dose is not available, precluding derivation of dose-response relationships required for risk assessments. Similarly, quality animal exposure or effects data are scarce for most NM. More importantly, there are no accepted benchmark particles against which NM data can be compared, which is essential for validation. Alternatively, one can use high quality epidemiological studies where available as well as chronic and sub-chronic inhalation studies in rodents on non-NM particles such as process-generated diesel exhaust nanoparticles, or asbestos with abundance of information on exposure, hazard, and dose-response relationships, for the purposes of assay validation.[101-105]
6 Next Steps: Considerations for Validation and Refinement of Cell Based In Vitro Alternatives
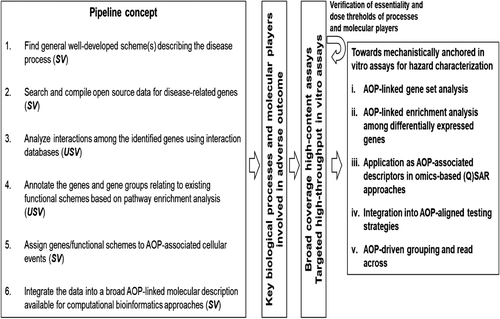
As described earlier, several AOPs of relevance to inhalation toxicity of NM are under development; however, only one of them (AOP173 for lung fibrosis) is near completion. For the rest of AOPs, for now, roadmaps describing putative mechanisms of toxicity exist. Regardless of their development status, none of the AOPs are quantitative, suggesting that generation of new data is warranted which will allow their refinement and validation of AOPs. Similarly, while specific assays and test systems/models for the measurement of the KEs in AOP173 are identified (Figure 4) and for measuring KEs identified in other AOPs, the specific biomolecules (genes, proteins) for targeting by the specific bioassays are still undergoing refinement. For example, the KEs of inflammation are mainly assessed by the targeted measurement of a select set of pro-inflammatory mediators known to be induced by NM,[16] the number and types of which vary across the studies, and specific rationale or guidance for their selection is not available. This is a critical issue and one that will require advanced data mining types of approaches involving mathematical and statistical algorithms to address it. For example, high-throughput and high-content data often referred to as “toxicogenomics” have been used in chemical toxicology and nanotoxicology to gain an understanding of the toxicity mechanisms and to identify and select KEs at all levels of biological organization. The resulting dataset consisting of millions of data points has also supported validation of MIEs and KEs, and identification of multi-variate biomarkers for targeted bioassay development.[26, 28, 35, 95] Such gene set- or pathway-based biomarkers are increasingly applicable to diverse predictive modeling approaches in toxicology and have been shown to have broad potential for identification and probabilistic prediction of human toxicity and AO.[95, 106-108] Recently establishment and validation of a gene panel consisting of 17 individual genes (Figure 4) potentially predictive of lung fibrosis was described in a bioinformatics study by Rahman et al. 2020.[95] The authors showed that the panel of 17 genes are associated with multiple KEs of the AOP173 for lung fibrosis and thus, enable measurement of more than one KE in a single assay. Moreover, the study established the statistical methodology to derive molecular targets that are experimentally validated, anchored to the KEs in the AOP, and an experimental test system for their effective measurement. Other examples of approaches to identify molecular targets associated with specific MIE/KE include bioinformatics-driven data integration pipelines aimed at providing bioinformatically employable molecular descriptions linked to AOPs[37] (Figure 6, left panel). The identification of key biological processes and molecular players involved in AOs supports development of broad coverage high-content (whole-genome microarray/next generation sequencing) and targeted high-throughput transcriptomics (qRT-PCR) assays performed in relevant model systems[109, 110] as described above (Figure 6, middle panels). The molecular descriptions serve as multi-variate biomarkers[41] eligible for supporting the development of gene set- or pathway-based first-tier toxicity testing strategies coupled to probabilistic modeling[111] (Figure 6, right panel). In addition, the molecular descriptors can be used to support mechanism-aware QSAR approaches.[63, 112] This type of molecular descriptors have also been used to establish dose-response relationships and derive points of departures,[35] useful for ranking of NM potency to induce inflammation,[113] identification properties responsible for the effects[63] and for prediction of AOs such as lung fibrosis.[95] The resulting big data and comprehensive knowledge further supports AOP-driven hazard characterization, including grouping and read across among substances, through implementation of omics-based toxicity prediction tools (Figure 6, right panel). Overall, data integration pipelines, such as the one described in Figure 6, constantly develop and become more automated through diverse efforts between the AOP- and the biological pathway-communities. For example, some efforts are aiming to allow AOPs to provide an “index” for the complexity of the biological pathways in pathway-databases, such as WikiPathways.[114] This allows computational scientists to dig deeper into the underlying molecular mechanisms outlined in the AOPs. The vast amount of data generated can help validate the toxicity mechanisms outlined in the AOPs, identify new bioassays and new molecular targets for measurement and in turn, aid in validation of the bioassays and results (Figure 6, feedback arrow from last panel).
7 Conclusion
This review presents the strategies to address the pressing need in the community to optimize and validate existing or novel animal reduction or replacement alternatives in NM toxicity testing; including cell based in vitro, organoid ex vivo and computational in silico methods, with an expectation that these alternatives will eventually help lift the burden placed on regulatory agencies to assess an ever growing number of NM in a faster, cheaper, and timely manner. The review demonstrates how the existing literature and more importantly, AOP thinking can be used to 1) identify the critical biological events (KEs) in the path of an AO, and 2) rationalise testing strategies involving combination of exposure models, test systems, endpoints, and assays. By using the existing AOP for lung fibrosis as an example, the review recommends combination of specific cell or tissue models, assays, and endpoints that can potentially be used for predicting in vivo occurrence of lung fibrosis. It is acknowledged that some physical chemical properties of NM such as solubility are important modifier/predictor of toxicity. The AOP example chosen to elaborate in this review concerns tissue fibrotic response that mainly originates from persistence of an insoluble material and sustained and chronic injury. However, the mechanism presented and the in vitro testing strategies outlined are also applicable to NM that are highly soluble. The proposed strategy is flexible and the individual components of it are expected to change as new knowledge and data become available. However, for now, the stage is set to embark on the new toxicity testing paradigms in nanotoxicology, success of which will rely on the active participation of academic, industry, and regulatory researchers in adopting the proposed strategies for generating and reporting much required toxicity data in a consistent manner. This will allow refinement and validation of the strategies and expedite the process of drafting of guidance documents for their uptake, and application in regulatory decision making.
Acknowledgements
S.H. acknowledges Saba Berhane, Jiao Jianli, Luna Rahman, and Silvia Solorio-Rodriguez for serving as Health Canada's internal reviewers. S.H. acknowledges help of Silvia Solorio-Rodriguez in formatting the manuscript according to journal's standards.S.H. acknowledges the funding from Health Canada's Genomics Research and Development Initiative. S.H., P.N., M.J.D.C., B.R.R., and U.V. acknowledge the support by the PATROLS project, the European Union's Horizon 2020 Research and Innovation Programme under grant agreement No 760813, and S.H. and U.V. acknowledge SmartNanoTox, grant agreement No 686098. B.R.R. acknowledges the support by the Adolphe Merkle Foundation. U.V. acknowledges “FFIKA, Focused Research Effort on Chemicals in the Working Environment” from the Danish Government. H.F.K. acknowledges the support of the Swiss Federal Office of Public Health (FOPH) and the German Chemicals Industry Association (VCI, Verband der Chemischen Industrie e.V.).
Conflict of Interest
The authors declare no conflict of interest.
Author Contributions
All authors were involved in writing the different sections of the manuscript. All authors read, commented, and proposed revisions to the entire manuscript. The final manuscript was compiled by S.H. All authors approved the final manuscript.
Biographies

Sabina Halappanavar is a research scientist at Health Canada, a Federal Government institution of Canada and an adjunct professor at the Department of Biology of University of Ottawa, Canada. She is investigating the early molecular origin of cardio-pulmonary diseases induced by inhalation toxicants using toxicogenomics tools. She has developed nanomaterial-relevant Adverse Outcome Pathways, in support of the potential development of animal reduction and replacement strategies and regulatory decision making. She serves on the Science Advisory Board of European nanotoxicology consortia and is an active contributor to the nanotoxicology research initiatives led by the Organisation for Economic Co-operation and Development.

Penny Nymark serves as an assistant professor at the Institute of Environmental Medicine, Karolinska Institute in Sweden, and member of the Swedish National Platform for Nanosafety. Her research and involvement in multiple EU-funded projects over the past 15 years has focused on mechanisms of particle-induced toxicity and development of novel animal-free, data-driven methods, including Adverse Outcome Pathways for safety assessment of nano- and advanced materials.

Harald F. Krug studied Chemistry and Biology at the University of Kassel and received his Ph.D. from the Georg-August University in Göttingen in Germany. After a postdoc period at the GSF Research Center Munich he took over the Department for Environmental Toxicology at the Research Center Karlsruhe. He habilitated at the TH Karlsruhe in 1996 in the field of Environmental Toxicology. From 2007 he taught at the University Berne and headed a laboratory for Nanomaterials-Biology Interaction at Empa and was a member of the board. Since 2017 he is retired but founded his own company NanoCASE GmbH.

Martin J. D. Clift is an associate professor of in vitro (nano)particle toxicology at Swansea University Medical School, developing advanced in vitro models to understand human health hazards associated with inhalation of nanomaterials. Martin is chair of the UK Animal Alternative Technologies Society and a member the British Toxicology Society Sub-Scientific Committee, Scientific Board of Animal Free Research, and UK Government Committee on the Medical Effects of Air Pollutants.

Barbara Rothen-Rutishauser has received her Ph.D. in 1996 in Cell Biology at the Swiss Federal Institute of Technology in Zurich (ETHZ). She worked as postdoc and group leader at ETHZ and University of Bern, Switzerland. Since 2011, she is a full professor in BioNanomaterials at the Adolphe Merkle Institute, University of Fribourg, Switzerland, sharing the position equally with Prof. Alke Fink. She is an expert in the field of cell-nanoparticle interactions, with a particular focus on the development of human 3D epithelial tissue models as alternative test methods to animal experiments.

Ulla Vogel (Ph.D) is a professor and a group leader at the National Research Centre for the Working Environment. She is a participant of past and present H2020 projects related to nanosafety. She is trained as biochemist, molecular biologist, and certified ERT toxicologist. She has worked with molecular epidemiology and the toxicology of inhaled aerosols including nanoparticles with focus on effects in lungs and secondary target organs (cardiovascular, liver, blood) since 1997. She is advisor to the Danish Working Environment Authority on toxicology.




